Activated Carbon from Palm Date Seeds for CO2 Capture
Abstract
:1. Introduction
2. Experiment
2.1. Preparation of the PDS Hydrochar
2.2. Activation of the PDS Hydrochar
2.3. Characterisation
2.4. CO2 Adsorption
3. Results and Discussion
3.1. Characterization of the PDS Carbon Powders
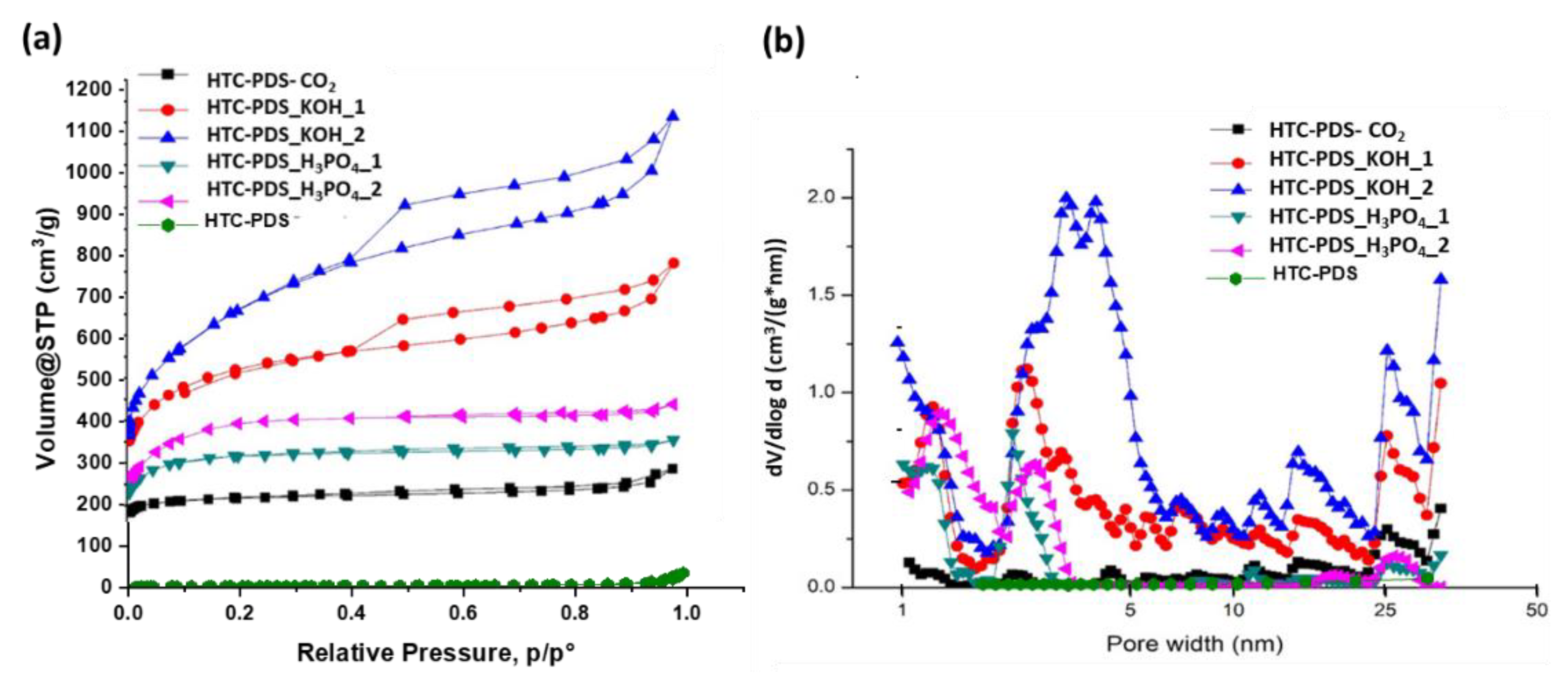
3.2. Porosity of the PDS Carbon Powders
3.3. CO2 Adsorption Studies
3.4. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siriwardane, R.V.; Shen, M.-S.; Fisher, E.P.; Poston, J.A. Adsorption of CO2 on Molecular Sieves and Activated Carbon. Energy Fuels 2001, 15, 279–284. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. Determination of the optimal pore size for improved CO2 adsorption in activated carbon fibers. J. Colloid Interface Sci. 2013, 389, 230–235. [Google Scholar] [CrossRef]
- Jadhav, P.D.; Chatti, R.V.; Biniwale, R.B.; Labhsetwar, N.K.; Devotta, S.; Rayalu, S.S. Monoethanol Amine Modified Zeolite 13X for CO2 Adsorption at Different Temperatures. Energy Fuels 2007, 21, 3555–3559. [Google Scholar] [CrossRef]
- Gray, M.L.; Soong, Y.; Champagne, K.J.; Pennline, H.; Baltrus, J.P.; Stevens, R.W.; Khatri, R.; Chuang, S.S.C.; Filburn, T. Improved immobilized carbon dioxide capture sorbents. Fuel Process. Technol. 2005, 86, 1449–1455. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.; Cnen, W.; Bai, H.; Hwang, J.F. Capture of CO2 from flue gas via multiwalled carbon nanotubes. Sci. Total Environ. 2009, 407, 3017–3023. [Google Scholar] [CrossRef] [PubMed]
- Pellerano, M.; Pré, P.; Kacem, M.; Delebarre, A. CO2 capture by adsorption on activated carbons using pressure modulation. Energy Procedia 2009, 1, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Bai, H.; Wu, B.; Su, F.; Hwang, J.F. Comparative study of CO2 capture by carbon nanotubes, activated carbons, and zeolites. Energy Fuels 2008, 22, 3050–3056. [Google Scholar] [CrossRef]
- Serna-Guerrero, R.; Belmabkhout, Y.; Sayari, A. Further investigations of CO2 capture using triamine-grafted pore-expanded mesoporous silica. Chem. Eng. J. 2010, 158, 513–519. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.-S.; Fisher, E.P.; Losch, J. Adsorption of CO2 on zeolites at moderate temperatures. Energy Fuels 2005, 19, 1153–1159. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Lin, Y. Adsorption and diffusion of carbon dioxide on metal−organic framework (MOF-5). Ind. Eng. Chem. Res. 2009, 48, 10015–10020. [Google Scholar] [CrossRef]
