Association between Body Composition and Bone Mineral Density in Children and Adolescents: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
3. Results
3.1. Characteristics of Eligible Studies
3.2. Risk of Bias
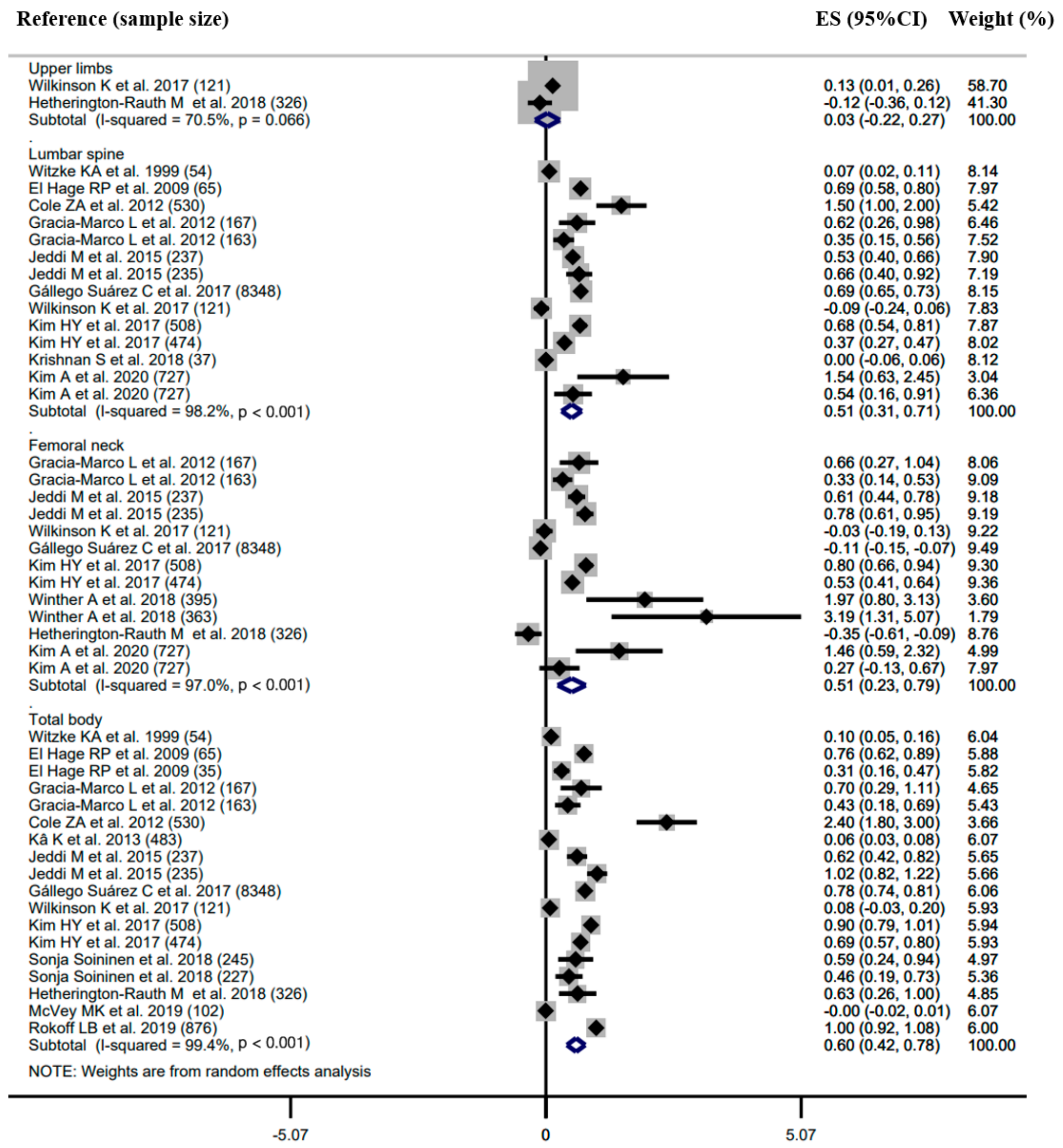
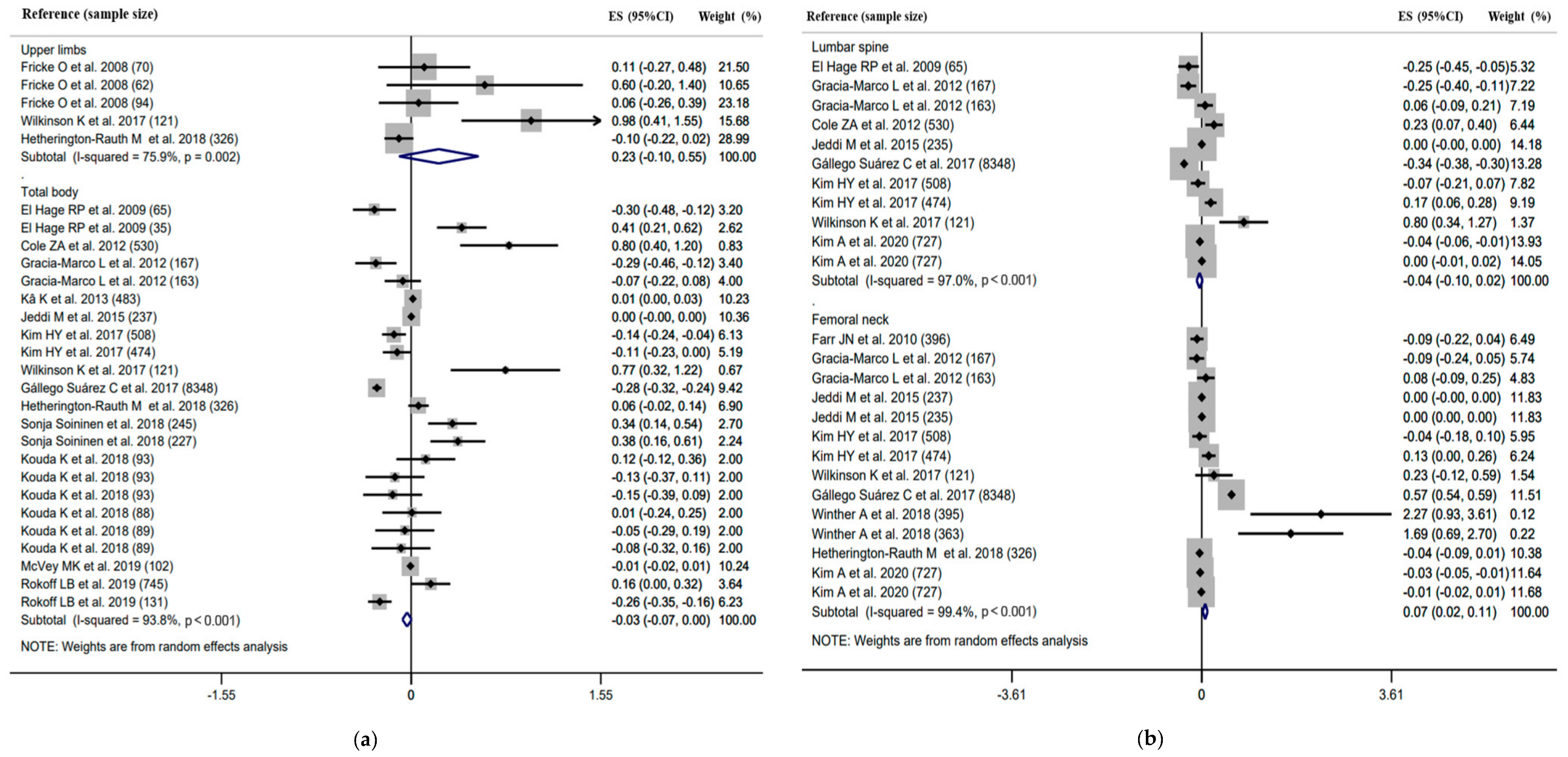
3.3. Meta-Analysis
3.3.1. Correlation between Body Composition and BMD
Subgroup Analysis
Sensitivity Analysis
Publication Bias
3.3.2. Association between Body Composition and BMD
Subgroup Analysis
Sensitivity Analysis
Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torres-Costoso, A.; López-Muñoz, P.; Martínez-Vizcaíno, V.; Álvarez-Bueno, C.; Cavero-Redondo, I. Association Between Muscular Strength and Bone Health from Children to Young Adults: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 1163–1190. [Google Scholar] [CrossRef]
- Zhu, X.; Zheng, H. Factors influencing peak bone mass gain. Front. Med. 2021, 15, 53–69. [Google Scholar] [CrossRef]
- Bland, V.L.; Bea, J.W.; Blew, R.M.; Roe, D.J.; Lee, V.R.; Funk, J.L.; Going, S.B. Influence of Changes in Soft Tissue Composition on Changes in Bone Strength in Peripubertal Girls: The STAR Longitudinal Study. J. Bone Miner. Res. 2020, 36, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [Green Version]
- Hamrick, M.W. A role for myokines in muscle-bone interactions. Exerc. Sport Sci. Rev. 2011, 39, 43–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapses, S.A.; Pop, L.C.; Wang, Y. Obesity is a concern for bone health with aging. Nutr. Res. 2017, 39, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Dolan, E.; Swinton, P.A.; Sale, C.; Healy, A.; O’Reilly, J. Influence of adipose tissue mass on bone mass in an overweight or obese population: Systematic review and meta-analysis. Nutr. Rev. 2017, 75, 858–870. [Google Scholar] [CrossRef]
- Sioen, I.; Lust, E.; De Henauw, S.; Moreno, L.A.; Jiménez-Pavón, D. Associations Between Body Composition and Bone Health in Children and Adolescents: A Systematic Review. Calcif. Tissue Int. 2016, 99, 557–577. [Google Scholar] [CrossRef] [PubMed]
- Ripka, W.L.; Modesto, J.D.; Ulbricht, L.; Gewehr, P.M. Obesity Impact Evaluated from Fat Percentage in Bone Mineral Density of Male Adolescents. PLoS ONE 2016, 11, e0163470. [Google Scholar] [CrossRef]
- Gállego Suárez, C.; Singer, B.H.; Gebremariam, A.; Lee, J.M.; Singer, K. The relationship between adiposity and bone density in U.S. children and adolescents. PLoS ONE 2017, 12, e0181587. [Google Scholar] [CrossRef]
