Oral Mucosa Status and Saliva Parameters of Multimorbid Adult Patients Diagnosed with End-Stage Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.1.1. Examined Group
2.1.2. Control Group
2.2. Exclusion Criteria
- R—patients diagnosed with chronic kidney disease (42 people).
- + H—patients diagnosed with chronic kidney disease and hypertension (79 people).
- + D—patients diagnosed with chronic kidney disease and diabetes (16 people).
- + H + D—patients diagnosed with chronic kidney disease, hypertension and diabetes (43 people).
- I drink water while swallowing the food (never, sometimes, often).
- I feel dryness in oral cavity while eating meals (never, sometimes, often).
- Feeling of dryness wakes me up and I drink at night (never, sometimes, often).
- I feel discomfort while eating dry meals (never, sometimes, often).
- I suck candies to decrease the feeling of dryness (never, sometimes, often).
- I have difficulties in swallowing food (never, sometimes, often).
- My skin on the face is dry (never, sometimes, often).
- My eyes are dry (never, sometimes, often).
- My lips are dry (never, sometimes, often).
- I often fell thirst, what is problematic (never, sometimes, often).
- I feel thirst during the day (never, sometimes, often).
- I feel thirst during the night (never, sometimes, often).
- I take thirst into consideration when planning my free time (never, sometimes, often).
- I feel thirst before hemodialysis (never, sometimes, often).
- I feel thirst during hemodialysis (never, sometimes, often).
- I feel thirst after hemodialysis (never, sometimes, often).
- Stimulated saliva secretion—the aim of the examination was to calculate the salivary flow rate (SFR). Patients were asked to chew a paraffin cube (1g) for 60 s and to swallow the saliva. In the next stage of the examination, while chewing the same paraffin cube, participants deposited saliva into the sterile test tube for 5 min. Salivary flow rate was calculated in ml/min. Patients who underwent hemodialysis were examined before and after the procedure.
- Buffer capacity of saliva—with the usage of a pipette, one drop of gathered saliva was placed onto each of the three test pads. After 2 min, the color of each pad was compared with the table provided by the producer. Each color was given a particular number of points that were added together, and on the basis of the recorded results, buffer capacity was classified as very low (0–5), low (6–9) or normal (10–12).
