Degradation of Minocycline by the Adsorption–Catalysis Multifunctional PVDF–PVP–TiO2 Membrane: Degradation Kinetics, Photocatalytic Efficiency, and Toxicity of Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Fiber Mats
2.3. Characterization of Fiber Mats
2.4. Photocatalysis Experiments
2.5. Analytical Procedures
3. Results and Discussion
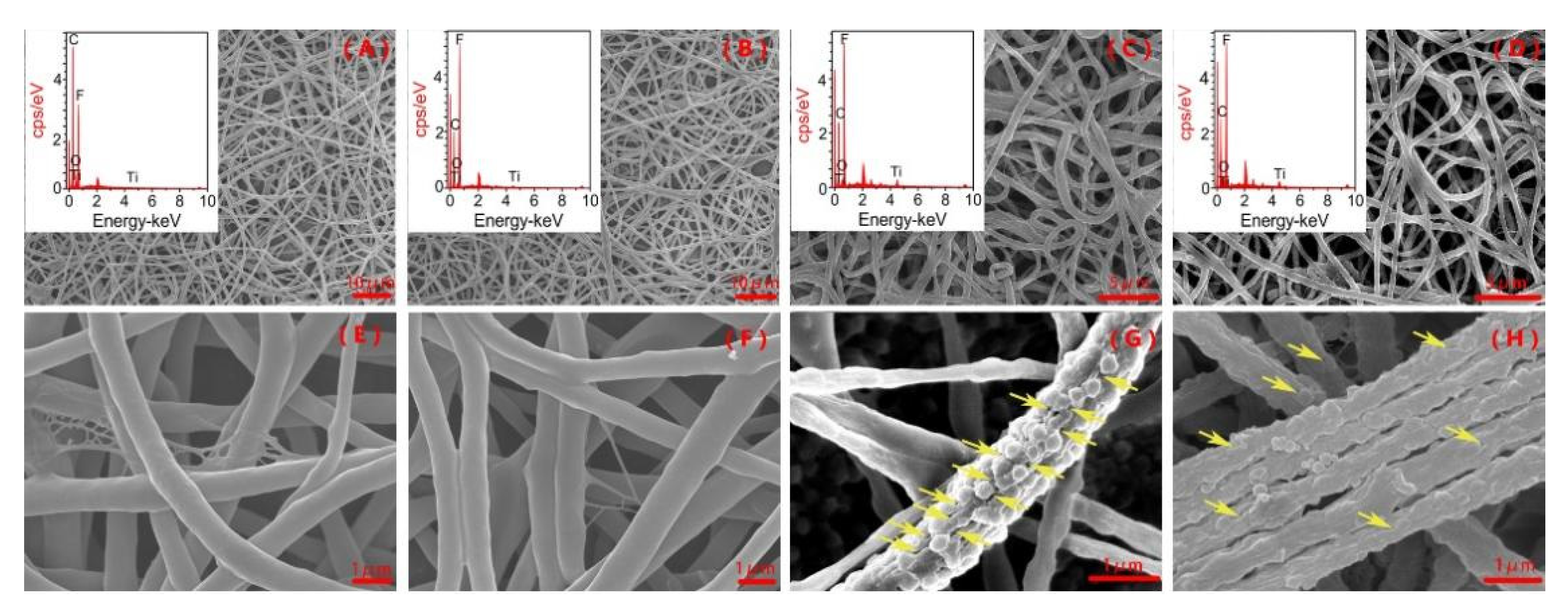
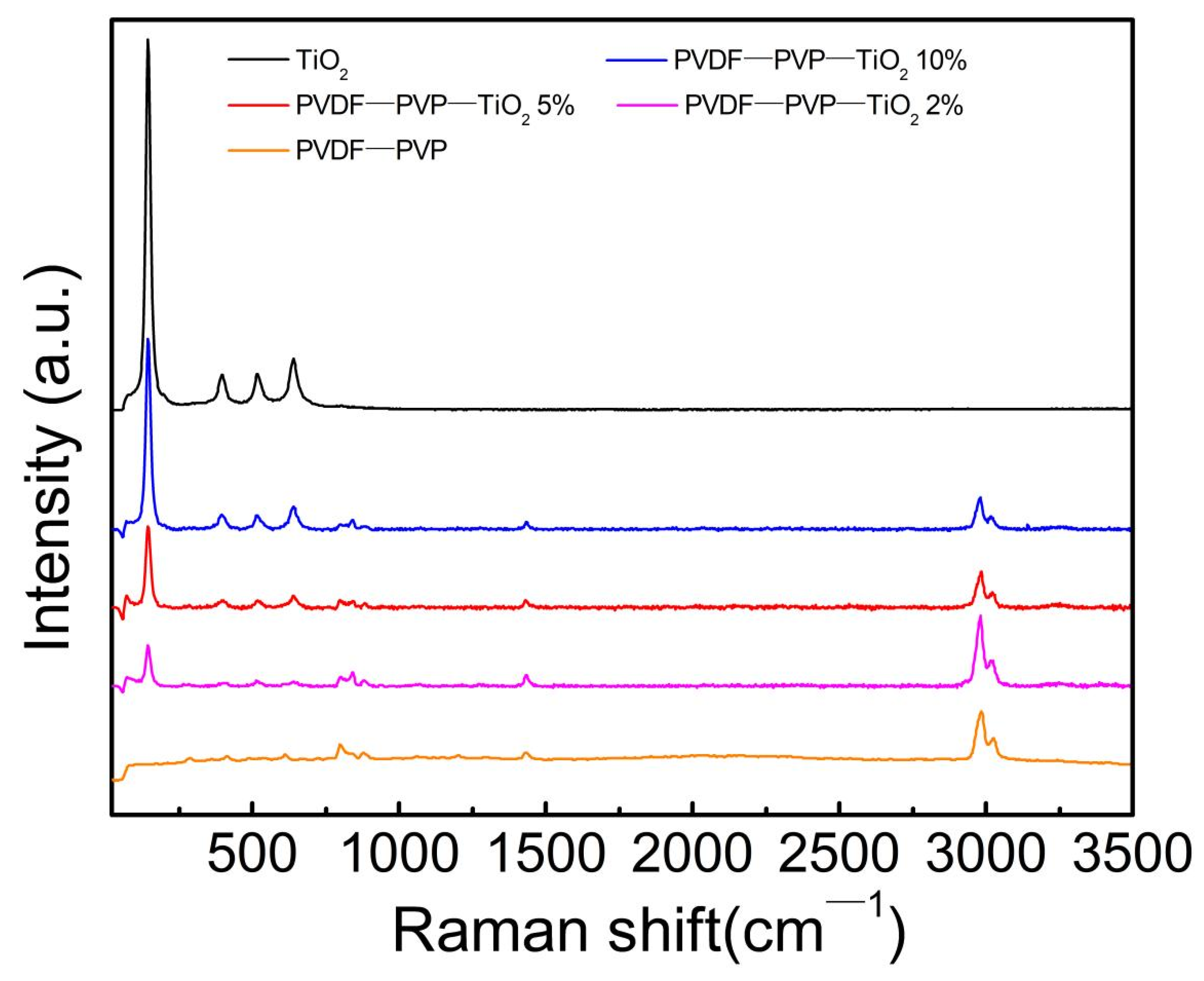
3.1. Characterization of Fiber Mats
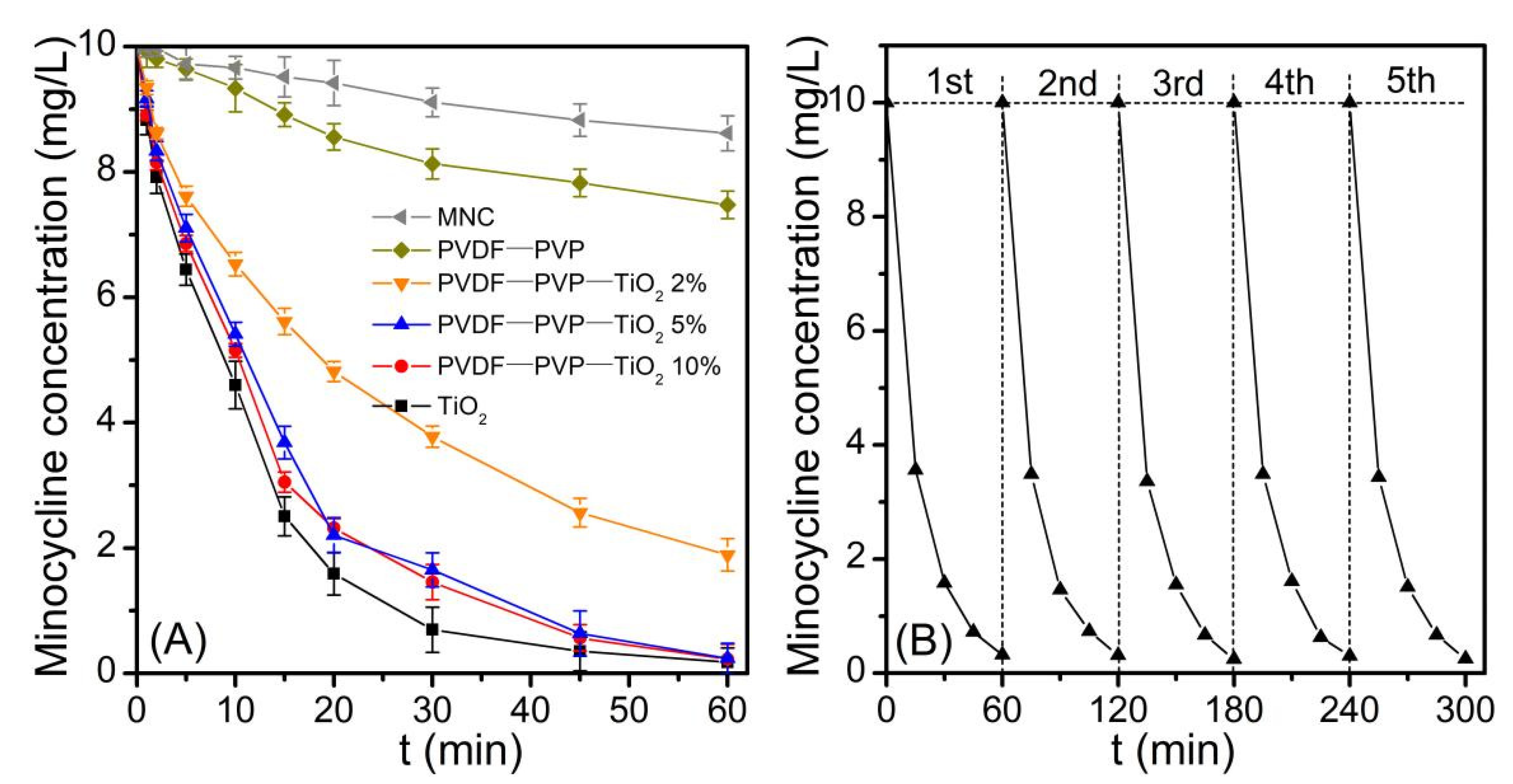
3.2. Photocatalytic Activity of PVDF–PVP–TiO2 Fiber Mats
3.3. Photocatalytic Degradation Kinetics of Minocycline
3.3.1. Effect of Initial Concentration of Minocycline
3.3.2. Effect of Initial pH
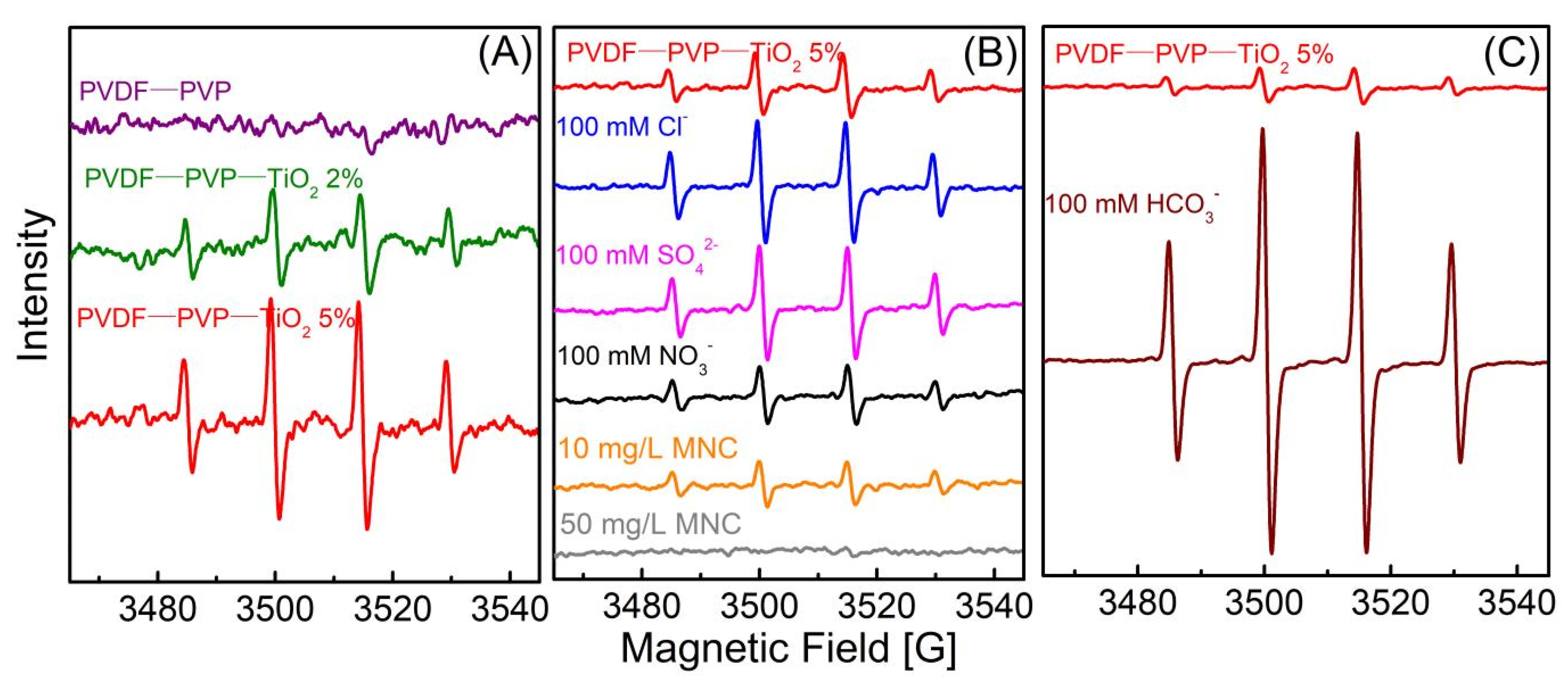
3.3.3. Effect of Inorganic Anions
3.3.4. Effect of DOM
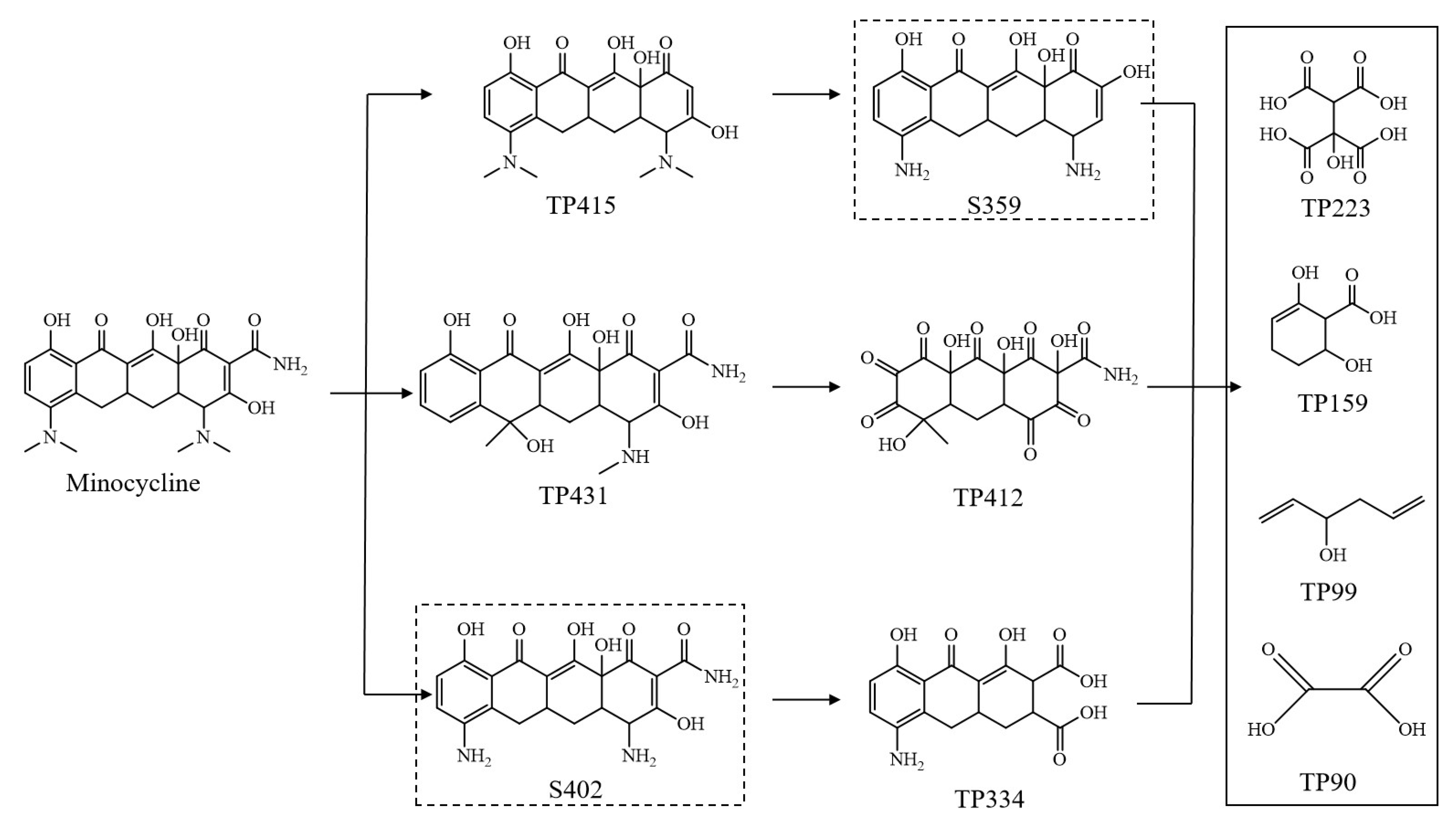
3.4. Degradation Mechanismand Toxicity Evaluation
3.5. Energy Consumption for Minocycline Photochemical Decomposition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, C.-G.; Javed, H.; Zhang, D.; Kim, J.-H.; Westerhoff, P.; Li, Q.; Alvarez, P.J.J. Porous Electrospun Fibers Embedding TiO2 for Adsorption and Photocatalytic Degradation of Water Pollutants. Environ. Sci. Technol. 2018, 52, 4285–4293. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, R.; We, Q.; Yang, M.; Cao, R.; Zong, X. Photocatalytic degradation of organic pollutants in wastewater by heteropolyacids: A review. J. Coord. Chem. 2021, 74, 1751–1764. [Google Scholar]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Guo, Y.; Ao, Y.; Wang, P.; Wang, C. Mediator-free direct dual-Z-scheme Bi2S3/BiVO4/MgIn2S4 composite photocata-lysts with enhanced visible-light-driven performance towards carbamazepine degradation. Appl. Catal. B Environ. 2019, 254, 479–490. [Google Scholar] [CrossRef]
- Kour, S.; Jasrotia, R.; Puri, P.; Verma, A.; Sharma, B.; Singh, V.P.; Kumar, R.; Kalia, S. Improving photocatalytic efficiency of MnFe2O4 ferrites via doping with Zn2+/La3+ ions: Photocatalytic dye degradation for water remediation. Environ. Sci. Pollut. Res. 2021, 1–16. [Google Scholar] [CrossRef]