- Arenillas, A.; Smith, K.; Drage, T.; Snape, C. CO2 capture using some fly ash-derived carbon materials. Fuel 2005, 84, 2204–2210. [Google Scholar] [CrossRef]
- Lua, A.C.; Guo, J. Preparation and characterization of activated carbons from oil-palm stones for gas-phase adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2001, 179, 151–162. [Google Scholar] [CrossRef]
- Guangzhi, Y.; Jinyu, Y.; Yuhua, Y.; Zhihong, T.; DengGuang, Y.; Junhe, Y. Preparation and CO2 adsorption properties of porous carbon from camphor leaves by hydrothermal carbonization and sequential potassium hydroxide activation. RSC Adv. 2017, 7, 4152–4160. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.A.; Tan, I.A.W.; Benhouria, A.; Asif, M.; Hameed, B.H. Mesoporous and adsorptive properties of palm date seed activated carbon prepared via sequential hydrothermal carbonization and sodium hydroxide activation. Chem. Eng. J. 2015, 270, 187–195. [Google Scholar] [CrossRef]
- El-Habba, M.S.; Al-Mulhim, F. The competitiveness of the Saudi Arabian date palm: An analytical study. Afr. J. Agric. Res. 2013, 8, 5260–5267. [Google Scholar]
- Nasser, R.A.-S. An evaluation of the use of midribs from common date palm cultivars grown in Saudi Arabia for energy production. BioResources 2014, 9, 4343–4357. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Bahkali, A.H. Valorization of date palm (Phoenix dactylifera) fruit processing by-products and wastes using bioprocess technology–Review. Saudi J. Biol. Sci. 2013, 20, 105–120. [Google Scholar] [CrossRef] [Green Version]
- Plaza, M.G.; González, A.S.; Pevida, C.; Pis, J.J.; Rubiera, F. Valorisation of spent coffee grounds as CO2 adsorbents for postcombustion capture applications. Appl. Energy 2012, 99, 272–279. [Google Scholar] [CrossRef] [Green Version]