- Khwanchuea, R.; Punsawad, C. Association between Anthropometric Indices, Body Composition and Bone Parameters in Thai Female Adolescents. Indian J. Pediatr. 2017, 84, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Jung, H.W.; Hong, H.; Kim, J.H.; Shin, C.H.; Yang, S.W.; Lee, Y.A. The Role of Overweight and Obesity on Bone Health in Korean Adolescents with a Focus on Lean and Fat Mass. J. Korean Med. Sci. 2017, 32, 1633–1641. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, K.; Vlachopoulos, D.; Klentrou, P.; Ubago-Guisado, E.; De Moraes, A.C.; Barker, A.R.; Williams, C.A.; Moreno, L.A.; Gracia-Marco, L. Soft tissues, areal bone mineral density and hip geometry estimates in active young boys: The PRO-BONE study. Eur. J. Appl. Physiol. 2017, 117, 833–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hetherington-Rauth, M.; Bea, J.W.; Blew, R.M.; Funk, J.L.; Hingle, M.D.; Lee, V.R.; Roe, D.J.; Wheeler, M.D.; Lohman, T.G.; Going, S.B. Relative contributions of lean and fat mass to bone strength in young Hispanic and non-Hispanic girls. Bone 2018, 113, 144–150. [Google Scholar] [CrossRef] [Green Version]
- Kouda, K.; Ohara, K.; Nakamura, H.; Fujita, Y.; Jaalkhorol, M.; Iki, M. Fat mass is positively associated with bone mass acquisition in children with small or normal lean mass: A three-year follow-up study. Bone 2018, 107, 222–227. [Google Scholar] [CrossRef]
- Krishnan, S.; Anderson, M.P.; Fields, D.A.; Misra, M. Abdominal obesity adversely affects bone mass in children. World J. Clin. Pediatr. 2018, 7, 43–48. [Google Scholar] [CrossRef]
- Soininen, S.; Sidoroff, V.; Lindi, V.; Mahonen, A.; Kröger, L.; Kröger, H.; Jääskeläinen, J.; Atalay, M.; Laaksonen, D.E.; Laitinen, T.; et al. Body fat mass, lean body mass and associated biomarkers as determinants of bone mineral density in children 6-8years of age—The Physical Activity and Nutrition in Children (PANIC) study. Bone 2018, 108, 106–114. [Google Scholar] [CrossRef] [Green Version]
- Winther, A.; Jørgensen, L.; Ahmed, L.A.; Christoffersen, T.; Furberg, A.S.; Grimnes, G.; Jorde, R.; Nilsen, O.A.; Dennison, E.; Emaus, N. Bone mineral density at the hip and its relation to fat mass and lean mass in adolescents: The Tromsø Study, Fit Futures. BMC Musculoskel. Disord. 2018, 19, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rokoff, L.B.; Rifas-Shiman, S.L.; Switkowski, K.M.; Young, J.G.; Rosen, C.J.; Oken, E.; Fleisch, A.F. Body composition and bone mineral density in childhood. Bone 2019, 121, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zhu, M.; Zheng, R.; Hu, Y.; Li, R.; Zhu, G.; Chen, L.; Xiong, F. Analysis of bone mass and its relationship with body composition in school-aged children and adolescents based on stage of puberty and site specificity: A retrospective case-control study. Medicine 2019, 98, e14005. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Baek, S.; Park, S.; Shin, J. Bone Mineral Density of Femur and Lumbar and the Relation between Fat Mass and Lean Mass of Adolescents: Based on Korea National Health and Nutrition Examination Survey (KNHNES) from 2008 to 2011. Int. J. Environ. Res. Public Health 2020, 17, 4471. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Cole, Z.A.; Harvey, N.C.; Kim, M.; Ntani, G.; Robinson, S.M.; Inskip, H.M.; Godfrey, K.M.; Cooper, C.; Dennison, E.M. Increased fat mass is associated with increased bone size but reduced volumetric density in pre pubertal children. Bone 2012, 50, 562–567. [Google Scholar] [CrossRef] [Green Version]