- Koe, W.S.; Lee, J.W.; Chong, W.C.; Pang, Y.L.; Sim, L.C. An overview of photocatalytic degradation: Photocatalysts, mechanisms, and development of photocatalytic membrane. Environ. Sci. Pollut. Res. 2019, 27, 2522–2565. [Google Scholar] [CrossRef] [PubMed]
- Abulizi, A.; Kadeer, K.; Maimaitizi, H.; Tursun, Y.; Talifu, D. In situ ultrasound-assisted ion exchange synthesis of sphere-like AgClxBr1−x composites with enhanced photocatalytic activity and stability. Environ. Sci. Pollut. Res. 2020, 27, 43615–43624. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, P.; Hou, J.; Qian, J.; Ao, Y.; Wang, C. Significantly enhanced visible light photocatalytic efficiency of phosphorus doped TiO2 with surface oxygen vacancies for ciprofloxacin degradation: Synergistic effect and intermediates analysis. J. Hazard. Mater. 2018, 351, 196–205. [Google Scholar] [CrossRef]
- Feng, X.; Wang, P.; Hou, J.; Qian, J.; Wang, C.; Ao, Y. Oxygen vacancies and phosphorus codoped black titania coated carbon nanotube composite photocatalyst with efficient photocatalytic performance for the degradation of aceta-minophen under visible light irradiation. Chem. Eng. J. 2018, 352, 947–956. [Google Scholar] [CrossRef]
- Qiu, L.; Cui, Y.; Tan, X.; Zheng, S.; Zhang, H.; Xu, J.; Wang, Q. Construction of Ag3PO4/Ag4P2O7 nanospheres sensitized hierarchical titanium dioxide nanotube mesh for photoelectrocatalytic degradation of methylene blue. Sep. Purif. Technol. 2019, 215, 619–624. [Google Scholar] [CrossRef]
- Wang, G.; Dai, J.; Luo, Q.; Deng, N. Photocatalytic degradation of bisphenol A by TiO2@ aspartic ac-id-β-cyclodextrin@ reduced graphene oxide. Sep. Purif. Technol. 2021, 254, 117574. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Westerhoff, P.; Hristovski, K.; Crittenden, J.C. Stability of commercial metal oxide nanoparticles in water. Water Res. 2008, 42, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mahalingam, H.; Singh, P.K. Polymer-supported titanium dioxide photocatalysts for environmental remedi-ation: A review. Appl. Catal. A 2013, 462, 178–195. [Google Scholar] [CrossRef]
