Surgical Treatment of “Large Uterine Masses” in Pregnancy: A Single-Center Experience
Abstract
:1. Introduction
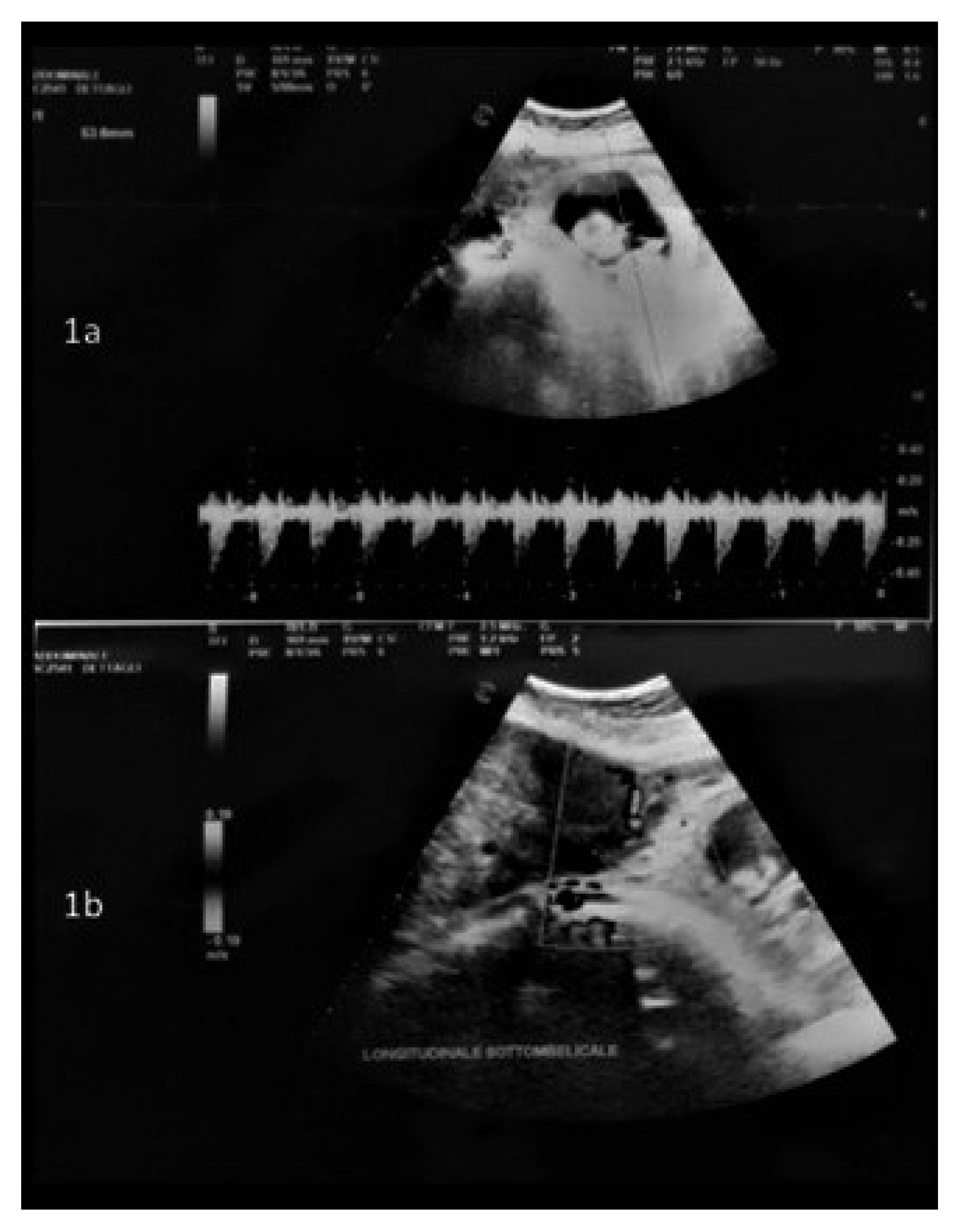
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Stewart, E.A.; Laughlin-Tommaso, S.K.; Catherino, W.H.; Lalitkumar, S.; Gupta, D.; Vollenhoven, B. Uterine fibroids. Nat. Rev. Dis. Primers 2016, 2, 16043. [Google Scholar] [CrossRef]
- Borah, B.J.; Nicholson, W.K.; Bradley, L.; Stewart, E.A. The impact of uterine leiomyomas: A national survey of affected women. Am. J. Obstet. Gynecol. 2013, 209, 319.e1–319.e20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, D.; Zhang, W.; Chames, M.C.; Guo, J. Myomectomy during cesarean delivery. Int. J. Gynaecol. Obstet. 2013, 121, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Padula, F.; Gulino, F.A. Management of uterine fibroids in pregnancy: Recent trends. Curr. Opin. Obstet. Gynecol. 2015, 27, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Ezzedine, D.; Norwitz, E.R. Are Women With Uterine Fibroids at Increased Risk for Adverse Pregnancy Outcome? Clin. Obstet. Gynecol. 2016, 59, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Mas, A.; Tarazona, M.; Dasí Carrasco, J.; Estaca, G.; Cristóbal, I.; Monleón, J. Updated approaches for management of uterine fibroids. Int. J. Womens Health 2017, 9, 607–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milazzo, G.N.; Catalano, A.; Badia, V.; Mallozzi, M.; Caserta, D. Myoma and myomectomy: Poor evidence concern in pregnancy. J. Obstet/Gynaecol. Res. 2017, 43, 1789–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Cruz, M.S.; Buchanan, E.M. Uterine Fibroids: Diagnosis and Treatment. Am. Fam. Physician 2017, 95, 100–107. [Google Scholar] [PubMed]
- Pritts, E.A.; Vanness, D.J.; Berek, J.S.; Parker, W.; Feinberg, R.; Feinberg, J.; Olive, D.L. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: A meta-analysis. Gynecol. Surg. 2015, 12, 165–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Luigi, G.; D’Alfonso, A.; Patacchiola, F.; Di Stefano, L.; Palermo, P.; Carta, G. Leiomyosarcoma: A rare malignant transformation of a uterine leiomyoma. Eur. J. Gynaecol. Oncol. 2015, 36, 84–87. [Google Scholar] [PubMed]
- Klatsky, P.C.; Tran, N.D.; Caughey, A.B.; Fujimoto, V.Y. Fibroids and reproductive outcomes: A systematic literature review from conception to delivery. Am. J. Obstet. Gynecol. 2008, 198, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Kaganov, H.; Ades, A.; Fraser, D.S. Preoperative Magnetic Resonance Imaging Diagnostic Features of Uterine Leiomyosarcomas: A Systematic Review. Int. J. Technol. Assess. Health Care 2018, 34, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Vitale, S.G.; Tropea, A.; Rossetti, D.; Carnelli, M.; Cianci, A. Management of uterine leiomyomas in pregnancy: Review of literature. Updates Surg. 2013, 65, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Wallis, L. FDA warns against power morcellation for hysterectomy and fibroids. Am. J. Nurs. 2014, 114, 16. [Google Scholar] [CrossRef] [PubMed]
