The Isolation of Culturable Bacteria in Ixodes ricinus Ticks of a Belgian Peri-Urban Forest Uncovers Opportunistic Bacteria Potentially Important for Public Health
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection and Preparation
2.3. DNA Extraction and High-Resolution Melting Analysis for Ixodes spp. Confirmation
2.4. Bacterial Identification
2.5. Antimicrobial Susceptibility Testing
2.6. WGS and In Silico Analysis of Resistance Genes
2.7. Bacterial Diversity
3. Results
3.1. Description of the Tick Culturable Bacteria
3.2. Diversity
3.3. Antibiotic Resistance Pattern and Genomic Characteristics of C. davisae
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kmet’, V.; Čaplová, Z. An update on the Ixodes ricinus microbiome. J. Microbiol. Biotechnol. Food Sci. 2019, 8, 1340–1342. [Google Scholar] [CrossRef]
- Lejal, E.; Estrada-Peña, A.; Marsot, M.; Cosson, J.F.; Rué, O.; Mariadassou, M.; Midoux, C.; Vayssier-Taussat, M.; Pollet, T. Taxon appearance from extraction and amplification steps demonstrates the value of multiple controls in tick microbiota analysis. Front. Microbiol. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Murrell, A.; Dobson, S.J.; Yang, X.; Lacey, E.; Barker, S.C. A survey of bacterial diversity in ticks, lice and fleas from Australia. Parasitol. Res. 2003, 89, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Pollet, T.; Sprong, H.; Lejal, E.; Krawczyk, A.I.; Moutailler, S.; Cosson, J.F.; Vayssier-Taussat, M.; Estrada-Peña, A. The scale affects our view on the identification and distribution of microbial communities in ticks. Parasites Vectors 2020, 13, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Medlock, J.M.; Hansford, K.M.; Bormane, A.; Derdakova, M.; Estrada-Peña, A.; George, J.-C.; Golovljova, I.; Jaenson, T.G.T.; Jensen, J.-K.; Jensen, P.M.; et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasites Vectors 2013, 6, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanwambeke, S.O.; Van doninck, J.; Artois, J.; Davidson, R.K.; Meyfroidt, P.; Jore, S. Forest classes and tree cover gradient: Tick habitat in encroached areas of southern Norway. Exp. Appl. Acarol. 2016, 68, 375–385. [Google Scholar] [CrossRef]
- Bonnet, S.I.; Binetruy, F.; Hernández-Jarguín, A.M.; Duron, O. The tick microbiome: Why non-pathogenic microorganisms matter in tick biology and pathogen transmission. Front. Cell. Infect. Microbiol. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Steiner, F.E.; Pinger, R.R.; Vann, C.N.; Grindle, N.; Civitello, D.; Clay, K.; Fuqua, C. Infection and co-infection rates of Anaplasma phagocytophilum variants, Babesia spp., Borrelia burgdorferi, and the rickettsial endosymbiont in Ixodes scapularis (Acari: Ixodidae) from sites in Indiana, Maine, Pennsylvania, and Wisconsin. J. Med. Entomol. 2008, 45, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Van Overbeek, L.; Gassner, F.; Van Der Plas, C.L.; Kastelein, P.; Nunes-Da Rocha, U.; Takken, W. Diversity of Ixodes ricinus tick-associated bacterial communities from different forests. FEMS Microbiol. Ecol. 2008, 66, 72–84. [Google Scholar] [CrossRef] [Green Version]