- Streeter, A.J.; Hosking, J.; Metcalf, B.S.; Jeffery, A.N.; Voss, L.D.; Wilkin, T.J. Body fat in children does not adversely influence bone development: A 7-year longitudinal study (EarlyBird 18). Pediatr. Obes. 2013, 8, 418–427. [Google Scholar] [CrossRef]
- El Hage, R.P.; Courteix, D.; Benhamou, C.L.; Jacob, C.; Jaffré, C. Relative importance of lean and fat mass on bone mineral density in a group of adolescent girls and boys. Eur. J. Appl. Physiol. 2009, 105, 759–764. [Google Scholar] [CrossRef]
- McVey, M.K.; Geraghty, A.A.; O’Brien, E.C.; McKenna, M.J.; Kilbane, M.T.; Crowley, R.K.; Twomey, P.J.; McAuliffe, F.M. The impact of diet, body composition, and physical activity on child bone mineral density at five years of age-findings from the ROLO Kids Study. Eur. J. Pediatr. 2020, 179, 121–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fricke, O.; Land, C.; Semler, O.; Tutlewski, B.; Stabrey, A.; Remer, T.; Schoenau, E. Subcutaneous fat and body fat mass have different effects on bone development at the forearm in children and adolescents. Calcif. Tissue Int. 2008, 82, 436–444. [Google Scholar] [CrossRef]
- Gracia-Marco, L.; Ortega, F.B.; Jiménez-Pavón, D.; Rodríguez, G.; Castillo, M.J.; Vicente-Rodríguez, G.; Moreno, L.A. Adiposity and bone health in Spanish adolescents. The HELENA study. Osteoporos. Int. 2012, 23, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Ivuskans, A.; Latt, E.; Maestu, J.; Saar, M.; Purge, P.; Maasalu, K.; Jurimae, T.; Jurimae, J. Bone mineral density in 11-13-year-old boys: Relative importance of the weight status and body composition factors. Rheumatol. Int. 2013, 33, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Viljakainen, H.T.; Pekkinen, M.; Saarnio, E.; Karp, H.; Lamberg-Allardt, C.; Mäkitie, O. Dual effect of adipose tissue on bone health during growth. Bone 2011, 48, 212–217. [Google Scholar] [CrossRef]
- El Hage, R.; Moussa, E.; Jacob, C. Bone mineral content and density in obese, overweight, and normal-weighted sedentary adolescent girls. J. Adolesc. Health 2010, 47, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Jeddi, M.; Dabbaghmanesh, M.H.; Ranjbar Omrani, G.; Ayatollahi, S.M.; Bagheri, Z.; Bakhshayeshkaram, M. Relative Importance of Lean and Fat Mass on Bone Mineral Density in Iranian Children and Adolescents. Int. J. Endocrinol. Metab. 2015, 13, e25542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeon, H.C.; Lee, K.; Kim, J.; Park, T.J.; Kang, D.W.; Park, D.J. The Relationship between Body Fat Percent and Bone Mineral Density in Korean Adolescents: The Fifth Korea National Health and Nutrition Examination Survey (KNHANES V-1), 2010. Korean J. Fam. Med. 2014, 35, 303–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K. Regional percent fat and bone mineral density in Korean adolescents: The Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV-3), 2009. Asia Pac. J. Clin. Nutr. 2013, 22, 69–73. [Google Scholar] [CrossRef]