- Mynbaev, O.A.; Sparic, R.; Stark, M.; Malvasi, A.; Marinelli, E.; Zaami, S.; Tinelli, A. The Medical Device Applied to Uterine Fibroids Morcellation: Analysis of Critical Biological Issues and Drawbacks from A Medical-Legal Prospective. Curr. Pharm. Des. 2020, 26, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Zaami, S.; Zupi, E.; Lazzeri, L.; Stark, M.; Malvasi, A.; Signore, F.; Marinelli, E. Medicolegal Issues in Power Morcellation: Cautionary Rules for Gynecologists to Avoid Unfavorable Outcomes. J. Minim. Invasive Gynecol. 2020, 27, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Committee Opinion No. 696: Nonobstetric Surgery During Pregnancy. Obstet. Gynecol. 2017, 129, 777–778. [CrossRef] [PubMed]
- Basso, A.; Catalano, M.R.; Loverro, G.; Nocera, S.; Di Naro, E.; Loverro, M.; Natrella, M.; Mastrolia, S.A. Uterine Fibroid Torsion during Pregnancy: A Case of Laparotomic Myomectomy at 18 Weeks’ Gestation with Systematic Review of the Literature. Case Rep. Obstet. Gynecol. 2017, 2017, 4970802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moruzzi, M.C.; Moro, F.; Bolomini, G.; Macchi, C.; Cavaliere, A.F.; Fagotti, A.; Scambia, G.; Testa, A.C. Intraoperative ultrasound assistance during myomectomy in pregnant woman. Ultrasound Obstet. Gynecol. 2020, 55, 840–841. [Google Scholar] [CrossRef] [PubMed]

| Case | Age | Ethnicity | BMI | Associated Pathologies | Education |
|---|---|---|---|---|---|
| 1 | 38 | Caucasian | 26 | Thrombophilia deficit ATIII | High school diploma Housewife |
| 2 | 35 | Caucasian | 22 | Gestational diabetes | High school diploma Housewife |
| 3 | 40 | Caucasian | 24 | Gestational hypertension | High school diploma Housewife |
| 4 | 28 | Caucasian | 27 | None | High school diploma Housewife |
| 5 | 36 | Caucasian | 27 | None | High school diploma Housewife |
| 6 | 38 | Caucasian | 28 | Endometriosis | High school diploma |
| 7 | 38 | Black African | 24 | None | Low education level |
| 8 | 33 | Caucasian | 30 | CMV in pregnancy | High school diploma |
| 9 | 37 | Caucasian | 34 | Gestational hypothyroidism | High school diploma |
| Case | Masses > 50 mm | US Characteristics | Intraoperative Data | Histologic Results |
|---|---|---|---|---|
| 1 | 1 | 90 mm left lateral mass, implantation base of 290 mm. Myometrial thickness: 10 mm Hypervascularity: none | LPT 280 mm SS fundic mass | LEIOMYOMA OF 290 mm |
| 2 | 2 | 173 × 87 × 116 mm mass, implantation base of 54 mm; 60 × 44 × 56 mm mass, implantation base of 46 mm. Both masses SS right fundic-posterolateral. Myometrial thickness: 11 mm Hypervascularity: none | LPT 200 mm SS fundic mass, with implantation base of 50 mm; 50 mm SS fundic mass; 2 anterior centimetric mass; EBL < 200 cc | LEIOMYOMAS of 4 myomatous nodules, the largest of 190 mm |
| 3 | 2 | 47 mm × 59 mm isthmic IM mass (dislocating uterine cervix); 21 mm × 26 mm right posterolateral IM mass; 196 × 105 mm right fundic-lateral SS sessile mass, implantation base of 62 mm. Myometrial thickness: 14 mm Hypervascularity: none | LPT 200 mm SS fundic-posterior mass; 2 SS mass of 50–60 mm; 3 SS mass of 20–30 mm; EBL 250 cc | LEIOMYOMAS of 6 myomatous nodules, dimensions range between 10 and 190 mm |