- Bouchelta, C.; Medjram, M.S.; Bertrand, O.; Bellat, J.-P. Preparation and characterization of activated carbon from date stones by physical activation with steam. J. Anal. Appl. Pyrolysis 2008, 82, 70–77. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.A.; El-Naas, M.H.; Abdallah, S. Removal of aluminum from aqueous solutions by adsorption on date-pit and BDH activated carbons. J. Hazard. Mater. 2008, 158, 300–307. [Google Scholar] [CrossRef] [PubMed]
- El Nemr, A.; Khaled, A.; Abdelwahab, O.; El-Sikaily, A. Treatment of wastewater containing toxic chromium using new activated carbon developed from date palm seed. J. Hazard. Mater. 2008, 152, 263–275. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Li, J.; Salamh, Y.; Al-Laqtah, N.; Walker, G.; Ahmad, M.N. Adsorption mechanisms of removing heavy metals and dyes from aqueous solution using date pits solid adsorbent. J. Hazard. Mater. 2010, 176, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Bouchelta, C.; Medjram, M.S.; Zoubida, M.; Chekkat, F.A.; Ramdane, N.; Bellat, J.-P. Effects of pyrolysis conditions on the porous structure development of date pits activated carbon. J. Anal. Appl. Pyrolysis 2012, 94, 215–222. [Google Scholar] [CrossRef]
- Abed, I.; Paraschiv, M.; Loubar, K.; Zagrouba, F.; Tazerout, M. Thermogravimetric investigation and thermal conversion kinetics of typical North-Africa and middle-east lignocellulosic wastes. BioResources 2012, 7, 1120–1220. [Google Scholar]
- Zhu, X.; Liu, Y.; Qian, F.; Zhou, C.; Zhang, S.; Chen, J. Role of Hydrochar Properties on the Porosity of Hydrochar-based Porous Carbon for Their Sustainable Application. ACS Sustain. Chem. Eng. 2015, 3, 833–840. [Google Scholar] [CrossRef]
- Peng, N.; Gai, C.; Peng, C. Enhancing hydrogen-rich syngas production and energy recovery efficiency by integrating hydrothermal carbonization pretreatment with steam gasification. Energy 2020, 210, 118655. [Google Scholar] [CrossRef]
- Heidari, M.; Salaudeen, S.; Arku, P.; Acharya, B.; Tasnim, S.; Dutta, A. Development of a mathematical model for hydrothermal carbonization of biomass: Comparison of experimental measurements with model predictions. Energy 2021, 214, 119020. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, X.; Yuan, Q.; Liu, R. Investigation of physico-chemical properties of hydrochar and composition of bio-oil from the hydrothermal treatment of dairy manure: Effect of type and usage volume of extractant. Waste Manag. 2020, 116, 157–165. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Balu, A.M.; van der Waal, J.C.; Luque, R. Biomass-derived porous carbon materials: Synthesis and catalytic applications. ChemCatChem 2015, 7, 1608–1629. [Google Scholar] [CrossRef]