- Saliva pH—the pH strip was placed in the sample of saliva obtained from the patient for 10 s. The obtained result was compared with the dental saliva pH indicator provided by the producer.
2.3. Statistical Analysis
3. Results
3.1. Feeling of Thirst
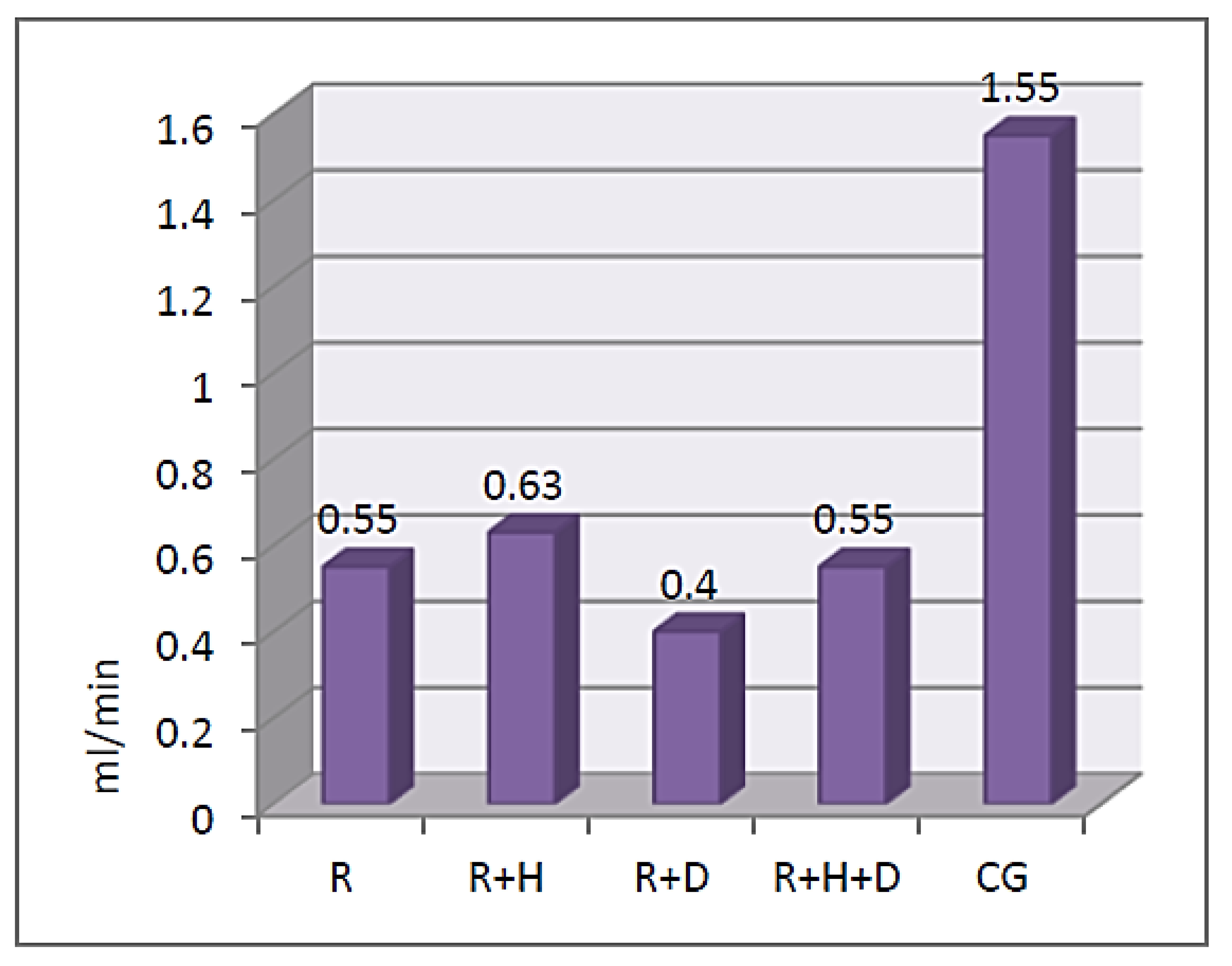
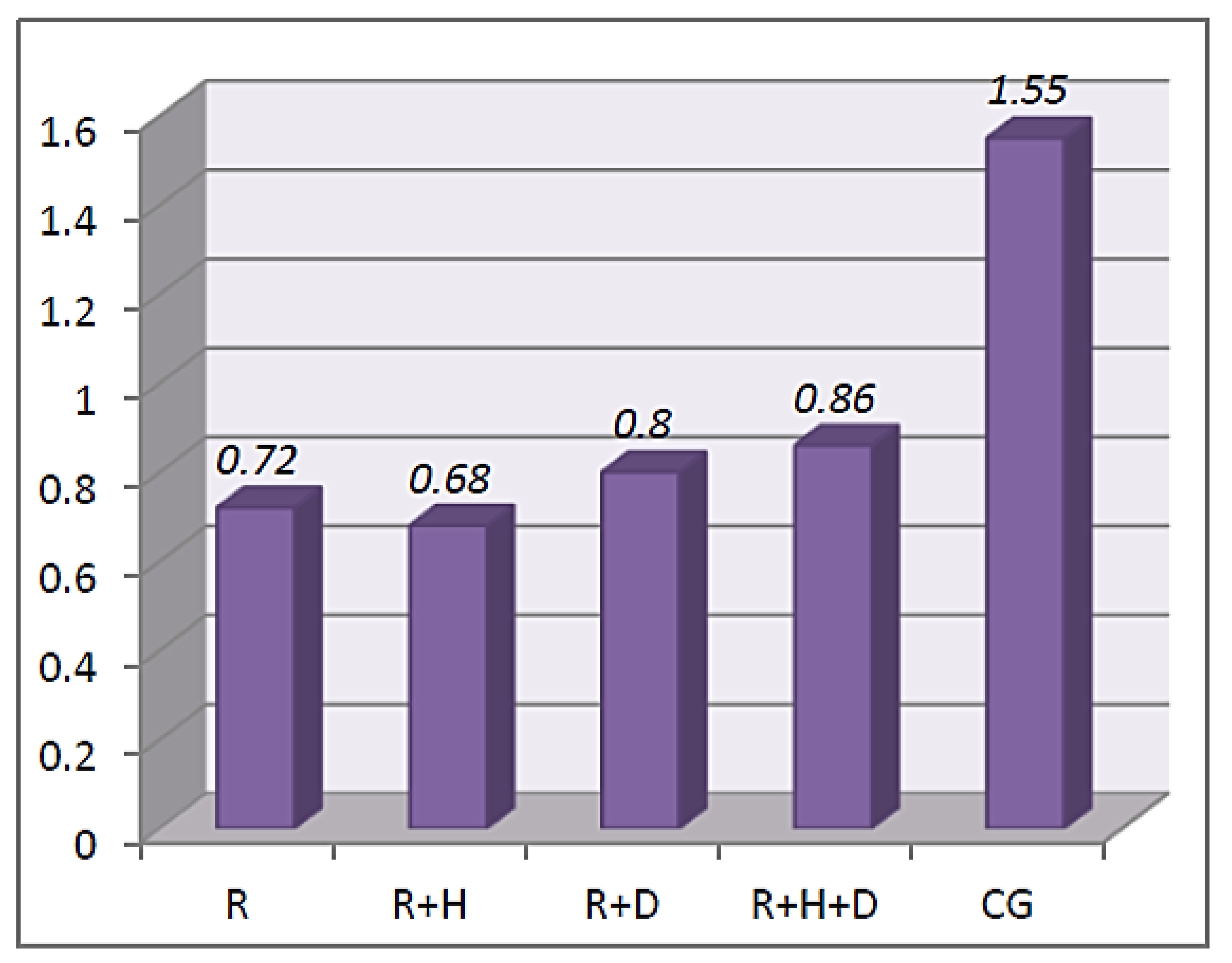
3.2. Saliva Parameters
Stimulated Saliva
3.3. Buffer Capacity
3.4. Saliva pH
3.5. Mucosa Status
4. Discussion
4.1. Secretion of Saliva
4.2. Buffer Capacity of Saliva
4.3. Dry Mouth Syndrome
4.4. Pathologies in Oral Mucosa
4.5. Halitosis
4.6. Taste Disorders
4.7. Limitations of the Work
4.8. Future Research Plan
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lebel, S.; Mutsaers, B.; Tomei, C.; Leclair, C.S.; Jones, G.; Petricone-Westwood, D.; Rutkowski, N.; Ta, V.; Trudel, G.; Laflamme, S.Z.; et al. Health anxiety and illness-related fears across diverse chronic illnesses: A systematic review on conceptualization, measurement, prevalence, course, and correlates. PLoS ONE 2020, 15, e0234124–e0234172. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Deng, Q.; Geng, Q.; Tang, Y.; Ma, J.; Ye, W.; Gan, Q.; Rehemayi, R.; Gao, X.; Zhu, C. Association of self-related health with chronic disease, mental health symptom and social relationship in older people. Sci. Rep. 2021, 11, 14653–14664. [Google Scholar] [CrossRef]
- Lyu, J.; Zhang, W.; Li, W.; Wang, S.; Zhang, J. Epidemic of chronic diseases and the related healthy lifestyle interventions in rural areas of Shandong Province, China. BMC Public Health 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Petersen, P.E.; Bourgeois, D.; Ogawa, H.; Estupinan-Day, S.; Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull. World Health Organ. 2005, 83, 661–669. [Google Scholar]
- Rasławska, J.; Dembowska, E. Przewlekła choroba nerek i dializoterapia a stan jamy ustnej. Den. Med. Probl. 2011, 48, 405–411. [Google Scholar]
- Proctor, R.; Kumar, N.; Stein, A.; Moles, D. Oral and dental aspects of chronic renal failure. J. Dent. Res. 2005, 84, 199–208. [Google Scholar] [CrossRef]
- Seraj, B.; Ahmadi, R.; Ramezani, N.; Mashayekhi, A.; Ahmadi, M. Oro-dental health status and salivary characteristics in children with chronic renal failure. J. Dent. (Teheran) 2011, 8, 146–151. [Google Scholar]