| 4 | 1 | 220 × 179 × 145 mm fundic right posterolateral SS mass Myometrial thickness: 10 mm Hypervascularity: none | LPT 250 mm SS mass; EBL 450 cc | LEIOMYOMA of 170 mm |
| 5 | 1 | 160 × 150 × 100 mm left anterolateral SS mass, implantation base of 92 mm. Myometrial thickness: 12 mm Hypervascularity: none | LPT 160 mm mass, implantation base of 80–90 mm; EBL 350 cc | APOPLECTIC LEIOMYOMA of 160 mm |
| 6 | 1 | 121 × 81 × 73 mm right anterior mass. Myometrial thickness: 13 mm Hypervascularity: none | LPT 120 mm antero-isthmic mass; 30 mm SS anterior mass; EBL 250 cc | LEIOMYOMAS: 1 of 140 mm; 1 of 43 mm |
| 7 | 2 | 87 × 66 × 49 mm left antero-isthmic IM-SS mass; 146 × 110 × 142 mm right fundic-lateral, implantation base of 89 mm. Myometrial thickness: 10 mm Hypervascularity: none | LPT 150 mm right fundic mass, with a wide implantation base that touches the gestational chamber; 70 mm left antero-isthmic IM mass; other small (10 mm) pedunculated mass | LEIOMYOMAS: 2 intramural nodules of 80–170 mm; Other fragments of 80 mm |
| 8 | 2 | 123 × 90 × 78.5 mm fundic mass; 78.5 × 64 × 76 mm fundic mass, implantation base of 120 mm. Myometrial thickness: 11 mm Hypervascularity: none | LPT 150 mm right fundic IM-SS mass; 90 mm anterior mass | LEIOMYOMAS: 2 myomatous nodules of 195 mm e 93 mm; 1 fibroid fragment of 155 mm; |
| 9 | 4 | 157 × 90 mm right fundic-lateral mass; 77 × 76 mm left lateral mass; implantation base of 157 mm; Other small masses observed. Myometrial thickness: 10 mm Hypervascularity: none | LPT 140 mm mass; 120 mm mass; 80 mm mass; 55 mm mass; 20 mm mass; 40 mm mass | LEIOMYOMAS: 140 mm; 120 mm; 80 mm; 55 mm; 20 mm; ADENOMYOMA 40 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavaliere, A.F.; Vidiri, A.; Gueli Alletti, S.; Fagotti, A.; La Milia, M.C.; Perossini, S.; Restaino, S.; Vizzielli, G.; Lanzone, A.; Scambia, G. Surgical Treatment of “Large Uterine Masses” in Pregnancy: A Single-Center Experience. Int. J. Environ. Res. Public Health 2021, 18, 12139. https://doi.org/10.3390/ijerph182212139
Cavaliere AF, Vidiri A, Gueli Alletti S, Fagotti A, La Milia MC, Perossini S, Restaino S, Vizzielli G, Lanzone A, Scambia G. Surgical Treatment of “Large Uterine Masses” in Pregnancy: A Single-Center Experience. International Journal of Environmental Research and Public Health. 2021; 18(22):12139. https://doi.org/10.3390/ijerph182212139
Chicago/Turabian StyleCavaliere, Anna Franca, Annalisa Vidiri, Salvatore Gueli Alletti, Anna Fagotti, Maria Concetta La Milia, Silvia Perossini, Stefano Restaino, Giuseppe Vizzielli, Antonio Lanzone, and Giovanni Scambia. 2021. "Surgical Treatment of “Large Uterine Masses” in Pregnancy: A Single-Center Experience" International Journal of Environmental Research and Public Health 18, no. 22: 12139. https://doi.org/10.3390/ijerph182212139
APA StyleCavaliere, A. F., Vidiri, A., Gueli Alletti, S., Fagotti, A., La Milia, M. C., Perossini, S., Restaino, S., Vizzielli, G., Lanzone, A., & Scambia, G. (2021). Surgical Treatment of “Large Uterine Masses” in Pregnancy: A Single-Center Experience. International Journal of Environmental Research and Public Health, 18(22), 12139. https://doi.org/10.3390/ijerph182212139