- Aivelo, T.; Norberg, A.; Tschirren, B. Bacterial microbiota composition of Ixodes ricinus ticks: The role of environmental variation, tick characteristics and microbial interactions. PeerJ 2019, 2019, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Carpi, G.; Cagnacci, F.; Wittekindt, N.E.; Zhao, F.; Qi, J.; Tomsho, L.P.; Drautz, D.I.; Rizzoli, A.; Schuster, S.C. Metagenomic profile of the bacterial communities associated with Ixodes ricinus ticks. PLoS ONE 2011, 6, 1–11. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Cabezas-Cruz, A.; Pollet, T.; Vayssier-Taussat, M.; Cosson, J.F. High Throughput Sequencing and network analysis disentangle the microbial communities of ticks and hosts within and between ecosystems. Front. Cell. Infect. Microbiol. 2018, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Vayssier-Taussat, M.; Moutailler, S.; Michelet, L.; Devillers, E.; Bonnet, S.; Cheval, J.; Hébert, C.; Eloit, M. Next generation sequencing uncovers unexpected bacterial pathogens in ticks in western Europe. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Stewart, P.E.; Bloom, M.E. Sharing the ride: Ixodes scapularis symbionts and their interactions. Front. Cell. Infect. Microbiol. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Rudolf, I.; Mendel, J.; Šikutová, S.; Švec, P.; Masaříková, J.; Nováková, D.; Buňková, L.; Sedlácek, I.; Hubáleks, Z. 16S rRNA gene-based identification of cultured bacterial flora from host-seeking Ixodes ricinus, Dermacentor reticulatus and Haemaphysalis concinna ticks, vectors of vertebrate pathogens. Folia Microbiol. 2009, 54, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.D.; Gofton, A.W.; Paparini, A.; Codello, A.; Greay, T.; Gillett, A.; Warren, K.; Irwin, P.; Ryan, U. Increased genetic diversity and prevalence of co-infection with Trypanosoma spp. in koalas (Phascolarctos cinereus) and their ticks identified using next-generation sequencing (NGS). PLoS ONE 2017, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Vayssier-Taussat, M.; Kazimirova, M.; Hubalek, Z.; Hornok, S.; Farkas, R.; Cosson, J.-F.; Bonnet, S.; Vourch, G.; Gasqui, P.; Mihalca, A.D.; et al. Emerging horizons for tick-borne pathogens: From the “one pathogen—one disease” vision to the pathobiome paradigm. Future Microbiol. 2015, 10, 2033–2043. [Google Scholar] [CrossRef] [Green Version]
- Bonnet, S.I.; Pollet, T. Update on the intricate tango between tick microbiomes and tick-borne pathogens. Parasite Immunol. 2021, 43, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Heylen, D.; De Coninck, E.; Jansen, F.; Madder, M. Differential diagnosis of three common Ixodes spp. ticks infesting songbirds of Western Europe: Ixodes arboricola, I. frontalis and I. ricinus. Ticks Tick-Borne Dis. 2014, 5, 693–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillyard, P.D. Ticks of North-West Europe; The Natura: London, UK, 1996. [Google Scholar]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef] [Green Version]
- Tomaiuolo, S.; Boarbi, S.; Fancello, T.; Michel, P.; Desqueper, D.; Grégoire, F.; Callens, J.; Fretin, D.; Devriendt, B.; Cox, E.; et al. Phylogeography of Human and Animal Coxiella burnetii Strains: Genetic Fingerprinting of Q Fever in Belgium. Front. Cell. Infect. Microbiol. 2021, 10, 1–15. [Google Scholar] [CrossRef]
- Leung, E.T.; Noronha, R.; Mirza, A.; Shenwai, R.; Mpatziakas, A. Shinydiversity—Understanding alpha and beta diversity through interactive visualizations. F1000Research 2018, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Whittaker, R.H. Vegetation of the Siskiyou Mountains, Oregon and California. Ecol. Monogr. 1960, 30, 279–338. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package; 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 November 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: http://www.R-project.org/ (accessed on 10 November 2021).