- Farr, J.N.; Chen, Z.; Lisse, J.R.; Lohman, T.G.; Going, S.B. Relationship of total body fat mass to weight-bearing bone volumetric density, geometry, and strength in young girls. Bone 2010, 46, 977–984. [Google Scholar] [CrossRef] [Green Version]
- Kâ, K.; Rousseau, M.C.; Lambert, M.; O’Loughlin, J.; Henderson, M.; Tremblay, A.; Alos, N.; Nicolau, B. Association between lean and fat mass and indicators of bone health in prepubertal caucasian children. Horm. Res. Paediatr. 2013, 80, 154–162. [Google Scholar] [CrossRef]
- Witzke, K.A.; Snow, C.M. Lean body mass and leg power best predict bone mineral density in adolescent girls. Med. Sci. Sports Exerc. 1999, 31, 1558–1563. [Google Scholar] [CrossRef]
- Júnior, I.F.; Cardoso, J.R.; Christofaro, D.G.; Codogno, J.S.; de Moraes, A.C.; Fernandes, R.A. The relationship between visceral fat thickness and bone mineral density in sedentary obese children and adolescents. BMC Pediatr. 2013, 13, 37. [Google Scholar] [CrossRef] [Green Version]
- Mosca, L.N.; Goldberg, T.B.; da Silva, V.N.; da Silva, C.C.; Kurokawa, C.S.; Bisi Rizzo, A.C.; Corrente, J.E. Excess body fat negatively affects bone mass in adolescents. Nutrition 2014, 30, 847–852. [Google Scholar] [CrossRef] [Green Version]
- Goulding, A.; Taylor, R.W.; Grant, A.M.; Murdoch, L.; Williams, S.M.; Taylor, B.J. Relationship of total body fat mass to bone area in New Zealand five-year-olds. Calcif. Tissue Int. 2008, 82, 293–299. [Google Scholar] [CrossRef]
- Nordström, P.; Nordström, G.; Thorsen, K.; Lorentzon, R. Local bone mineral density, muscle strength, and exercise in adolescent boys: A comparative study of two groups with different muscle strength and exercise levels. Calcif. Tissue Int. 1996, 58, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, I.; Yamaguchi, T.; Yamamoto, M.; Yamauchi, M.; Kurioka, S.; Yano, S.; Sugimoto, T. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2009, 94, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Ferron, M.; Wei, J.; Yoshizawa, T.; Del Fattore, A.; DePinho, R.A.; Teti, A.; Ducy, P.; Karsenty, G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 2010, 142, 296–308. [Google Scholar] [CrossRef] [Green Version]
- Fricke, O.; Schoenau, E. The ’Functional Muscle-Bone Unit’: Probing the relevance of mechanical signals for bone development in children and adolescents. Growth Horm. IGF Res. 2007, 17, 1–9. [Google Scholar] [CrossRef]
