Characterization of Chemical and Bacterial Concentrations in Floor Dust Samples in Southeast Texas Households
Abstract
:1. Introduction
2. Materials and Methods
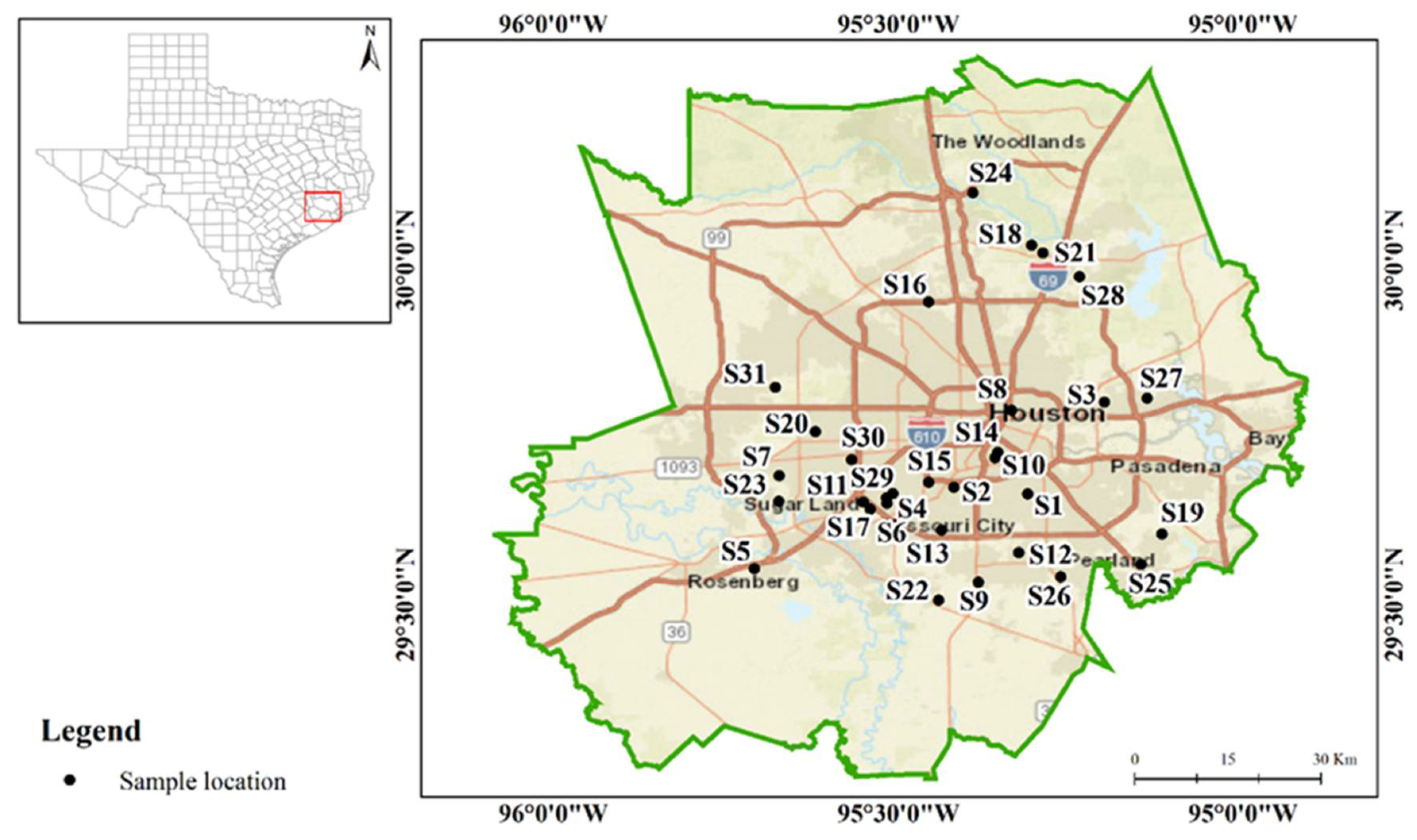
2.1. Study Area and Sample Collection
2.2. Chemical Analysis
2.3. Statistical and Spatial Analyses
2.4. Scanning Electron Microscope (SEM) and Energy Dispersive Spectrometer (EDS) Analysis