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.O. Diversity and Evenness: A Unifying Notation and Its Consequences. Ecology 1973, 54, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Koleff, P.; Gaston, K.J.; Lennon, J.J. Measuring beta diversity for presence-absence data. J. Anim. Ecol. 2003, 72, 367–382. [Google Scholar] [CrossRef] [Green Version]
- Otto, M. Staphylococcus epidermidis—The “accidental” pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [Green Version]
- Bryksin, A.V.; Matsumura, I. Rational design of a plasmid origin that replicates efficiently in both gram-positive and gram-negative bacteria. PLoS ONE 2010, 5, 1–9. [Google Scholar] [CrossRef]
- Malele, I.; Nyingilili, H.; Lyaruu, E.; Tauzin, M.; Bernard Ollivier, B.; Cayol, J.L.; Fardeau, M.L.; Geiger, A. Bacterial diversity obtained by culturable approaches in the gut of Glossina pallidipes population from a non sleeping sickness focus in Tanzania: Preliminary results 06 Biological Sciences 0605 Microbiology. BMC Microbiol. 2018, 18, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Martin, P.A.W.; Schmidtmann, E.T. Isolation of aerobic microbes from Ixodes scapularis (Acari: Ixodidae), the vector of Lyme disease in the Eastern United States. J. Econ. Entomol. 1998, 91, 864–868. [Google Scholar] [CrossRef]
- Egyed, L.; Makrai, L. Cultivable internal bacterial flora of ticks isolated in Hungary. Exp. Appl. Acarol. 2014, 63, 107–122. [Google Scholar] [CrossRef]
- Okła, H.; Sosnowska, M.; Jasik, K.P.; Słodki, J.; Wojtyczka, R.D.; Wojtyczka, R.D. Nonspecific bacterial flora isolated from the body surface and inside Ixodes ricinus ticks. Polish J. Microbiol. 2012, 61, 205–209. [Google Scholar] [CrossRef]
- Stojek, N.M.; Dutkiewicz, J. Studies on the occurrence of gram-negative bacteria in ticks: Ixodes ricinus as a potential vector of Pasteurella. Ann. Agric. Environ. Med. 2004, 11, 319–322. [Google Scholar] [PubMed]
- Lernout, T.; De Regge, N.; Tersago, K.; Fonville, M.; Suin, V.; Sprong, H. Prevalence of pathogens in ticks collected from humans through citizen science in Belgium. Parasites Vectors 2019, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dordet-Frisoni, E.; Dorchies, G.; De Araujo, C.; Talon, R.; Leroy, S. Genomic diversity in Staphylococcus xylosus. Appl. Environ. Microbiol. 2007, 73, 7199–7209. [Google Scholar] [CrossRef] [Green Version]