- Brame, J.; Long, M.; Li, Q.; Alvarez, P. Trading oxidation power for efficiency: Differential inhibition of pho-to-generated hydroxyl radicals versus singlet oxygen. Water Res. 2014, 60, 259–266. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Son, A.; Seid, M.G.; Shim, S.; Lee, S.H.; Chung, Y.C.; Lee, C.; Lee, J.; Hong, S.W. Photocatalytic applications of paper-like poly(vinylidene fluoride)-titanium dioxide hybrids fabricated using a combination of electrospinning and electrospraying. J. Hazard. Mater. 2015, 285, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.-Y.; Chung, J.W.; Kwak, S.-Y. Dependence of photocatalytic and antimicrobial activity of electrospun polymeric nanofiber composites on the positioning of Ag–TiO2 nanoparticles. Compos. Sci. Technol. 2015, 117, 9–17. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, S.; Wang, J.; Bu, L.; Deng, J.; Zhou, S. Role of reactive nitrogen species in ranitidine degradation in UV/chloramine process: Transformation pathways and NDMA formation. Chem. Eng. J. 2021, 404, 126557. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, J.-H.; Seo, G.; Park, H. Risk of Cancer Following the Use of N-Nitrosodimethylamine (NDMA) Contaminated Ranitidine Products: A Nationwide Cohort Study in South Korea. J. Clin. Med. 2021, 10, 153. [Google Scholar] [CrossRef]
- Engelward, B.P. Implications of an epidemiological study showing an association between in utero NDMA exposure and childhood cancer. Environ. Mol. Mutagen. 2021, 62, 288–292. [Google Scholar] [CrossRef]
- Le Roux, J.; Gallard, H.; Croué, J.-P. Chloramination of nitrogenous contaminants (pharmaceuticals and pesticides): NDMA and halogenated DBPs formation. Water Res. 2011, 45, 3164–3174. [Google Scholar] [CrossRef]
- Leavey-Roback, S.L.; Krasner, S.W.; Suffet, I.M. Veterinary antibiotics used in animal agriculture as NDMA precursors. Chemosphere 2016, 164, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zheng, W.; Xiao, Y.; Du, B.; Zhang, X.; Wen, M.; Lai, C.; Huang, Y.; Sheng, L. Multifunctional, Robust, and Porous PHBV—GO/MXene Composite Membranes with Good Hydrophilicity, Antibacterial Activity, and Platelet Adsorption Performance. Polymers 2021, 13, 3748. [Google Scholar] [CrossRef]