2.5. Heavy Metal Enrichment
2.6. Health Risk Assessment
2.7. Bacterial Analysis
3. Results
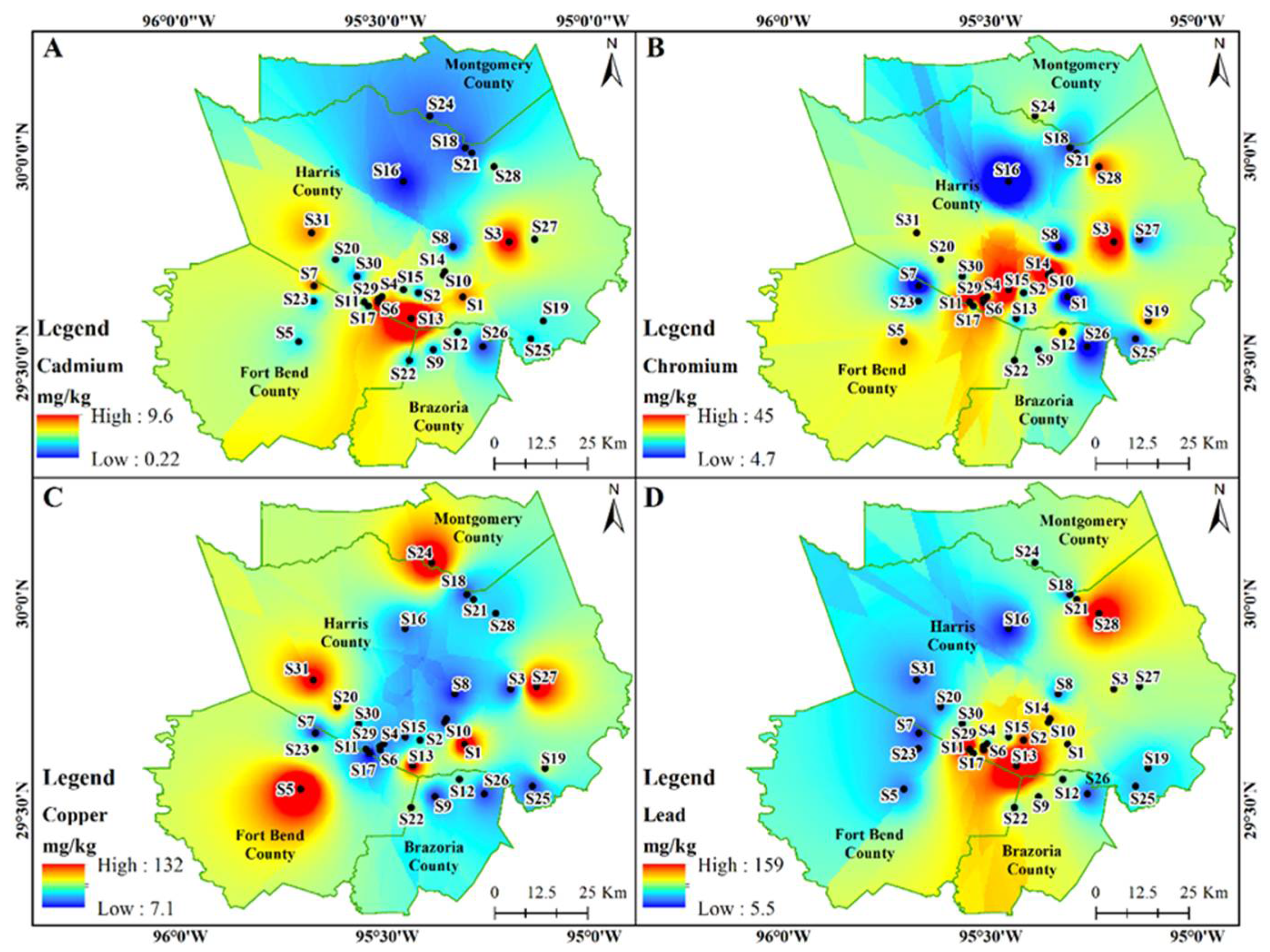
3.1. Chemical Analysis
3.2. Scanning Electron Microscopy (SEM)
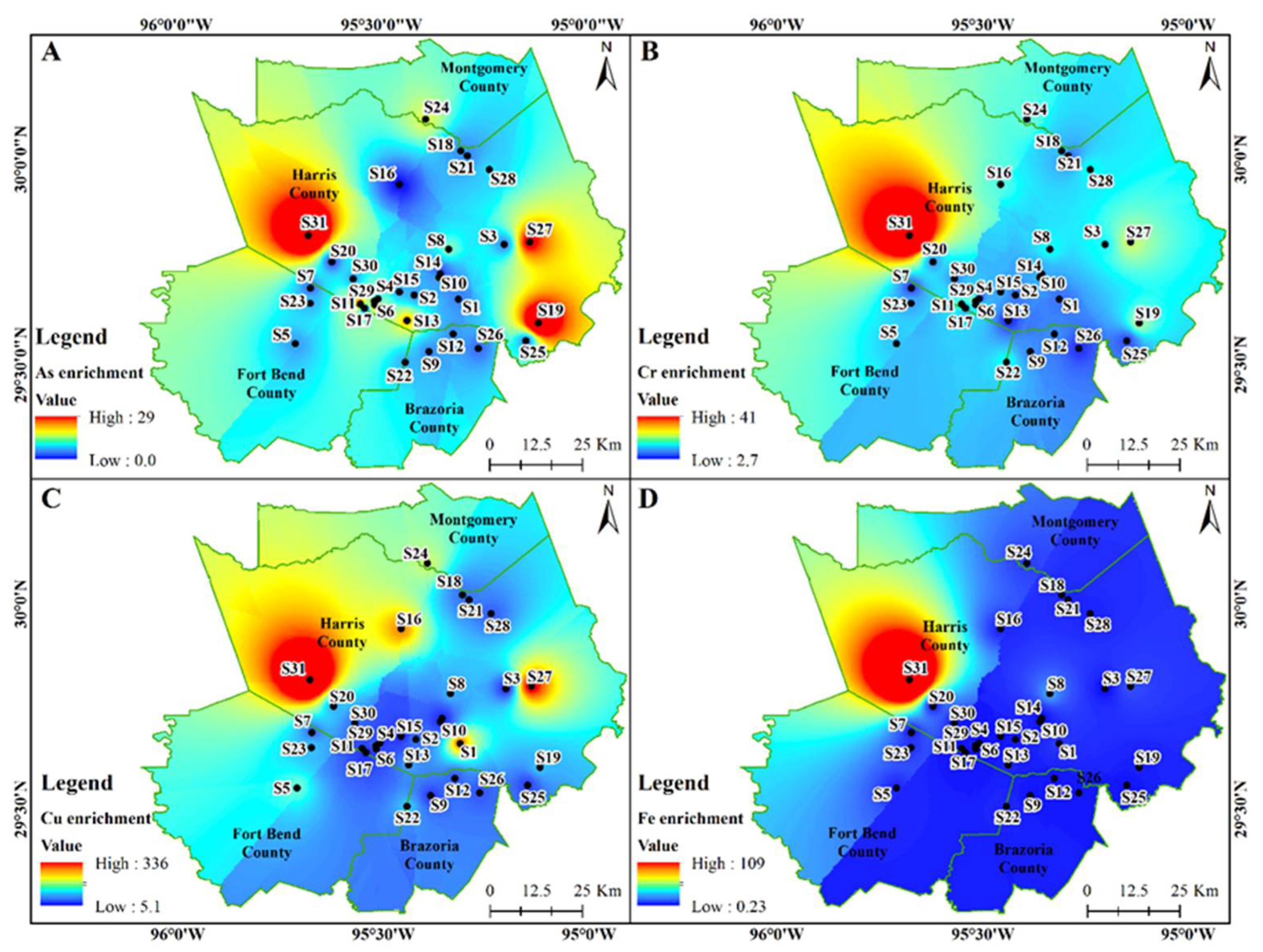
3.3. Enrichment of Measured Metals
3.4. Health Risk Assessment Results
3.5. Bacteria Isolate Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OSHA. Indoor Air Quality in Commercial and Institutional Buildings. 2011. Available online: https://www.osha.gov/sites/default/files/publications/3430indoor-air-quality-sm.pdf (accessed on 1 October 2021).
- WHO. Household Air Pollution and Health. 2018. Available online: https://webstore.iea.org/weo-2017-special-report-energy-access-outlook (accessed on 1 October 2021).