- Kloos, W.E.; Zimmerman, R.J.; Smith, R.F. Preliminary studies on the characterization and distribution of Staphylococcus and Micrococcus species on animal skin. Appl. Environ. Microbiol. 1976, 31, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namvar, A.E.; Bastarahang, S.; Abbasi, N.; Ghehi, G.S.; Farhadbakhtiarian, S.; Arezi, P.; Hosseini, M.; Baravati, S.Z.; Jokar, Z.; Chermahin, S.G.; et al. Clinical characteristics of Staphylococcus epidermidis: A systematic review. GMS Hyg. Infect. Control 2014, 9, 1–10. [Google Scholar] [CrossRef]
- Guizzo, M.G.; Neupane, S.; Kucera, M.; Perner, J.; Frantová, H.; da Silva Vaz, I.; de Oliveira, P.L.; Kopacek, P.; Zurek, L. Poor unstable midgut microbiome of hard ticks contrasts with abundant and stable monospecific microbiome in ovaries. Front. Cell. Infect. Microbiol. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Abate, G.; Qureshi, S.; Mazumder, S.A. Cedecea davisae bacteremia in a neutropenic patient with acute myeloid leukemia. J. Infect. 2011, 63, 83–85. [Google Scholar] [CrossRef]
- Thompson, D.K.; Sharkady, S.M. Expanding spectrum of opportunistic Cedecea infections: Current clinical status and multidrug resistance. Int. J. Infect. Dis. 2020, 100, 461–469. [Google Scholar] [CrossRef]
- Dalamaga, M.; Karmaniolas, K.; Arsenis, G.; Pantelaki, M.; Daskalopoulou, K.; Papadavid, E.; Migdalis, I. Cedecea lapagei bacteremia following cement-related chemical burn injury. Burns 2008, 34, 1205–1207. [Google Scholar] [CrossRef]
- Martiny, A.C. High proportions of bacteria are culturable across major biomes. ISME J. 2019, 13, 2125–2128. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Matsuzawa, H.; Tamaki, H.; Tagawa, M.; Toyama, T.; Kamagata, Y.; Mori, K. Isolation of novel bacteria including rarely cultivated phyla, Acidobacteria and Verrucomicrobia, from the roots of emergent plants by simple culturing method. Microbes Environ. 2017, 32, 288–292. [Google Scholar] [CrossRef] [Green Version]
- van Treuren, W.; Ponnusamy, L.; Brinkerhoff, R.J.; Gonzalez, A.; Parobek, C.M.; Juliano, J.J.; Andreadis, T.G.; Falco, R.C.; Ziegler, L.B.; Hathaway, N.; et al. Variation in the microbiota of Ixodes ticks with regard to geography, species, and sex. Appl. Environ. Microbiol. 2015, 81, 6200–6209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina-Menor, E.; Gimeno-Valero, H.; Pascual, J.; Peretó, J.; Porcar, M. High culturable bacterial diversity from a European desert: The Tabernas desert. Front. Microbiol. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Binetruy, F.; Dupraz, M.; Buysse, M.; Duron, O. Surface sterilization methods impact measures of internal microbial diversity in ticks. Parasites Vectors 2019, 12, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Randolph, S.E. The shifting landscape of tick-borne zoonoses: Tick-borne encephalitis and Lyme borreliosis in Europe. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2001, 356, 1045–1056. [Google Scholar] [CrossRef] [Green Version]
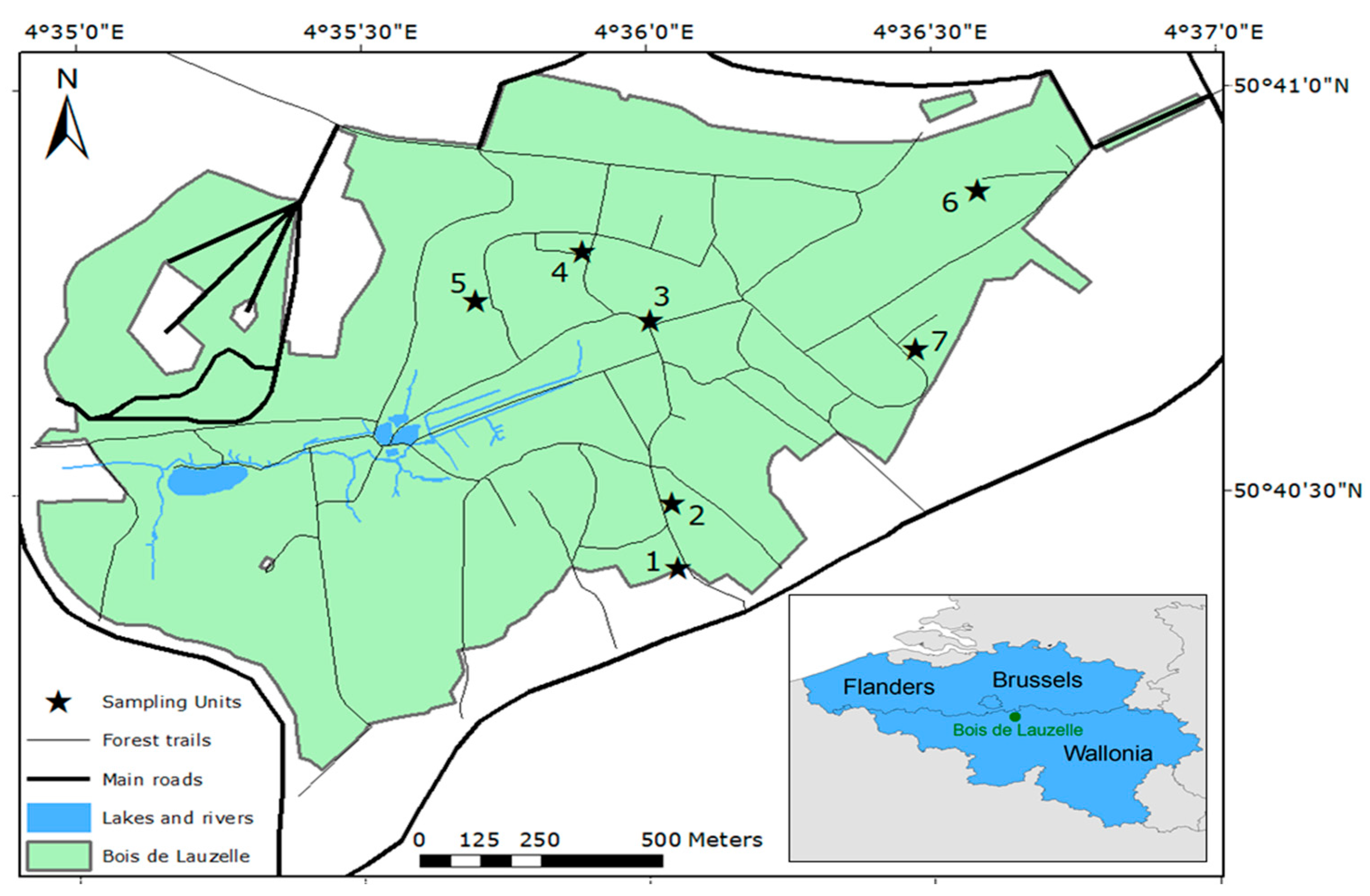


| Sites | Latitude (Degrees) | Longitude (Degrees) | Forest Type | Soil Vegetation | Soils |
|---|---|---|---|---|---|
| Site 1 | 4.6021 | 50.6729 | Deciduous | Grass | Loamy |
| Site 2 | 4.6018 | 50.6742 | Deciduous | Brambles | Loamy-Sand |
| Site 3 | 4.6014 | 50.6781 | Deciduous | Grass | Loamy |
| Site 4 | 4.5994 | 50.6794 | Coniferous | Grass | Sandy |
| Site 5 | 4.5963 | 50.6784 | Deciduous | Moss | Sandy |
| Site 6 | 4.6111 | 50.6807 | Deciduous | Brambles | Sandy |
| Site 7 | 4.6089 | 50.6772 | Coniferous | Brambles | Loamy-Sand |
| Site | Larvae | Nymphs | Females | Males | Ticks |
|---|---|---|---|---|---|
| Site 1 | 0 | 36 | 19 | 3 | 58 |
| Site 2 | 23 | 248 | 10 | 12 | 293 |
| Site 3 | 10 | 71 | 4 | 1 | 86 |
| Site 4 | 38 | 131 | 3 | 6 | 178 |
| Site 5 | 12 | 66 | 2 | 2 | 82 |
| Site 6 | 0 | 60 | 1 | 5 | 66 |
| Site 7 | 3 | 175 | 5 | 8 | 191 |
| Total | 86 | 787 | 44 | 37 | 954 |
| Sites | Pools Tested | Species Richness | Abundance | H |
|---|---|---|---|---|
| Site 1 | 15 | 3 | 17 | 0.68 |
| Site 2 | 69 | 5 | 18 | 1/11 |
| Site 3 | 20 | 2 | 4 | 0.56 |
| Site 4 | 35 | 6 | 8 | 1.73 |
| Site 5 | 18 | 7 | 7 | 1.95 |
| Site 6 | 17 | 1 | 1 | 0 |
| Site 7 | 47 | 9 | 13 | 2.06 |
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Site 6 | Site 7 | |
|---|---|---|---|---|---|---|---|
| Site 1 | - | ||||||
| Site 2 | 0.75 | - | |||||
| Site 3 | 0.60 | 0.43 | - | ||||
| Site 4 | 0.78 | 0.82 | 0.75 | - | |||
| Site 5 | 0.80 | 1 | 1 | 0.85 | - | ||
| Site 6 | 0.50 | 1 | 1 | 1 | 0.75 | - | |
| Site 7 | 0.83 | 0.86 | 0.82 | 0.87 | 0.88 | 0.80 | - |
| Antibiotic Class | Antibiotic Abbreviation | Antibiotic | Cedecea davisae (Tick) | Escherichia coli ATCC 25922 | Enterobacterales (EUCAST Clinical Breakpoints 1 January 2021) and ECOFF (*) | |||
|---|---|---|---|---|---|---|---|---|
| MIC (µg/mL) | Int. | MIC (µg/mL) | Int. | S≤ | R> | |||
| Aminoglycosides | GEN | Gentamicin | ≤0.5 | S | ≤0.5 | S | 2 | 2 |
| STR | Streptomycin | ≤4 | S * | >16 * | ||||