- Moghadam, M.T.; Lesage, G.; Mohammadi, T.; Mericq, J.P.; Mendret, J.; Heran, M.; Faur, C.; Brosillon, S.; Hemmati, M.; Naeimpoor, F. Improved antifouling properties of TiO2/PVDF nanocomposite membranes in UV-coupled ultrafiltration. J. Appl. Polym. Sci. 2015, 132, 132–153. [Google Scholar] [CrossRef]
- Dong, Z.-Q.; Ma, X.-H.; Xu, Z.-L.; Gu, Z.-Y. Superhydrophobic modification of PVDF–SiO2 electrospun nanofiber membranes for vacuum membrane distillation. RSC Adv. 2015, 5, 67962–67970. [Google Scholar] [CrossRef]
- Vild, A.; Teixeira, S.; Kühn, K.; Cuniberti, G.; Sencadas, V. Orthogonal experimental design of titanium dioxide—Poly(methyl methacrylate) electrospun nanocomposite membranes for photocatalytic applications. J. Environ. Chem. Eng. 2016, 4, 3151–3158. [Google Scholar] [CrossRef] [Green Version]
- Cossich, E.; Bergamasco, R.; de Amorim, M.P.; Martins, P.; Marques, J.; Tavares, C.; Lanceros-Méndez, S.; Sencadas, V. Development of electrospun photocatalytic TiO2-polyamide-12 nanocomposites. Mater. Chem. Phys. 2015, 164, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Scarisoreanu, M.; Ilie, A.; Dutu, E.; Badoi, A.; Dumitrache, F.; Tanasa, E.; Mihailescu, N.; Mihailescu, I. Direct nanocrystallite size investigation in microstrained mixed phase TiO2 nanoparticles by PCA of Raman spectra. Appl. Surf. Sci. 2019, 470, 507–519. [Google Scholar] [CrossRef]
- Buonomenna, M.; Macchi, P.; Davoli, M.; Drioli, E. Poly(vinylidene fluoride) membranes by phase inversion: The role the casting and coagulation conditions play in their morphology, crystalline structure and properties. Eur. Polym. J. 2007, 43, 1557–1572. [Google Scholar] [CrossRef]
- He, T.; Zhou, Z.; Xu, W.; Ren, F.; Ma, H.; Wang, J. Preparation and photocatalysis of TiO2–fluoropolymer electrospun fiber nanocomposites. Polymer 2009, 50, 3031–3036. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Z.; Wang, C.; Li, X.; Wang, C.-C. Photocatalytic degradation of DOM in urban stormwater runoff with TiO2 nanoparticles under UV light irradiation: EEM-PARAFAC analysis and influence of co-existing inorganic ions. Environ. Pollut. 2018, 243, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Sivalingam, G.; Chattopadhyay, S.; Madras, G. Enzymatic degradation of poly (ε-caprolactone), poly (vinyl acetate) and their blends by lipases. Chem. Eng. Sci. 2003, 58, 2911–2919. [Google Scholar] [CrossRef]