- Tan, S.Y.; Praveena, S.M.; Abidin, E.Z.; Cheema, M.S. A review of heavy metals in indoor dust and its human health-risk implications. Rev. Environ. Health 2016, 31, 447–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arar, S.; Al-Hunaiti, A.; Masad, M.H.; Maragkidou, A.; Wraith, D.; Hussein, T. Elemental Contamination in Indoor Floor Dust and Its Correlation with PAHs, Fungi, and Gram+/− Bacteria. Int. J. Environ. Res. Public Health 2019, 16, 3552. [Google Scholar] [CrossRef] [Green Version]
- Rasmussena, P.E.; Levesquea, C.; Cheniera, M.; Gardnera, H.D. Contribution of metals in resuspended dust to indoor and personal inhalation exposures: Relationships between PM10 and settled dust. Build. Environ. 2018, 143, 513–522. [Google Scholar] [CrossRef]
- Lin, Y.; Fang, F.; Wang, F.; Xu, M. Pollution distribution and health risk assessment of heavy metals in indoor dust in Anhui rural, China. Environ. Monit. Assess. 2015, 187, 565. [Google Scholar] [CrossRef]
- Hejami, A.A.; Davis, M.; Prete, D.; Lu, J.; Wang, S. Heavy metals in indoor settled dusts in Toronto, Canada. Sci. Total Environ. 2020, 703, 134895. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, S.; Chauhan, A.S.; Verma, R.; Gupta, T. Identification and quantification of indoor air pollutant sources within a residential academic campus. Sci. Total Environ. 2016, 569–570, 46–52. [Google Scholar] [CrossRef]
- Yang, L.; Liu, L.; Xiong, Y.; Zhang, G.; Wen, H.; Lei, J.; Guo, L.; Lyu, Y. A comparative study on physicochemical characteristics of household dust from a metropolitan city and a remote village in China. Atmos. Pollut. 2016, 7, 1090–1100. [Google Scholar] [CrossRef]
- Hu, J.; Li, N.; Lv, Y.; Liu, J.; Xie, J.; Zhang, H. Investigation on Indoor Air Pollution and Childhood Allergies in Households in Six Chinese Cities by Subjective Survey and Field Measurements. Int. J. Environ. Res. Public Health 2017, 14, 979. [Google Scholar] [CrossRef]
- Sabzevari, E.; Sobhanardakani, S. Analysis of Selected Heavy Metals in Indoor Dust Collected from City of Khorramabad, Iran: A Case Study. Jundishapur J. Health Sci. 2018, 10, e67382. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Patlolla, A.K.; Centeno, J.A. Carcinogenic and Systemic Health Effects Associated with Arsenic Exposure—A Critical Review. Toxicol. Pathol. 2003, 31, 575–588. [Google Scholar] [CrossRef]
- USEPA. Chromium Compounds. 2000. Available online: https://www.epa.gov/sites/production/files/2016-09/documents/chromium-compounds.pdf (accessed on 1 October 2021).
- USEPA. Review of Dust-Lead Post Abatement Clearance Levels. EPA-HQ-OPPT-202-0063 FRL-10018-61. 2020. Available online: https://www.federalregister.gov/documents/2021/01/07/2020-28565/review-of-dust-lead-post-abatement-clearance-levels (accessed on 1 October 2021).
- Sridhar, B.B.M.; Johnson, J.; Mosuro, A. Impact of land cover changes on the soil and water quality of Greens Bayou watershed. Water Air Soil Pollut. 2020, 231, 510. [Google Scholar] [CrossRef]
- Bukunmi-Omidiran, T.; Sridhar, B.B.M. Evaluation of spatial and temporal water and soil quality in the Buffalo and Brays Bayou watersheds of Houston, Texas. Remote Sens. Appl. Soc. Environ. 2021, 21, 100455. [Google Scholar] [CrossRef]
- USEPA. Method 3050B: Acid Digestion of Sediments, Sludges, and Soils, Revision 2; USEPA: Washington, DC, USA, 1996. [Google Scholar]
- Schober, P.; Christa, B.; Schwarte, L. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- TCEQ. Subchapter C: Affected Property Assessment. 1999. Available online: https://www.tceq.texas.gov/assets/public/legal/rules/rules/pdflib/350c.pdf (accessed on 1 October 2021).
- Bern, C.W. Improved enrichment factor calculations through principal component analysis: Examples from soils near breccia pipe uranium mines, Arizona, USA. Environ. Pollut. 2019, 248, 90–100. [Google Scholar] [CrossRef]
- Barbieri, M. The Importance of Enrichment Factor (EF) and Geoaccumulation Index (Igeo) to Evaluate the Soil Contamination. J. Geol. Geophys. 2016, 5, 237. [Google Scholar] [CrossRef]
- Vermont Department of Health. Residential Soil Values (RSVs) and Commercial Soil Values (CSVs). 2020. Available online: https://www.healthvermont.gov/sites/default/files/documents/pdf/ENV_ECP_GeneralScreeningValues_Soil.pdf (accessed on 1 October 2021).