- Ho-Pham, L.T.; Nguyen, U.D.; Nguyen, T.V. Association between lean mass, fat mass, and bone mineral density: A meta-analysis. J. Clin. Endocrinol. Metab. 2014, 99, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Mizuma, N.; Mizuma, M.; Yoshinaga, M.; Iwamoto, I.; Matsuo, T.; Douchi, T.; Osame, M. Difference in the relative contribution of lean and fat mass components to bone mineral density with generation. J. Obstet. Gynaecol. Res. 2006, 32, 184–189. [Google Scholar] [CrossRef]
- Marwaha, R.K.; Garg, M.K.; Bhadra, K.; Tandon, N. Bone mineral content has stronger association with lean mass than fat mass among Indian urban adolescents. Indian J. Endocrinol. Metab. 2015, 19, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Garnett, S.P.; Högler, W.; Blades, B.; Baur, L.A.; Peat, J.; Lee, J.; Cowell, C.T. Relation between hormones and body composition, including bone, in prepubertal children. Am. J. Clin. Nutr. 2004, 80, 966–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, K.L.; Li, H.; Yang, W.Y.; Hou, J.L.; Xu, Y.; Xiao, S.M. Analysis of the Association between Fat Mass Distribution and Bone Mass in Chinese Male Adolescents at Different Stages of Puberty. Nutrients 2021, 13, 2163. [Google Scholar] [CrossRef] [PubMed]
- Hoy, C.L.; Macdonald, H.M.; McKay, H.A. How does bone quality differ between healthy-weight and overweight adolescents and young adults? Clin. Orthop. Relat. Res. 2013, 471, 1214–1225. [Google Scholar] [CrossRef] [Green Version]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. Vitamin C and Bone Health: Evidence from Cell, Animal and Human Studies. Curr. Drug Targets 2018, 19, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, D.; Adikaram, S.G.S.; Atapattu, N.; Kendaragama, K.; Senevirathne, J.T.N.; Jayasekera, H.D.; Wickramasinghe, V.P. Vitamin D supplementation in obese Sri Lankan children: A randomized controlled trial. BMC Pediatr. 2020, 20, 426. [Google Scholar] [CrossRef] [PubMed]
- Reyman, M.; Verrijn Stuart, A.A.; van Summeren, M.; Rakhshandehroo, M.; Nuboer, R.; de Boer, F.K.; van den Ham, H.J.; Kalkhoven, E.; Prakken, B.; Schipper, H.S. Vitamin D deficiency in childhood obesity is associated with high levels of circulating inflammatory mediators, and low insulin sensitivity. Int. J. Obes. 2014, 38, 46–52. [Google Scholar] [CrossRef]
- Badr, S.; Legroux-Gérot, I.; Vignau, J.; Chauveau, C.; Ruschke, S.; Karampinos, D.C.; Budzik, J.F.; Cortet, B.; Cotten, A. Comparison of regional bone marrow adiposity characteristics at the hip of underweight and weight-recovered women with anorexia nervosa using magnetic resonance spectroscopy. Bone 2019, 127, 135–145. [Google Scholar] [CrossRef]
- Fazeli, P.K.; Bredella, M.A.; Freedman, L.; Thomas, B.J.; Breggia, A.; Meenaghan, E.; Rosen, C.J.; Klibanski, A. Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. J. Bone Miner. Res. 2012, 27, 1864–1871. [Google Scholar] [CrossRef]