- Gupta, M.; Gupta, M. Oral conditions in renal disorders and treatment considerations-A review for pediatric dentist. Saudi Dent. J. 2015, 27, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Oyetola, E.O.; Owotade, F.J.; Agbelusi, G.A.; Fatusi, O.A.; Sanusi, A.A. Oral findings in chronic kidney disease: Implications for management in developing countries. BMC Oral Health 2015, 15, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, K.M.; Mahmood, M.A.; Abdulraheam, R.H.; Ahmed, S.M. Oral and dental findings in patients with end stage renal disease undergoing maintenance hemodialysis in Sulaimani City. J. Interdiscipl. Med. Dent. Sci. 2015, 3, 182–186. [Google Scholar]
- Patil, S.; Khandelwal, S.; Doni, B.; Rahman, F.; Kaswan, S. Oral manifestations in chronic renal failure patients attending two hospitals in north Karnataka, India. OHDM 2012, 11, 100–106. [Google Scholar]
- Yadav, A.; Deepak, U.; Misra, N.; Shiva Kumar, G.C.; Kaur, A. Oral manifestations in renal failure patients undergoing Dialysis. Int. J. Med. Sci. Public Health 2015, 4, 1015–1018. [Google Scholar] [CrossRef] [Green Version]
- Sokołowska-Trelka, A.; Grzebieluch, W.; Dubiński, B. Problemy stomatologiczne u chorych na schyłkową niewydolność nerek. Dent. Med. Probl. 2005, 42, 351–356. [Google Scholar]
- Wilczyńska-Borawska, M.; Małyszko, J.; Stokowska, W. Stan zdrowia zębów i przyzębia pacjentów z przewlekłą niewydolnością nerek i dializowanych a niedożywienie- co jest skutkiem a co przyczyną, na podstawie piśmiennictwa i badań własnych. Nefrol. Dial. Pol. 2009, 13, 259–261. [Google Scholar]
- Summers, A.A.; Tilakaratne, W.M.; Fortune, F.; Ashman, N. Schorzenia nerek a jama ustna. Nefrol. Nadciś Tętn. 2008, 2, 31–44. [Google Scholar]
- Swapna, L.A.; Reddy, R.S.; Ramesh, T.; Reddy, R.L.; Vijayalaxmi, N.; Karmar, P.; Pradeep, K. Oral health status in haemodialysis patients. J. Clin. Diagn. Res. 2013, 7, 2047–2050. [Google Scholar] [CrossRef]
- Łabij-Reduta, B.; Żółtko, J.; Borawski, J.; Naumnik, B. Suchość jamy ustnej pacjentów przewlekle hemodializowanych-znaczenie, diagnostyka i leczenie. Nefrol. Dial. Pol. 2015, 19, 50–54. [Google Scholar]