- Yadav, I.C.; Devi, N.L.; Singh, V.K.; Li, J.; Zhang, G. Spatial distribution, source analysis, and health risk assessment of heavy metals contamination in house dust and surface soil from four major cities of Nepal. Chemosphere 2019, 218, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Kurt-Karakus, P.B. Determination of heavy metals in indoor dust from Istanbul, Turkey: Estimation of the health risk. Environ. Int. 2012, 50, 47–55. [Google Scholar] [CrossRef]
- NCEA. Exposure Factors Handbook: 2011 Edition; USEPA: Washington, DC, USA, 2011. [Google Scholar]
- Ge, J.; Woodward, L.A.; Li, Q.X.; Wang, J. Composition, distribution, and risk assessment of organochlorine pesticides in soils from the Midway Atoll, North Pacific Ocean. Sci. Total Environ. 2013, 452–453, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Adedoyin, F.T.; Sridhar, B.B.M.; Rosenzweig, J.A. Characterization of bacterial populations in urban and rural Houston watershed soil samples following a flooding event. Front. Environ. Microbiol. 2021, 7, 22–34. [Google Scholar] [CrossRef]
- USEPA. Region 4 Ecological Risk Assessment Supplemental Guidance. 2018. Available online: https://www.epa.gov/sites/default/files/2018-03/documents/era_regional_supplemental_guidance_report-march-2018_update.pdf (accessed on 1 October 2021).
- Hanover. Aluminum Wiring. 2017. Available online: https://www.hanover.com/resources/risk-management-businesses/hanover-risk-solutions/aluminum-wiring. (accessed on 1 October 2021).
- USGS. Do We Take Minerals for Granted? 2009. Available online: https://www.usgs.gov/energy-and-minerals/mineral-resources-program/science/do-we-take-minerals-granted?qt-science_center_objects=0#qt-science_center_objects (accessed on 1 October 2021).
- USEPA. Protect Your Family from Sources of Lead. 2020. Available online: https://www.epa.gov/lead/protect-your-family-sources-lead (accessed on 1 October 2021).
- Healthy Stuff. Vinyl Flooring Follow-Up Report. 2021. Available online: https://www.ecocenter.org/healthy-stuff/pages/vinyl-flooring-follow-report (accessed on 1 October 2021).
- ATSDR. Cadmium Toxicity. Where Is Cadmium Found? 2013. Available online: https://www.atsdr.cdc.gov/csem/cadmium/Where-Cadmium-Found.html (accessed on 1 October 2021).
- Mäki, J.M.; Kirjavainen, P.V.; Täubel, M.; Piippo-Savolainen, E.; Backman, K.; Hyvärinen, A.; Tuoresmäki, P.; Jayaprakash, B.; Heinrich, J.; Herberth, G.; et al. Associations between dog keeping and indoor dust microbiota. Sci. Rep. 2021, 11, 5341. [Google Scholar] [CrossRef]
- Madureira, J.; Paciência, I.; Fernandes, E.O. Levels and Indoor–Outdoor Relationships of Size-Specific Particulate Matter in Naturally Ventilated Portuguese Schools. J. Toxicol. Environ. Health Part A 2012, 75, 1423–1436. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Rajkumar, P. Characterization of minerals in air dust particles in the state of Tamilnadu, India through FTIR, XRD and SEM analyses. Infrared Phys. Technol. 2014, 67, 30–41. [Google Scholar] [CrossRef]
- Schaider, L.A.; Senn, D.B.; Brabander, D.J.; McCarthy, K.D.; Shine, J.P. Characterization of Zinc, Lead, and Cadmium in mine waste: Implications for Transport, Exposure, and Bioavailability. Environ. Sci. Technol. 2007, 41, 4164–4171. [Google Scholar] [CrossRef] [PubMed]