- Akune, T.; Ohba, S.; Kamekura, S.; Yamaguchi, M.; Chung, U.I.; Kubota, N.; Terauchi, Y.; Harada, Y.; Azuma, Y.; Nakamura, K.; et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Investig. 2004, 113, 846–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woods, G.N.; Ewing, S.K.; Sigurdsson, S.; Kado, D.M.; Eiriksdottir, G.; Gudnason, V.; Hue, T.F.; Lang, T.F.; Vittinghoff, E.; Harris, T.B.; et al. Greater Bone Marrow Adiposity Predicts Bone Loss in Older Women. J. Bone Miner. Res. 2020, 35, 326–332. [Google Scholar] [CrossRef]
- Park, Y.M.; Jankowski, C.M.; Swanson, C.M.; Hildreth, K.L.; Kohrt, W.M.; Moreau, K.L. Bone Mineral Density in Different Menopause Stages is Associated with Follicle Stimulating Hormone Levels in Healthy Women. Int. J. Environ. Res. Public Health 2021, 18, 1200. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.B.; Krum, S.A. Estrogen receptors alpha and beta in bone. Bone 2016, 87, 130–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wawrzkiewicz-Jałowiecka, A.; Lalik, A.; Soveral, G. Recent Update on the Molecular Mechanisms of Gonadal Steroids Action in Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 5226. [Google Scholar] [CrossRef] [PubMed]


| Author, Year | Country | Design | Sample Size (M, F) | Age (y) | Body Composition | Assessment | BMD Sites | Covariates | |
|---|---|---|---|---|---|---|---|---|---|
| Body Composition/BMD | r | β | |||||||
| Witzke KA et al. 1999 | USA | Cross-sectional study | 54 (0, 54) | 14.6 ± 0.50 | LM, FM | DXA a | WB, FN, LS (L2~L4) | None | LM: months past menarche, height, bone-free LM, leg strength, leg power, FM |
| Fricke O et al. 2008 | Germany | Cross-sectional study | 295 (139, 156) | Prepubertal M: 8.7 ± 1.90 F: 8.5 ± 1.60 Puberty M: 14.5 ± 2.40 F: 14.4 ± 2.80 | LM, FM, BF% | pQCT d | Arms | None | FM: sex, height, fat area, muscle area |
| Goulding et al. 2008 | New Zealand | Cross-sectional study | 194 (113, 81) | M: 5.0 ± 0.05, F: 5.0 ± 0.05 | LM, FM | DXA b | TBLH | LM/FM: sex, weight | NA |
| El Hage RP et al. 2009 | France | Cross-sectional study | 100 (65, 35) | M: 15.2 ± 0.60, F: 15.2 ± 0.70 | LM, FM, BF% | DXA a | WB, LS (L1~L4) | None | LM/FM: FM (or LM) |
| El Hage R et al. 2010 | Lebanon | Cross-sectional study | 65 (0, 65) | Obese: 15.5 ± 2.70 Overweight: 15.0 ± 1.80 Normal: 15.6 ± 1.60 | LM, FM, BF% | DXA a | WB | None | NA |
| Farr JN et al. 2010 | USA | Cross-sectional study | 396 (0, 396) | Tertiles of FM Lowest: 10.3 ± 1.00 Middle: 10.9 ± 1.10 Highest:11.0 ± 1.10 | LM, FM, BF% | DXA b/pQCT d | FN | None | FM: muscle cross-sectional area, maturity, bone length, physical activity, ethnicity |
| Viljakainen HT et al. 2011 | Finland | Cross-sectional study | 186 (73, 113) | M: 11.7 (7.7–18.1) F: 13.2 (7.4–18.8) | BF% | DXA a | WB, FN, LS (L1~L4) | BF%: age, pubertal development | NA |
| Cole ZA et al. 2012 | England | Longitudinal study | 499 (253, 246) | M: 6.6 ± 0.20 F: 6.6 ± 0.20 | LM, FM | DXA a | WB, LS | NA | FM: LM |