- Titirici, M.M.; Thomas, A.; Antonietti, M. Replication and coating of silica templates by hydrothermal carbonization. Adv. Funct. Mater. 2007, 17, 1010–1018. [Google Scholar] [CrossRef]
- Liu, J.; Yang, T.; Wang, D.-W.; Lu, G.Q.M.; Zhao, D.; Qiao, S.Z. A facile soft-template synthesis of mesoporous polymeric and carbonaceous nanospheres. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, X.; Shi, S.; Thiphuong, N.; Chen, M. Synergistical enhancement of the electrochemical properties of lignin-based activated carbon using NH3·H2O dielectric barrier discharge plasma. RSC Adv. 2017, 7, 7392–7400. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.-M.; Yang, S.-J.; Ma, C.-C.M.; Xie, X. Preparation and electrochemical activities of iridium-decorated graphene as the electrode for all-vanadium redox flow batteries. Electrochim. Acta 2012, 77, 232–236. [Google Scholar] [CrossRef]
- Alazmi, A.; El Tall, O.; Hedhili, M.N.; Costa, P.M. The impact of surface chemistry and texture on the CO2 uptake capacity of graphene oxide. Inorg. Chim. Acta 2018, 482, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Barron, A.R. The effect of KOH concentration on chemical activation of porous carbon sorbents for carbon dioxide uptake and carbon dioxide–methane selectivity: The relative formation of micro-(<2 nm) versus meso-(>2 nm) porosity. Sustain. Energy Fuels 2017, 1, 806–813. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Chang, K.-H.; Huang, Y.-L.; Lee, Y.-F.; Tien, H.-W.; Li, S.-M.; Lee, Y.-H.; Liu, C.-H.; Ma, C.-C.M.; Hu, C.-C. A powerful approach to fabricate nitrogen-doped graphene sheets with high specific surface area. Electrochem. Commun. 2012, 14, 39–42. [Google Scholar] [CrossRef]
- Islam, M.A.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous activated carbon prepared from NaOH activation of rattan (Lacosperma secundiflorum) hydrochar for methylene blue removal. Ecotoxicol. Environ. Saf. 2017, 138, 279–285. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Xue, R.; Shen, Z. Formation of graphite-potassium intercalation compounds during activation of MCMB with KOH. Carbon 2003, 41, 1862–1864. [Google Scholar] [CrossRef]