- Doyi, N.Y.; Strezov, V.; Isley, C.F.; Yazdanparast, T.; Taylor, M.P. The relevance of particle size distribution and bioaccessibility on human health risk assessment for trace elements measured in indoor dust Israel. Sci. Total Environ. 2020, 733, 137931. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, G.; Shen, M.; Hu, R.; Sun, M.; Liu, Y. Characteristics and health risk assessment of heavy metals in indoor dust from different functional areas in Hefei, China. Environ. Pollut. 2019, 25, 839–849. [Google Scholar] [CrossRef]
- Gopal, N.; Hill, C.; Ross, P.R.; Beresford, T.P.; Fenelon, M.A.; Cotter, P.D. The prevalence and control of Bacillus and related spore-forming bacteria in the dairy industry. Front. Microbiol. 2015, 6, 1418. [Google Scholar] [CrossRef]
- Huang, Y.; Flint, S.H.; Palmer, J.S. Bacillus cereus spores and toxins—The potential role of biofilms. Food Microbiol. 2020, 90, 103493. [Google Scholar] [CrossRef]
- Cattani, F.; Barth, V.C., Jr.; Nasário, J.S.R.; Ferreira, C.A.S.; Oliveira, S.D. Detection and quantification of viable Bacillus cereus group species in milk by propidium monoazide quantitative real-time PCR. J. Dairy Sci. 2016, 99, 2617–2624. [Google Scholar] [CrossRef]
- Elliot, T.; Worthington, T.; Osman, H.; Gill, M. Medical Microbiology & Infection, 4th ed.; Blackwell: Oxford, UK, 2007. [Google Scholar]
- Pappas, R.S. Toxic elements in tobacco and in cigarette smoke: Inflammation and sensitization. Metallomics 2011, 3, 1181–1198. [Google Scholar] [CrossRef] [Green Version]





| Habitat | Al | As | Cd | Cr | Cu | Fe | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|
| Mean | 3738 | 3.6 | 1.9 | 23 | 53 | 2939 | 12 | 38 | 368 |
| Home Type | |||||||||
| Apartment (n = 7) | 5103 a | 2.3 | 1.6 | 26 | 35 b | 1304 | 9.4 | 32 | 221 |
| Single Family (n = 24) | 3179 b | 4.1 | 2.1 | 22 | 61 a | 3608 | 14 | 41 | 428 |
| Home Age | |||||||||
| Under 10 years (n = 4) | 3687 | 3.0 | 1.8 ab | 29 | 56.5 | 8254 | 13 | 40 ab | 464 |
| 10 to 30 years (n = 17) | 3176 | 2.5 | 1.2 b | 21 | 46.4 | 1930 | 11 | 25 b | 297 |
| Over 30 years (n = 10) | 4662 | 5.6 | 3.2 a | 24 | 62.6 | 1896 | 14 | 58 a | 434 |
| Floor Type | |||||||||
| Carpet (n = 14) | 3805 | 4.3 | 1.6 | 24 | 38 b | 1674 | 10 | 42 ab | 241 b |
| Partial carpet (n = 12) | 3507 | 2.8 | 1.7 | 21 | 67 a | 4752 | 13 | 22 b | 390 ab |
| No carpet (n = 5) | 4102 | 3.5 | 3.3 | 25 | 65 a | 2131 | 17 | 64 a | 673 a |
| Pets | |||||||||
| No (n = 20) | 3195 b | 3.0 | 1.4 | 24 | 53 | 3507 | 12 | 34 | 333 |
| Yes (n = 11) | 4725 a | 4.6 | 2.9 | 23 | 54 | 1907 | 12 | 45 | 432 |
| Heating | |||||||||
| Electric (n = 16) | 4103 | 2.4 | 1.2 b | 22 | 48 | 1682 | 11 | 28 | 254 |
| Gas (n = 15) | 3348 | 4.8 | 2.7 a | 25 | 59 | 4280 | 14 | 49 | 491 |
| 1 Background | 30,000 | 5.9 | 0 | 30 | 15 | 15,000 | 10 | 30 | 15 |
| 2 ESV | N/A | 18 | 0.36 | 23 | 28 | N/A | 38 | 11 | 46 |
| Habitat | Na | Mg | K | Ca | Mn | TB | EB |
|---|---|---|---|---|---|---|---|
| Mean | 17,387 | 2900 | 2872 | 8030 | 48.2 | 47,714 | 11,833 |
| Home Type | |||||||
| Multi-family (n = 7) | 9868 | 2858 | 2040 | 4415 | 33 b | 21,100 | 4733 b |
| Single-family (n = 24) | 20,463 | 2917 | 3212 | 9508 | 54 a | 58,360 | 14,673 a |
| Home Age | |||||||
| Under 10 years (n = 4) | 9300 | 2632 | 4125 | 4282 | 66 | 94,750 a | 14,750 |