- Akar, H.; Akar, G.C.; Carrero, J.J.; Steinvinkel, P.; Lindholm, B. Systemic consequences of poor oral health in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Wilczyńska-Borawska, M.; Baginska, J.; Borawski, J. Is xerostomia a risk factor for cardiovascular morbidity and mortality in mainatnce hemodialysis patients? Med. Hypotheses 2012, 79, 544–548. [Google Scholar] [CrossRef]
- Bossola, M.; Tazza, L. Xerostomia in patients on chronic hemodialysis. Nat. Rev. Nephrol. 2012, 8, 176–182. [Google Scholar] [CrossRef]
- Kowalski, J. Lekopochodny przerost dziąseł-przegląd literatury. Nowa Stomatol. 2010, 4, 180–182. [Google Scholar]
- Livada, R.; Shiloah, J. Calcium channel blocker-induced gingival enlargement. J. Hum. Hypertens. 2014, 28, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Saha, A.; Das, A.K.; Nair, V.; Biswas, D. Anit-hypertensive drug induced gingival enlargement—A case report. JODAGH 2017, 10, 7–11. [Google Scholar]
- Tonsekar, P.; Tonsekar, V. Calcium-Channel-Blocker-Influenced Gingival Enlargement: A Conundrum Demystified. Oral 2021, 1, 236–249. [Google Scholar] [CrossRef]
- Miranda, J.; Brunet, L.; Roset, P.; Berini, L.; Farré, M.; Mendieta, C. Prevalence and risk of gingival overgrowth in patients treated with diltiazem or verapamil. J. Clin. Periodontol. 2005, 32, 294–298. [Google Scholar] [CrossRef]
- Lauritano, D.; Lucchese, A.; Di Stasio, D.; Della Vella, F.; Cura, F.; Palmieri, A.; Carinci, F. Molecular Aspects of Drug-Induced Gingival Overgrowth: An In Vitro Study on Amlodipine and Gingival Fibroblasts. Int. J. Mol. Sci. 2019, 20, 2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llorca, S.C.; Serra, M.P.M.; Silvestre, F.J. Drug-induced burning syndrome: A new etiological diagnosis. Med. Oral Patol. Oral Cir. Bucal. 2008, 13, e167–e170. [Google Scholar]
- Sękiewicz, A.; Pytiakowska, A.; Borakowska-Siennicka, M. Badanie zaburzeń wydzielania śliny u pacjentów leczonych z powodu schorzeń kardiologicznych. Nowa Stomatol. 2013, 1, 40–45. [Google Scholar]
- Knaś, M.; Zarzycki, W.; Karaszewski, J. Ocena ultrasonograficzna ślinianek podżuchwowych u chorych z cukrzycą typu I. Stomatol. Współczesna 2013, 20, 24–29. [Google Scholar]
- Zalewska, A.; Knaś, M.; Gumiężny, G.; Niczyporuk, M.; Waszkiel, D.; Przystupa, A.W.; Zarzycki, W. Salivary exoglycosidases in gestational diabetes. Postępy Hig. Med. Dosw. 2013, 67, 315–320. [Google Scholar] [CrossRef]
- Melkowska, M.; Banach, J. Patomechanizmy powstania zmian patologicznych w przyzębiu w przebiegu cukrzycy-przegląd piśmiennictwa. Dent. Med. Prob. 2009, 46, 10–109. [Google Scholar]