| Carbapenem | MERO | Meropenem | 0.12 | S | ≤0.03 | S | 2 | 8 |
| Cephalosporins | FOT | Cefotaxime | ≤0.25 | S | ≤0.25 | S | 1 | 2 |
| FOX | Cefoxitin | >16 | R * | 8 * | 8 * | |||
| TAZ | Ceftazidime | ≤0.5 | S | ≤0.5 | S | 1 | 4 | |
| Diterpenes | TIA | Tiamulin | >4 | |||||
| Fluoroquinolones | CIP | Ciprofloxacin | ≤0.015 | S | ≤0.015 | S | 0.25 | 0.5 |
| NAL | Nalidixic Acid | ≤4 | S * | ≤4 | S * | >8 | ||
| Macrolides, lincosamides and streptogramins | AZI | Azithromycin | 16 | 4 | ||||
| Penicillins | AMP | Ampicillin | >64 | R | 4 | S | 8 | 8 |
| Tetracyclines | TET | Tetracycline | ≤2 | S * | ≤2 | S * | >8 * | |
| TGC | Tigecycline | ≤0.25 | S * | ≤0.25 | S * | 1 | >0.5 | |
| Miscellaneous agent | CHL | Chloramphenicol | ≤8 | S | ≤8 | S | 8 | 8 |
| COL | Colistin | >16 | R | ≤1 | S | 2 | 2 | |
| KAN | Kanamycin | ≤4 | ||||||
| MUP | Mupirocin | 256 | ||||||
| RIF | Rifampicin | >0.5 | ||||||
| SMX | Sulfamethoxazole | >1024 | 64 | |||||
| TMP | Trimethoprim | ≤0.25 | S | 0.5 | S | 4 | 4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rousseau, R.; Vanwambeke, S.O.; Boland, C.; Mori, M. The Isolation of Culturable Bacteria in Ixodes ricinus Ticks of a Belgian Peri-Urban Forest Uncovers Opportunistic Bacteria Potentially Important for Public Health. Int. J. Environ. Res. Public Health 2021, 18, 12134. https://doi.org/10.3390/ijerph182212134
Rousseau R, Vanwambeke SO, Boland C, Mori M. The Isolation of Culturable Bacteria in Ixodes ricinus Ticks of a Belgian Peri-Urban Forest Uncovers Opportunistic Bacteria Potentially Important for Public Health. International Journal of Environmental Research and Public Health. 2021; 18(22):12134. https://doi.org/10.3390/ijerph182212134
Chicago/Turabian StyleRousseau, Raphaël, Sophie O. Vanwambeke, Cécile Boland, and Marcella Mori. 2021. "The Isolation of Culturable Bacteria in Ixodes ricinus Ticks of a Belgian Peri-Urban Forest Uncovers Opportunistic Bacteria Potentially Important for Public Health" International Journal of Environmental Research and Public Health 18, no. 22: 12134. https://doi.org/10.3390/ijerph182212134
APA StyleRousseau, R., Vanwambeke, S. O., Boland, C., & Mori, M. (2021). The Isolation of Culturable Bacteria in Ixodes ricinus Ticks of a Belgian Peri-Urban Forest Uncovers Opportunistic Bacteria Potentially Important for Public Health. International Journal of Environmental Research and Public Health, 18(22), 12134. https://doi.org/10.3390/ijerph182212134