| 10 to 30 years (n = 17) | 24,178 | 3063 | 2823 | 9709 | 45 | 35,227 b | 10,909 |
| Over 30 years (n = 10) | 10,566 | 2773 | 2324 | 7217 | 45 | 39,250 ab | 11,583 |
| Floor Type | |||||||
| Carpet (n = 14) | 20,306 | 3249 | 2850 | 6104 | 41 | 60,618 | 13,636 |
| Partial carpet (n = 12) | 14,507 | 2859 | 3235 | 11,634 | 55 | 35,625 | 10,313 |
| No carpet (n = 5) | 16,128 | 2021 | 2062 | 4771 | 51 | 25,100 | 8000 |
| Pets | |||||||
| No (n = 20) | 19,714 | 3038 | 2644 | 10,520 | 50 | 36,192 | 10,362 |
| Yes (n = 11) | 13,157 | 2649 | 3286 | 3502 | 44 | 66,438 | 14,225 |
| Heating | |||||||
| Electric (n = 16) | 20,535 | 2875 | 2401 | 6096 | 41 | 53,810 | 9000 |
| Gas (n = 15) | 14,031 | 2927 | 3374 | 10,092 | 56 | 42,173 | 14,409 |
| 1 Background | N/A | N/A | N/A | N/A | 300 | N/A | N/A |
| 2 ESV | N/A | N/A | N/A | N/A | 220 | N/A | N/A |
| Al | As | Cd | Cr | Cu | Fe | Ni | Pb | Zn | Na | Mg | K | Ca | Mn | TB | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | 0.42 * | ||||||||||||||
| Cd | 0.36 * | 0.54 ** | |||||||||||||
| Cr | 0.65 ** | 0.57 ** | 0.38 * | ||||||||||||
| Cu | −0.16 | 0.33 | 0.28 | 0.08 | |||||||||||
| Fe | −0.20 | 0.36 * | 0.26 | 0.32 | 0.57 ** | ||||||||||
| Ni | 0.09 | 0.41 * | 0.15 | 0.27 | 0.61 ** | 0.62 ** | |||||||||
| Pb | 0.54 ** | 0.59 ** | 0.56 ** | 0.56 ** | 0.26 | 0.26 | 0.36 * | ||||||||
| Zn | −0.06 | 0.38 * | 0.37 * | 0.25 | 0.70 ** | 0.78 ** | 0.67 ** | 0.39 * | |||||||
| Na | −0.47 ** | −0.38 * | −0.27 | −0.59 ** | 0.07 | −0.23 | −0.05 | −0.50 ** | −0.30 | ||||||
| Mg | 0.06 | 0.23 | −0.24 | 0.19 | −0.23 | 0.18 | −0.07 | −0.04 | −0.11 | −0.31 | |||||
| K | −0.11 | 0.29 | 0.15 | 0.21 | 0.42 * | 0.48 ** | 0.38 * | 0.00 | 0.37 * | 0.31 | 0.04 | ||||
| Ca | −0.52 ** | −0.20 | −0.33 | −0.34 | −0.06 | 0.2 | −0.07 | −0.31 | −0.02 | −0.15 | 0.53 ** | −0.35 | |||
| Mn | −0.05 | 0.29 | 0.20 | 0.32 | 0.38 * | 0.85 ** | 0.57 ** | 0.34 | 0.63 ** | −0.38 * | 0.29 | 0.31 | 0.29 | ||
| TB | 0.19 | 0.45 * | −0.08 | 0.40 | 0.40 | 0.38 | 0.43 * | 0.42 | 0.347 | −0.18 | 0.10 | 0.46 * | −0.30 | 0.38 | |
| EB | 0.15 | 0.56 ** | 0.13 | 0.36 | 0.55 ** | 0.44 * | 0.54 ** | 0.515 * | 0.45 * | −0.06 | 0.03 | 0.56 ** | −0.25 | 0.42 | 0.88 ** |
| SEM Particle | C | O | Na | Al | Si | S | Cl | K | Ca |
|---|---|---|---|---|---|---|---|---|---|
| Fiber (1) | 49.9 | 34.1 | 3.4 | 0.6 | 2.3 | 1.0 | 2.4 | 1.1 | 1.8 |
| Skin (2) | 60.2 | 28.4 | 2.1 | 0.4 | 1.6 | 1.3 | 1.5 | 1.1 | 2.1 |
| Sand (3) | 69.9 | 10.3 | 1.5 | 1.6 | 10.2 | 1.3 | 1.5 | 0.9 | 2.0 |
| Ca Salt (4) | 35.5 | 40.2 | 0.7 | 0.6 | 1.6 | 9.1 | 0.9 | 0.5 | 10.8 |
| Na Salt (5) | 15.9 | 55.7 | 21.2 | 0.5 | 1.4 | 0.5 | 0.6 | 0.5 | 0.7 |
| County | Risk | As | Cd | Cr | Ni |
|---|---|---|---|---|---|
| Harris | LCR ingestion | 8.59 × 10−5 | 4.42 × 10−4 | 1.67 × 10−4 | 1.61 × 10−4 |
| LCR dermal | 2.01 × 10−6 | 1.03 × 10−5 | 3.91 × 10−6 | 3.77 × 10−6 | |
| LCR inhalation | 5.41 × 10−10 | 2.78 × 10−9 | 1.05 × 10−9 | 1.02 × 10−9 | |
| TLCR | 8.79 × 10−5 | 4.52 × 10−4 | 1.71 × 10−4 | 1.65 × 10−4 | |
| Brazoria | LCR ingestion | 3.58 × 10−5 | 2.03 × 10−4 | 1.33 × 10−4 | 1.30 × 10−4 |
| LCR dermal | 8.37 × 10−7 | 4.74 × 10−6 | 3.12 × 10−6 | 3.04 × 10−6 | |
| LCR inhalation | 2.26 × 10−10 | 1.28 × 10−9 | 8.41 × 10−10 | 8.21 × 10−10 | |