- Serafin, J.; Baca, M.; Biegun, M.; Mijowska, E.; Kaleńczuk, R.J.; Sreńscek-Nazzal, J.; Michalkiewicz, B. Direct conversion of biomass to nanoporous activated biocarbons for high CO2 adsorption and supercapacitor applications. Appl. Surf. Sci. 2019, 497, 143722. [Google Scholar] [CrossRef]
- Qazvini, O.T.; Babarao, R.; Telfer, S.G. Selective capture of carbon dioxide from hydrocarbons using a metal-organic framework. Nat. Commun. 2021, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ogungbenro, A.E.; Quang, D.V.; Al-Ali, K.A.; Vega, L.F.; Abu-Zahra, M.R. Physical synthesis and characterization of activated carbon from date seeds for CO2 capture. J. Environ. Chem. Eng. 2018, 6, 4245–4252. [Google Scholar] [CrossRef]
- González, A.; Plaza, M.; Rubiera, F.; Pevida, C. Sustainable biomass-based carbon adsorbents for post-combustion CO2 capture. Chem. Eng. J. 2013, 230, 456–465. [Google Scholar] [CrossRef] [Green Version]
- Su, W.; Wang, R.; Zhao, T. CO2-imprinted Sustainable Carbon Derived from Sunflower Heads for Highly Effective Capture of CO2 from Flue Gas. Aerosol Air Qual. Res. 2020, 20, 180–192. [Google Scholar] [CrossRef] [Green Version]
- Díez, N.; Álvarez, P.; Granda, M.; Blanco, C.; Santamaría, R.; Menéndez, R. CO2 adsorption capacity and kinetics in nitrogen-enriched activated carbon fibers prepared by different methods. Chem. Eng. J. 2015, 281, 704–712. [Google Scholar] [CrossRef] [Green Version]

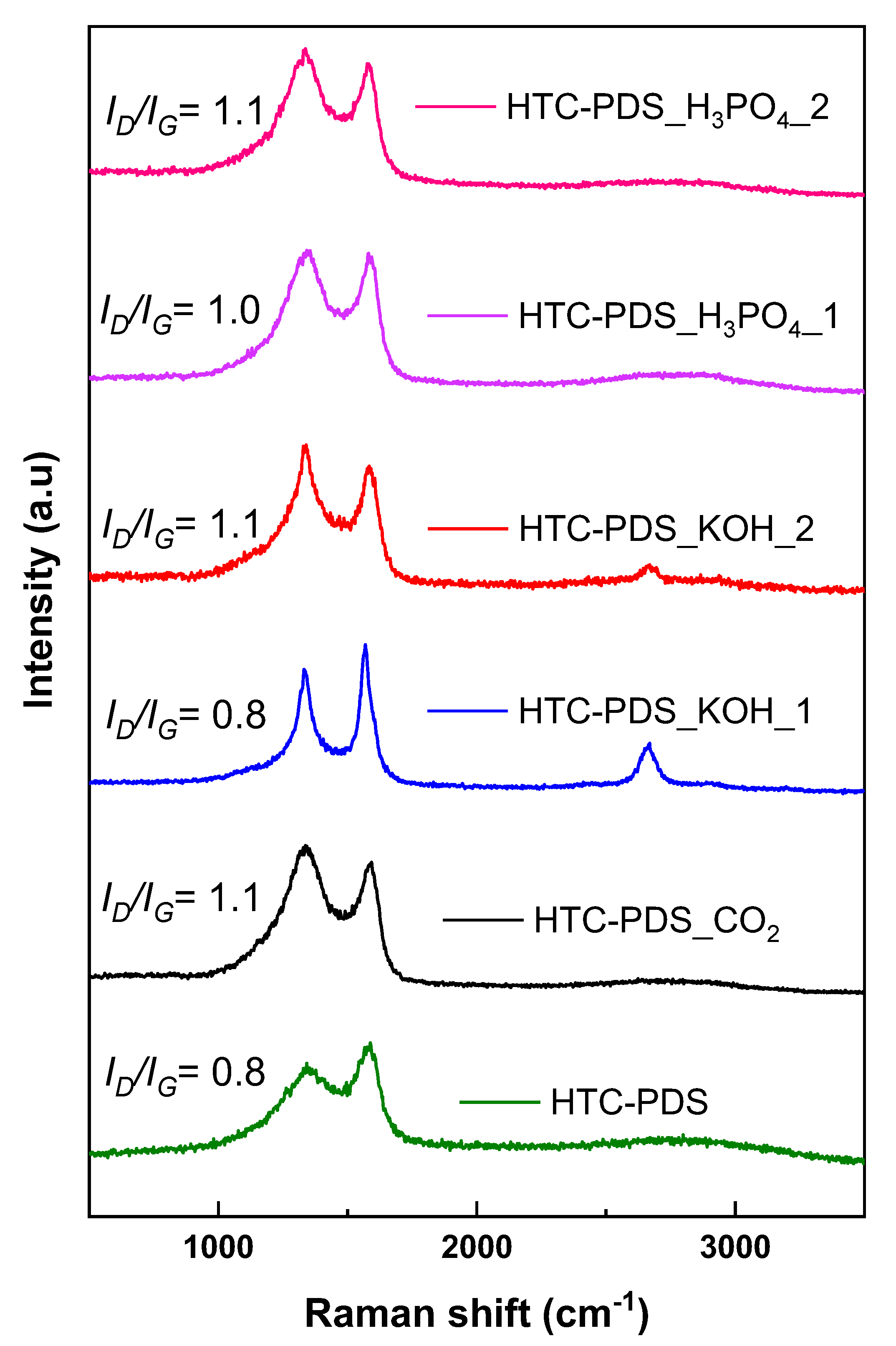

| Sample | Elemental Composition (wt %) | C/O Ratio | K (ppm) | |||
|---|---|---|---|---|---|---|
| C | N | H | O | |||
| HTC-PDS | 67.6 | 1.4 | 5.1 | 25.9 | 2.6 | 586 |
| HTC-PDS_CO2 activation | 81.1 | 1.7 | 1.4 | 15.8 | 5.1 | n.a. |
| HTC-PDS_KOH_1 | 87.8 | 0.6 | 0.3 | 11.3 | 7.7 | 8840 |
| HTC-PDS_KOH_2 | 57.4 | 0.4 | 0.5 | 41.7 | 1.4 | 8415 |
| HTC-PDS_H3PO4_1 | 69.4 | 1.4 | 2.0 | 27.2 | 2.5 | n.a. |
| HTC-PDS_H3PO4_2 | 70.2 | 1.3 | 2.0 | 26.5 | 2.6 | n.a. |
| Sample | SBET (m2 g−1) | SDR (m2 g−1) | SDFT (m2 g−1) | *μV (cm3 g−1) | PV (cm3 g−1) |
|---|---|---|---|---|---|