- Sampaio, N.; Mello, S.; Alves, C. Dental caries-associated risk factors and type 1 diabetes mellitus. Pediatric Endocrinol. Diabetes Metab. 2011, 17, 152–157. [Google Scholar]
- Preferansow, E.; Gołębiewska, M.; Górska, M. Ocena stanu uzębienia u pacjentów z niewyrównaną cukrzycą typu 1 i typu 2. Protet. Stomatol. 2009, 4, 242–248. [Google Scholar]
- Koziołek, M.; Kiedrowicz, M.; Kiedrowicz, B.; Dembowska, E.; Syrenicz, A. Objawy chorób endokrynologicznych u pacjentów leczonych stomatologicznie. Dent. Med. Probl. 2011, 48, 229–235. [Google Scholar]
- Chavez, E.M.; Borell, L.N.; Taylor, G.W.; Ship, J.A. A longitudinal analysis of salivary flow in control subjects and older adults with type 2 diabetes. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 91, 166–173. [Google Scholar] [CrossRef]
- Paprotna-Cnota, A.; Postek-Stefańska, L. Stan zdrowia jamy ustnej chorych na cukrzycę. Mag. Stomatol. 2005, 15, 29–31. [Google Scholar]
- Burzyńska, B.; Mierzwińska-Nastalska, E. Stan jamy ustnej pacjentów chorych na cukrzycę. Dent. Med. Probl. 2011, 48, 412–416. [Google Scholar]
- Jaruchowska, M.; Węgrzyn, R.; Kuczyńska, M.; Jasińska, A.; Androsz-Kowalska, O.; Krajewski, J.; Jedynasty, K.; Franek, E. Ocena występowania liszaja płaskiego (OLP) u pacjentów z cukrzycą. Nowa Stomatol. 2008, 2, 65–69. [Google Scholar]
- Dorocka-Bobkowska, B.; Zozulińska-Ziółkiewska, B.; Wierusz- Wysocka, B.; Hędzelek, W.; Szumala- Kąkol, A.; Budtz-Jörgensen, E. Candida associated denture stomatitis in type 2 diabetes mellitus. Diabetes Res. Clin. Pr. 2010, 7, 81–86. [Google Scholar] [CrossRef]
- Trzcionka, A.; Twardawa, H.; Mocny-Pachońska, K.; Tanasiewicz, M. Oral cavity status of long-term hemodialized patients vs. their socio-economic status. Med. Pr. 2020, 71, 279–288. [Google Scholar] [CrossRef]
- Trzcionka, A.; Twardawa, H.; Mocny-Pachońska, K.; Tanasiewicz, M. Periodontal treatment needs of hemodialized patients. Healthcare 2021, 9, 139. [Google Scholar] [CrossRef]