- Doong, R.A.; Chen, C.H.; Maithreepala, R.A.; Chang, S.M. The influence of pH and cadmium sulfide on the photo-catalytic degradation of 2-chlorophenol in titanium dioxide suspensions. Water Res. 2001, 35, 2873–2880. [Google Scholar] [CrossRef]
- Hu, X.; Jia, L.; Cheng, J.; Sun, Z. Magnetic ordered mesoporous carbon materials for adsorption of minocycline from aqueous solution: Preparation, characterization and adsorption mechanism. J. Hazard. Mater. 2019, 362, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Brigante, M.; Schulz, P.C. Remotion of the antibiotic tetracycline by titania and titania–silica composed materials. J. Hazard. Mater. 2011, 192, 1597–1608. [Google Scholar] [CrossRef]
- Maeng, S.K.; Cho, K.; Jeong, B.; Lee, J.; Lee, Y.; Lee, C.; Choi, K.J.; Hong, S.W. Substrate-immobilized electrospun TiO2 nanofibers for photocatalytic degradation of pharmaceuticals: The effects of pH and dissolved organic matter characteristics. Water Res. 2015, 86, 25–34. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Y.; Chen, J.; Xu, L.; Huang, H.; Niu, J. High-efficiency electrochemical degradation of antiviral drug abacavir using a penetration flux porous Ti/SnO2–Sb anode. Chemosphere 2019, 225, 304–310. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Puma, G.L.; Wang, C.; Wang, P.; Zhang, W.; Wang, Q. Mechanism and experimental study on the photocatalytic performance of Ag/AgCl@ chiral TiO2 nanofibers photocatalyst: The impact of wastewater components. J. Hazard. Mater. 2015, 285, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Yin, K.; Liu, C.; Luo, J.; Crittenden, J.; Zhang, W.; Luo, S.; He, Q.; Deng, Y.; Liu, H.; et al. The role of re-active oxygen species and carbonate radical in oxcarbazepine degradation via UV, UV/H2O2: Kinetics, mechanisms and toxicity evaluation. Water Res. 2018, 147, 204–213. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Duan, X.; Fu, Y.; Dionysiou, D. Photochemical degradation of oxytetracycline: Influence of pH and role of carbonate radical. Chem. Eng. J. 2015, 276, 113–121. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Duan, X.; Fu, Y.; Fatta-Kassinos, D.; Dionysiou, D.D. Significant role of UV and carbonate radical on the degradation of oxytetracycline in UV-AOPs: Kinetics and mechanism. Water Res. 2016, 95, 195–204. [Google Scholar] [CrossRef]
- Conte, P.; Kucerik, J. Water Dynamics and Its Role in Structural Hysteresis of Dissolved Organic Matter. Environ. Sci. Technol. 2016, 50, 2210–2216. [Google Scholar] [CrossRef] [Green Version]
- Uyguner-Demirel, C.S.; Birben, N.C.; Bekbolet, M. Elucidation of background organic matter matrix effect on photo-catalytic treatment of contaminants using TiO2: A review. Catal. Today 2017, 284, 202–214. [Google Scholar] [CrossRef]
- Piccapietra, F.; Gil Allué, C.; Sigg, L.; Behra, R. Intracellular Silver Accumulation in Chlamydomonas reinhardtii upon Exposure to Carbonate Coated Silver Nanoparticles and Silver Nitrate. Environ. Sci. Technol. 2012, 46, 7390–7397. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Chen, M.; Schlautman, M.A.; Hur, J. Dynamic exchanges between DOM and POM pools in coastal and in-land aquatic ecosystems: A review. Sci. Total Environ. 2016, 551, 415–428. [Google Scholar] [CrossRef]
- Parvin, F.; Nayna, O.K.; Tareq, S.M.; Rikta, S.Y.; Kamal, A.K. Facile synthesis of iron oxide nanoparticle and synergistic effect of iron nanoparticle in the presence of sunlight for the degradation of DOM from textile wastewater. Appl. Water Sci. 2018, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Liu, L.; Su, J.; Liang, J.; Wu, B.; Zuo, J.; Zuo, Y. Role of humic substances in the photodegradation of naproxen under simulated sunlight. Chemosphere 2017, 187, 261–267. [Google Scholar] [CrossRef]
- Porras, J.; Giannakis, S.; Torres-Palma, R.A.; Fernandez, J.J.; Bensimon, M.; Pulgarin, C. Fe and Cu in humic acid ex-tracts modify bacterial inactivation pathways during solar disinfection and photo-Fenton processes in water. Appl. Catal. B-Environ. 2018, 235, 75–83. [Google Scholar] [CrossRef]