| Gracia-Marco L et al. 2012 | Spain | Cross-sectional study | 330 (167, 163) | M: 14.7 ± 1.30 F: 14.7 ± 1.10 | LM, FM | DXA a | WB, LS, FN | NA | LM/FM: height, calcium intake, sexual maturation, average physical activity, WB FM (or LM) |
| Júnior IF et al. 2013 | Brazil | Cross-sectional study | 175 (83, 92) | 11.1 ± 2.60 | BF% | DXA b | WB | None | NA |
| Kâ K et al. 2013 | Canada | Cross-sectional study | 483 (305, 178) | 9.4 ± 0.90 | LM, FM | DXA b | WB | LM/FM: age, height | LM/FM: age, sex, height, daily calcium, vitamin D intake, daily physical activity, vitamin and mineral intake in the two previous weeks, bone or joint problems including history of fracture, FM (or LM) |
| Lee K et al. 2013 | Korea | Cross-sectional study | 710 (365, 345) | 10.0–19.0 | BF% | DXA a | Arms, WB, LS, FN | NA | BF%: age, weight, height, serum 25(OH) vitamin D level, calcium intake, menarche status |
| Ivuskans A et al. 2013 | Estonia | Cross-sectional study | 264 (264, 0) | Normal: 12.1 ± 0.77 Overweight: 11.9 ± 0.76 | LM, FM, BF% | DXA b | WB, FN, LS (L2~L4) | FM: age, pubertal status | NA |
| Streeter AJ et al. 2013 | England | Prospective longitudinal study | 347 (NA, NA) | Baseline M: 8.9 ± 0.02 F: 8.9 ± 0.02 Follow up 9, 10, 11, 12, 13, 14, 15, 16 | BF% | DXA b | TBLH | None | BF%: age at peak height velocity |
| Mosca LN et al. 2014 | Brazil | Cross-sectional study | 377 (170, 207) | 10.0–19.0 | LM, FM, BF% | DXA a | WB, FN LS (L1~L4) | None | NA |
| Jeon HC et al. 2014 | Korea | Cross-sectional study | 795 (433, 362) | M: 15.2 ± 0.13 F: 15.0 ± 0.13 | BF% | DXA a | WB, LS, FN | NA | BF%: age, menarche status, height, weight, serum 25-OH vitamin D, physical activity, energy intake, calcium intake, LM of whole body |
| Jeddi M et al. 2015 | Iran | Cross-sectional study | 469 (235, 234) | 9.0–18.0 | LM, FM, BF% | DXA a | WB, LS, FN | None | LM/FM: age, sex, stage of puberty, level of 25-hydroxy vitamin D, FM (or LM) |
| Ripka WL et al. 2016 | Brazil | Cross-sectional study | 318 (318, 0) | 14.9 ± 1.52 | LM, FM, BF% | DXA a | Arms, WB, FN, LS | None | NA |
| Khwanchuea R et al. 2017 | Thailand | Cross-sectional study | 135 (0, 135) | 16.1 ± 0.49 | LM, FM, BF% | DXA c | WB, LS (L2~L4) | None | NA |
| Kim HY et al. 2017 | Korea | Cross-sectional study | 982 (508, 474) | 15.6 ± 0.10 | LM, FM | DXA a | FN, WB, LS (L1~L4) | NA | LM/FM: age, vitamin D deficiency, insufficient Ca intake, physically inactive, homeostasis model assessment of insulin resistance, FM (or LM) |
| Wilkinson K et al. 2017 | England | Cross-sectional study | 121 (121, 0) | 13.1 ± 1.00 | LM, FM | DXA b | Arms, TBLH, FN, LS (L1~L4) | NA | LM/FM: height, age, physical activity, FM (or LM) |
| Gállego Suárez C et al. 2017 | USA | Cross-sectional study | 8348 (4745, 3603) | 13.0 ± 4.40 | LM, FM, BF% | DXA a | WB, LS | NA | FM/BF%: gender, race, LM |
| Sonja Soininen et al. 2018 | Finland | Cross-sectional study | 472 (227, 245) | 7.6 ± 0.40 | LM, FM, BF% | DXA b | TBLH | NA | LM/FM: age, sex, height, FM (or LM) |