- Teratani, G.; Awano, S.; Soh, I.; Yoshida, A.; Kinoshita, N.; Hamasaki, T.; Takata, Y.; Sonoki, K.; Nakamura, H.; Ansai, T. Oral health in patients on haemodialysis for diabetic nephropathy in chronic glomerulonephris. Clin. Oral Invest. 2013, 17, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Vesterinen, M.; Ruokonen, H.; Furuholm, J.; Honkanen, E.; Meurman, J.H. Oral health in predialysis patients with emphasis on diabetic nephropathy. Clin. Oral Invest. 2011, 15, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Reddy, S.S.; Umesh, L.; Devi, B.K.Y.; Santana, N.; Rakesh, N. Oral and salivary changes among renal patients undergoing hemodialysis: A cross-sectional study. Indian J. Nephrol. 2013, 23, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Wilczyńska-Borawska, M.; Borawski, J.; Myśliwiec, M.; Stokowska, W. Wpływ zabiegu hemodializy na pH śliny pacjentów z niewydolnością nerek. Czas. Stomatol. 2005, 7, 480–485. [Google Scholar]
- Souza, C.M.; Braosi, A.P.R.; Luszczyszyn, S.M.; Casagrande, R.W.; Pecpits-Filho, R.; Riela, M.C.; Ignácio, S.A.; Trevilatto, P.C. Oral health in Brazilian patients with chronic renal disease. Rev. Med. Chil. 2008, 136, 741–746. [Google Scholar] [CrossRef]







| Question | Answer | Number of Answers (Percentage Distribution) | p | ||||
|---|---|---|---|---|---|---|---|
| CG | R | R + H | R + D | R + H + D | |||
| I drink water while swallowing the food. | never | 22 (46%) | 13 (31%) | 24 (30%) | 5 (31%) | 11 (26%) | 0.007 |
| sometimes | 20 (42%) | 18 (43%) | 25 (32%) | 7 (44%) | 9 (21%) | ||
| often | 6 (13%) | 11 (26%) | 30 (38%) | 4 (25%) | 23 (53%) | ||
| I feel dryness in oral cavity while eating meals. | never | 26 (54%) | 24 (57%) | 36 (46%) | 6 (38%) | 25 (58%) | 0.101 |
| sometimes | 17 (35%) | 11 (26%) | 17 (22%) | 6 (38%) | 8 (19%) | ||
| often | 5 (10%) | 7 (17%) | 26 (33%) | 4 (25%) | 10 (23%) | ||
| Feeling of dryness wakes me up and I drink at night. | never | 23 (48%) | 20 (48%) | 23 (29%) | 9 (56%) | 15 (35%) | 0.176 |
| sometimes | 17 (35%) | 14 (33%) | 28 (35%) | 3 (19%) | 15 (35%) | ||
| often | 8 (17%) | 8 (19%) | 28 (35%) | 4 (25%) | 13 (30%) | ||
| I feel discomfort while eating dry meals. | never | 27 (56%) | 24 (57%) | 41 (52%) | 10 (63%) | 19 (44%) | 0.242 |
| sometimes | 12 (25%) | 12 (29%) | 15 (19%) | 1 (6%) | 8 (19%) | ||
| often | 9 (19%) | 6 (14%) | 23 (29%) | 5 (31%) | 16 (37%) | ||
| I suck candies to decrease the feeling of dryness. | never | 39 (81%) | 24 (57%) | 34 (43%) | 6 (38%) | 26 (60%) | 0.001 |
| sometimes | 6 (13%) | 11 (26%) | 17 (22%) | 7 (44%) | 9 (21%) | ||
| often | 3 (6%) | 7 (17%) | 28 (35%) | 3 (19%) | 8 (19%) | ||