- Canonica, S.; Laubscher, H.-U. Inhibitory effect of dissolved organic matter on triplet-induced oxidation of aquatic contaminants. Photochem. Photobiol. Sci. 2008, 7, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Wenk, J.; Von Gunten, U.; Canonica, S. Effect of dissolved organic matter on the transformation of contaminants in-duced by excited triplet states and the hydroxyl radical. Environ. Sci. Technol. 2011, 45, 1334–1340. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Xiong, Z.; Lai, B. Effect of initial pH on the tetracycline (TC) removal by zero-valent iron: Adsorption, oxidation and reduction. Chem. Eng. J. 2018, 343, 492–499. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Ma, Y.-L.; Yang, J.; Wang, L.-Q.; Lv, J.-M.; Ren, C.-J. Aqueous tetracycline degradation by H2O2 alone: Removal and transformation pathway. Chem. Eng. J. 2017, 307, 15–23. [Google Scholar] [CrossRef]
- Beck, S.; Ryu, H.; Boczek, L.A.; Cashdollar, J.L.; Jeanis, K.M.; Rosenblum, J.S.; Lawal, O.R.; Linden, K.G. Evaluating UV-C LED disinfection performance and investigating potential dual-wavelength synergy. Water Res. 2017, 109, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Benotti, M.J.; Stanford, B.D.; Wert, E.C.; Snyder, S. Evaluation of a photocatalytic reactor membrane pilot system for the removal of pharmaceuticals and endocrine disrupting compounds from water. Water Res. 2009, 43, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Stancl, H.O.N.; Hristovski, K.; Westerhoff, P. Hexavalent chromium removal using UV-TiO2/ceramic membrane reactor. Environ. Eng. Sci. 2015, 32, 676–683. [Google Scholar] [CrossRef]

| Product | Acute Toxicity | Chronic Toxicity | ||||
|---|---|---|---|---|---|---|
| (mg/L) | (ChV) (mg/L) | |||||
| Fish (LC50) | Daphnid (LC50) | Green Algae (EC50) | Fish | Daphnid | Green Algae | |
| Minocyline | 1.78 × 102 | 6.85 × 100 | 9.14 × 101 | 2.06 × 101 | 8.04 × 100 | 9.62 × 100 |
| TP431 | 4.94 × 102 | 1.08 × 101 | 2.97 × 102 | 8.63 × 101 | 2.64 × 101 | 2.26 × 101 |
| TP415 | 6.35 × 101 | 3.98 × 100 | 2.86 × 101 | 5.21 × 100 | 2.50 × 100 | 3.97 × 100 |