| TLCR | 3.67 × 10−5 | 2.08 × 10−4 | 1.37 × 10−4 | 1.33 × 10−4 | |
| Fort Bend | LCR ingestion | 2.56 × 10−5 | 3.13 × 10−4 | 1.12 × 10−4 | 9.30 × 10−5 |
| LCR dermal | 5.97 × 10−7 | 7.31 × 10−6 | 2.61 × 10−6 | 2.17 × 10−6 | |
| LCR inhalation | 1.61 × 10−10 | 1.97 × 10−9 | 7.05 × 10−10 | 5.86 × 10−10 | |
| TLCR | 2.62 × 10−5 | 3.20 × 10−4 | 1.15 × 10−4 | 9.52 × 10−5 | |
| Montgomery | LCR ingestion | 8.77 × 10−5 | 1.18 × 10−4 | 1.58 × 10−4 | 1.98 × 10−4 |
| LCR dermal | 2.05 × 10−6 | 2.76 × 10−6 | 3.68 × 10−6 | 4.62 × 10−6 | |
| LCR inhalation | 5.53 × 10−10 | 7.45 × 10−10 | 9.93 × 10−10 | 1.25 × 10−9 | |
| TLCR | 8.97 × 10−5 | 1.21 × 10−4 | 1.61 × 10−4 | 2.02 × 10−4 |
| Sample | Colony Color | Ribotyping/BIOLOG | Gram Stain | Catalase | Oxidase |
|---|---|---|---|---|---|
| S1 | White | Bacillus sp. MG2–11 | + | + | + |
| S4 | Yellow | Sporosarcina koreensis | + | + | − |
| Milk White | Bacillus megaterium strain EN2 | + | + | + | |
| S6 | Gray | Bacillus bingmayongensis strain SCSB-19 | + | + | + |
| Grey whitish | Bacillus muralis strain QT332 | + | + | + | |
| S7 | Orange | Arthrobacter sp. strain N5 | + | + | − |
| S8 | Oyster white | Brevibacterium sp. strain A9 | + | + | + |
| Creamy | Bacillus oceanisediminis strain NFS-CAP-3 | + | + | + | |
| S9 | White | Bacillus aryabhattai strain G3 | + | + | + |
| S10 | Yellow-orange | Bacillus firmus strain S2 | + | + | + |
| S11 | Yellow | Lysinibacillus sphaericus strain D9 | + | + | + |
| S12 | Milk White | Bacillus megaterium strain EN2 | + | + | + |
| S15 | Creamy-Yellowish | Bacillus koreensis strain EFBL-YM2 | + | + | + |
| Orange | Chryseobacterium spp. | − | + | + | |
| S24 | Gray | Aerococcus spp. strain DE018 | + | − | − |
| Creamy | Klebsiella aerogenes | − | − | − | |
| S25 | Milk White | Bacillus megaterium strain TSM3 | + | + | + |
| S28 | White | Bacillus megaterium strain TSM3 | + | + | + |
| S30 | Yellow | Pantoea sp. strain JZ108 | − | + | − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, F.R.; Ali, H.H.; Rosenzweig, J.A.; Vrinceanu, D.; Maruthi Sridhar, B.B. Characterization of Chemical and Bacterial Concentrations in Floor Dust Samples in Southeast Texas Households. Int. J. Environ. Res. Public Health 2021, 18, 12399. https://doi.org/10.3390/ijerph182312399
Davis FR, Ali HH, Rosenzweig JA, Vrinceanu D, Maruthi Sridhar BB. Characterization of Chemical and Bacterial Concentrations in Floor Dust Samples in Southeast Texas Households. International Journal of Environmental Research and Public Health. 2021; 18(23):12399. https://doi.org/10.3390/ijerph182312399
Chicago/Turabian StyleDavis, Felica R., Hanan H. Ali, Jason A. Rosenzweig, Daniel Vrinceanu, and Balaji Bhaskar Maruthi Sridhar. 2021. "Characterization of Chemical and Bacterial Concentrations in Floor Dust Samples in Southeast Texas Households" International Journal of Environmental Research and Public Health 18, no. 23: 12399. https://doi.org/10.3390/ijerph182312399
APA StyleDavis, F. R., Ali, H. H., Rosenzweig, J. A., Vrinceanu, D., & Maruthi Sridhar, B. B. (2021). Characterization of Chemical and Bacterial Concentrations in Floor Dust Samples in Southeast Texas Households. International Journal of Environmental Research and Public Health, 18(23), 12399. https://doi.org/10.3390/ijerph182312399