| I have difficulties in swallowing food. | never | 40 (83%) | 31 (74%) | 56 (71%) | 11 (69%) | 32 (74%) | 0.120 |
| sometimes | 8 (17%) | 9 (21%) | 10 (13%) | 2 (13%) | 6 (14%) | ||
| often | 0 (0%) | 2 (5%) | 13 (16%) | 3 (19%) | 5 (12%) | ||
| My skin on the face is dry. | never | 21 (44%) | 20 (48%) | 23 (29%) | 5 (31%) | 18 (42%) | 0.268 |
| sometimes | 19 (40%) | 12 (29%) | 26 (33%) | 5 (31%) | 13 (30%) | ||
| often | 8 (17%) | 10 (24%) | 30 (38%) | 6 (38%) | 12 (28%) | ||
| My eyes are dry. | never | 34 (71%) | 27 (64%) | 39 (49%) | 5 (31%) | 25 (58%) | 0.006 |
| sometimes | 8 (17%) | 11 (26%) | 14 (18%) | 8 (50%) | 9 (21%) | ||
| often | 6 (13%) | 4 (10%) | 26 (33%) | 3 (19%) | 9 (21%) | ||
| My lips are dry. | never | 27 (56%) | 24 (57%) | 29 (37%) | 6 (38%) | 20 (47%) | 0.084 |
| sometimes | 14 (29%) | 14 (33%) | 24 (30%) | 6 (38%) | 10 (23%) | ||
| often | 7 (15%) | 4 (10%) | 26 (33%) | 4 (25%) | 13 (30%) | ||
| Question | Answer | Number of Answers (Percentage Distribution) | p | ||||
|---|---|---|---|---|---|---|---|
| CG | R | R + H | R + D | R + H + D | |||
| I often feel thirst, what is problematic. | never | 32 (67%) | 13 (31%) | 27 (34%) | 8 (50%) | 14 (33%) | 0.000 |
| sometimes | 14 (29%) | 17 (40%) | 17 (22%) | 5 (31%) | 11 (26%) | ||
| often | 2 (4%) | 12 (29%) | 35 (44%) | 3 (19%) | 18 (42%) | ||
| I feel thirst during the day. | never | 16 (33%) | 10 (24%) | 11 (14%) | 4 (25%) | 6 (14%) | 0.014 |
| sometimes | 23 (48%) | 21 (50%) | 28 (35%) | 6 (38%) | 17 (40%) | ||
| often | 9 (19%) | 11 (26%) | 40 (51%) | 6 (38%) | 20 (47%) | ||
| I feel thirst during the night. | never | 31 (65%) | 21 (50%) | 29 (37%) | 7 (44%) | 18 (42%) | 0.031 |
| sometimes | 12 (25%) | 15 (36%) | 25 (32%) | 6 (38%) | 10 (23%) | ||
| often | 5 (10%) | 6 (14%) | 25 (32%) | 3 (19%) | 15 (35%) | ||
| I take thirst into consideration when planning my free time. | never | 43 (90%) | 29 (69%) | 39 (49%) | 9 (56%) | 26 (60%) | 0.000 |
| sometimes | 4 (8%) | 11 (26%) | 20 (25%) | 5 (31%) | 7 (16%) | ||
| often | 1 (2%) | 2 (5%) | 20 (25%) | 2 (13%) | 10 (23%) | ||
| I feel thirst before hemodialysis. | never | - | 25 (60%) | 39 (49%) | 7 (44%) | 24 (56%) | 0.431 |
| sometimes | - | 10 (24%) | 13 (16%) | 4 (25%) | 10 (23%) | ||
| often | - | 7 (17%) | 27 (34%) | 5 (31%) | 9 (21%) | ||
| I feel thirst during hemodialysis. | never | - | 26 (62%) | 41 (52%) | 8 (50%) | 20 (47%) | 0.762 |