| TP412 | 6.41 × 106 | 1.48 × 108 | 4.86 × 104 | 2.18 × 102 | 1.06 × 105 | 2.82 × 103 |
| TP334 | 7.75 × 102 | 3.91 × 101 | 3.71 × 101 | 7.44 × 101 | 3.25 × 101 | 4.55 × 101 |
| TP223 | 1.01 × 108 | 3.54 × 107 | 3.51 × 106 | 5.58 × 106 | 8.88 × 105 | 3.10 × 105 |
| TP159 | 5.84 × 101 | 6.97 × 100 | 2.92 × 103 | 5.33 × 100 | 7.26 × 10−1 | 2.91 × 102 |
| TP99 | 2.27 × 100 | 3.04 × 10−1 | 2.95 × 101 | 7.60 × 10−2 | 1.70 × 10−2 | 4.32 × 100 |
| TP90 | 1.68 × 105 | 6.75 × 104 | 1.21 × 104 | 1.09 × 104 | 2.52 × 103 | 1.47 × 103 |
| S402 | 3.29 × 101 | 3.24 × 100 | 4.78 × 100 | 1.68 × 101 | 9.86 × 10−1 | 1.07 × 100 |
| S359 | 1.43 × 101 | 1.67 × 100 | 5.02 × 101 | 1.54 × 100 | 1.93 × 10−1 | 5.80 × 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Sun, Y.; Zhang, F.; Wu, Y. Degradation of Minocycline by the Adsorption–Catalysis Multifunctional PVDF–PVP–TiO2 Membrane: Degradation Kinetics, Photocatalytic Efficiency, and Toxicity of Products. Int. J. Environ. Res. Public Health 2021, 18, 12339. https://doi.org/10.3390/ijerph182312339
Zhou C, Sun Y, Zhang F, Wu Y. Degradation of Minocycline by the Adsorption–Catalysis Multifunctional PVDF–PVP–TiO2 Membrane: Degradation Kinetics, Photocatalytic Efficiency, and Toxicity of Products. International Journal of Environmental Research and Public Health. 2021; 18(23):12339. https://doi.org/10.3390/ijerph182312339
Chicago/Turabian StyleZhou, Chengzhi, Yanlong Sun, Fan Zhang, and Yuandong Wu. 2021. "Degradation of Minocycline by the Adsorption–Catalysis Multifunctional PVDF–PVP–TiO2 Membrane: Degradation Kinetics, Photocatalytic Efficiency, and Toxicity of Products" International Journal of Environmental Research and Public Health 18, no. 23: 12339. https://doi.org/10.3390/ijerph182312339
APA StyleZhou, C., Sun, Y., Zhang, F., & Wu, Y. (2021). Degradation of Minocycline by the Adsorption–Catalysis Multifunctional PVDF–PVP–TiO2 Membrane: Degradation Kinetics, Photocatalytic Efficiency, and Toxicity of Products. International Journal of Environmental Research and Public Health, 18(23), 12339. https://doi.org/10.3390/ijerph182312339