| Krishnan S et al. 2018 | USA | Cross-sectional study | 37 (19, 18) | Overweight: 15.6 ± 2.12 Normal weight: 16.5 ± 2.59 | LM | DXA a | WB, LS | NA | LM: waist to hip ratio, truck to total fat ratio, percent trunk fat, C-reactive protein, total activity time, apo CIII ratio, gender, homeostatic model assessment-estimated insulin resistance |
| Kouda K et al. 2018 | Japan | Longitudinal study | 545 (279, 266) | Baseline M: 11.2 ± 0.30 F: 11.1 ± 0.30 Follow up 14.0 | LM, FM | DXA a | WB | NA | FM: pubic hair appearance, sedentary behavior, height |
| Winther A et al. 2018 | Norway | Cross-sectional study | 759 (364, 395) | M: 16.7 ± 0.40 F: 16.6 ± 0.40 | LM, FM | DXA b | FN | NA | LM/FM: age, height, sexual maturity, physical activity levels, calcium intake, vitamin D levels, alcohol consumption, smoking habits |
| Hetherington-Rauth M et al. 2018 | USA | Cross-sectional study | 326 (0, 326) | 10.8 ± 1.10 | LM, FM, BF% | DXA b/pQCT d | Arms, FN, WB | NA | LM/FM: maturity offset, height, ethnicity, FM (or LM) |
| McVey MK et al. 2019 | Ireland | Cross-sectional study | 102 (47, 55) | 5.1 ± 0.13 | LM, FM | DXA b | WB | None | LM/FM: sex, maternal BMD, maternal education level, membership of intervention/control group, breastfeeding status |
| Rokoff LB et al. 2019 | USA | Cross-sectional study | 876 (430, 446) | 7.7 ± 1.00 | LM, FM | DXA a | TBLH | NA | FM: maternal education, pubertal status, physical activity, environmental tobacco smoke exposure, 25(OH)D plasma concentration, maternal marital status, annual household income, sex, ethnicity, height, age, fat-free mass |
| Song C et al. 2019 | China | Cross-sectional study | 1179 (581, 598) | M: 11.8 ± 3.71 F: 12.4 ± 3.81 | LM, FM | DXA a | TBLH | None | NA |
| Kim A et al. 2020 | Korea | Cross-sectional study | 1454 (727, 727) | Total: 15.1 ± 0.60 M: 15.1 ± 0.08 F: 15.1 ± 0.09 | LM, FM | DXA b | LS, FN | NA | LM/FM: age, weight, walking, muscle-strengthening exercises, nutrition (intake of calcium and serum vitamin D) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, K.-L.; Yang, W.-Y.; Hou, J.-L.; Li, H.; Feng, H.; Xiao, S.-M. Association between Body Composition and Bone Mineral Density in Children and Adolescents: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 12126. https://doi.org/10.3390/ijerph182212126
Deng K-L, Yang W-Y, Hou J-L, Li H, Feng H, Xiao S-M. Association between Body Composition and Bone Mineral Density in Children and Adolescents: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(22):12126. https://doi.org/10.3390/ijerph182212126
Chicago/Turabian StyleDeng, Kai-Li, Wan-Yu Yang, Jin-Li Hou, Hui Li, Hao Feng, and Su-Mei Xiao. 2021. "Association between Body Composition and Bone Mineral Density in Children and Adolescents: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 22: 12126. https://doi.org/10.3390/ijerph182212126