| HTC-PDS_CO2 | 858 | 949 | 910 | 0.34 | 0.39 |
| HTC-PDS_KOH_1 | 1906 | 2189 | 1867 | 0.78 | 1.06 |
| HTC-PDS_KOH_2 | 2335 | 2552 | 2122 | 0.90 | 1.54 |
| HTC-PDS_H3PO4_1 | 1218 | 1403 | 1251 | 0.50 | 0.50 |
| HTC-PDS_H3PO4_2 | 1439 | 1674 | 1086 | 0.60 | 0.60 |
| Precursor | Activation Method | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Structure (ID/IG) | CO2 Adsorption (mmol g−1) | Ref. |
|---|---|---|---|---|---|---|
| Commercial activated carbon | - | 698 | 0.21 | n.a. | 2.18 | [42] |
| Palm date seeds (UAE) | Physical activation (under CO2) | 798 | 0.28 | n.a. | 3.20 | [42] |
| Olive stones/almond shells | Physical activation (under CO2) | 1113 | 0.51 | n.a. | 1.02 | [43] |
| CO2–MIP * | Chemical activation (KOH) | - | - | n.a. | 1.71 | [44] |
| Camphor leaves | Chemical activation (KOH) | 1633 | 0.98 | n.a. | 0.80 | [14] |
| Oil-based pitch | Chemical activation (KOH) | 1720 | 0.98 | n.a. | 1.90 | [45] |
| Activated biocarbon | Chemical activation (KOH) | 1968 | 1.14 | 0.6 | 1.67 | [40] |
| Palm date seeds (KSA) | Chemical activation (KOH) | 1906 | 1.06 | 1.1 | 5.44 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alazmi, A.; Nicolae, S.A.; Modugno, P.; Hasanov, B.E.; Titirici, M.M.; Costa, P.M.F.J. Activated Carbon from Palm Date Seeds for CO2 Capture. Int. J. Environ. Res. Public Health 2021, 18, 12142. https://doi.org/10.3390/ijerph182212142
Alazmi A, Nicolae SA, Modugno P, Hasanov BE, Titirici MM, Costa PMFJ. Activated Carbon from Palm Date Seeds for CO2 Capture. International Journal of Environmental Research and Public Health. 2021; 18(22):12142. https://doi.org/10.3390/ijerph182212142
Chicago/Turabian StyleAlazmi, Amira, Sabina A. Nicolae, Pierpaolo Modugno, Bashir E. Hasanov, Maria M. Titirici, and Pedro M. F. J. Costa. 2021. "Activated Carbon from Palm Date Seeds for CO2 Capture" International Journal of Environmental Research and Public Health 18, no. 22: 12142. https://doi.org/10.3390/ijerph182212142
APA StyleAlazmi, A., Nicolae, S. A., Modugno, P., Hasanov, B. E., Titirici, M. M., & Costa, P. M. F. J. (2021). Activated Carbon from Palm Date Seeds for CO2 Capture. International Journal of Environmental Research and Public Health, 18(22), 12142. https://doi.org/10.3390/ijerph182212142