| sometimes | - | 9 (21%) | 22 (28%) | 3 (19%) | 13 (30%) | ||
| often | - | 7 (17%) | 16 (20%) | 5 (31%) | 10 (23%) | ||
| I feel thirst after hemodialysis. | never | - | 20 (48%) | 24 (30%) | 7 (44%) | 17 (40%) | 0.451 |
| sometimes | - | 10 (24%) | 21 (27%) | 4 (25%) | 7 (16%) | ||
| often | - | 12 (29%) | 34 (43%) | 5 (31%) | 19 (44%) | ||
| Kruskal–Wallis Test Results | |||
|---|---|---|---|
| X2 | p | ε2 | |
| [H+] | 0.34 | 0.987 | 0.00 |
| Pathology | Chi-Squared Test Results | |||
|---|---|---|---|---|
| X2 | df | p | η | |
| Pain | 2.39 | 4 | 0.664 | 0.10 |
| Burning mouth syndrome | 3.44 | 4 | 0.487 | 0.12 |
| Dry mouth syndrome | 17.06 | 4 | 0.002 | 0.27 |
| Taste disorders | 4.60 | 4 | 0.331 | 0.14 |
| Ulcerations | 4.35 | 4 | 0.360 | 0.14 |
| White patches | 4.25 | 4 | 0.373 | 0.14 |
| Red patches | 1.47 | 4 | 0.831 | 0.08 |
| Malformations | 3.47 | 4 | 0.482 | 0.12 |
| Ecchymosis | 14.72 | 4 | 0.005 | 0.25 |
| Candidiasis | 15.77 | 4 | 0.003 | 0.26 |
| Herpes simplex | 7.49 | 4 | 0.112 | 0.18 |
| Geographic tongue | 1.56 | 4 | 0.816 | 0.08 |
| Fissured tongue | 24.62 | 4 | 0.000 | 0.33 |
| Halitosis | 6.00 | 4 | 0.199 | 0.16 |
| Signs of previous operations | 1.89 | 4 | 0.755 | 0.09 |
| Trauma-related lesions | 11.56 | 4 | 0.021 | 0.23 |
| Overgrowth gingiva | 2.18 | 4 | 0.703 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trzcionka, A.; Twardawa, H.; Mocny-Pachońska, K.; Korkosz, R.; Tanasiewicz, M. Oral Mucosa Status and Saliva Parameters of Multimorbid Adult Patients Diagnosed with End-Stage Chronic Kidney Disease. Int. J. Environ. Res. Public Health 2021, 18, 12515. https://doi.org/10.3390/ijerph182312515
Trzcionka A, Twardawa H, Mocny-Pachońska K, Korkosz R, Tanasiewicz M. Oral Mucosa Status and Saliva Parameters of Multimorbid Adult Patients Diagnosed with End-Stage Chronic Kidney Disease. International Journal of Environmental Research and Public Health. 2021; 18(23):12515. https://doi.org/10.3390/ijerph182312515
Chicago/Turabian StyleTrzcionka, Agata, Henryk Twardawa, Katarzyna Mocny-Pachońska, Rafał Korkosz, and Marta Tanasiewicz. 2021. "Oral Mucosa Status and Saliva Parameters of Multimorbid Adult Patients Diagnosed with End-Stage Chronic Kidney Disease" International Journal of Environmental Research and Public Health 18, no. 23: 12515. https://doi.org/10.3390/ijerph182312515






