Reaction Times among Batik Workers: The Influence of Gender and Occupational Lead Exposure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Outcome Measures
2.4. Sample Size Considerations
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Educational Scientific and Cultural Organization. Subsidiary body for the examination of nominations to the representative list of the intangible cultural heritage of humanity. In Convention for the Safeguarding of the Intangible Cultural Heritage; United Nations Educational Scientific and Cultural Organization: Abu Dhabi, United Arab Emirates, 2009; p. 8. [Google Scholar]
- Vincent, A. Batik industry of indonesia: The rise, fall and prospects. Stud. Bus. Econ. 2010, 5, 156–170. [Google Scholar]
- Kementerian Perindustrian Republik Indonesia. Dilanda Pandemi, Ekspor Batik Indonesia Mampu Tembus USD 21,5 Juta. 2020. Available online: https://www.kemenperin.go.id/artikel/22039/Dilanda-Pandemi,-Ekspor-Batik-Indonesia-Mampu-Tembus-USD-21,5-Juta (accessed on 5 February 2021).
- Junaidi, M.S.; Fatoni, R.; Fatimah, S. The Analysis of Occupational Safety and Health of the Batik Industry. Adv. Sustain. Sci. Eng. Technol. 2020, 2, 2. [Google Scholar] [CrossRef]
- Nankongnab, N.; Silpasuwan, P.; Markkanen, P.; Kongtip, P.; Woskie, S. Occupational Safety, Health, and Well-being Among Home-based Workers in the Informal Economy of Thailand. New Solut. A J. Environ. Occup. Health Policy 2015, 25, 212–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soebaryo, R.W. Batik Manufacturing Workers. Kanerva’s Occup. Dermatol. 2012, 1, 1289–1295. [Google Scholar] [CrossRef]
- Febriana, S.A.; Ogiwati, K.; Tanziha, I.; Roto, R.; Sarian, F.D.; Prakoeswa, C.R.S.; Thursina, C.; Suhartini, S.; Vicaria, L.D.; Priyambodo, D.Y.; et al. Initiating “Healthy Batik Village”/“Desa Batik Sehat” to empower batik workers through collaborative health, environmental and social interventions. Res. Sq. 2021, 1–29, (preprint). [Google Scholar] [CrossRef]
- George Foundation. Implementing a National Program in Developing Countries. In Proceedings of the International Conference on Lead Poisoning, Prevention and Treatment, Bangalore, India, 8–10 February 1999. [Google Scholar]
- Obeng-Gyasi, E. Lead Exposure and Oxidative Stress—A Life Course Approach in U.S. Adults. Toxics 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagliuca, A.; Mufti, G.J.; Baldwin, D.; Lestas, A.N.; Wallis, R.M.; Bellingham, A.J. Lead poisoning: Clinical, biochemical, and haematological aspects of a recent outbreak. J. Clin. Pathol. 1990, 43, 277–281. [Google Scholar] [CrossRef]
- Can, S.; Bağci, C.; Ozaslan, M.; Bozkurt, A.I.; Cengiz, B.; Cakmak, E.A.; Kocabas, R.; Karadağ, E.; Tarakçioğlu, M. Occupational lead exposure effect on liver functions and biochemical parameters. Acta Physiol. Hung. 2008, 95, 395–403. [Google Scholar] [CrossRef]
- Harari, F.; Sallsten, G.; Christensson, A.; Petkovic, M.; Hedblad, B.; Forsgard, N.; Melander, O.; Nilsson, P.M.; Borné, Y.; Engström, G.; et al. Blood Lead Levels and Decreased Kidney Function in a Population-Based Cohort. Am. J. Kidney Dis. 2018, 72, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Obeng-Gyasi, E.; Ferguson, A.; Stamatakis, K.; Province, M. Combined Effect of Lead Exposure and Allostatic Load on Cardiovascular Disease Mortality—A Preliminary Study. Int. J. Environ. Res. Public Health 2021, 18, 6879. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Rauch, S.; Auinger, P.; Allen, R.W.; Hornung, R.W. Low-level lead exposure and mortality in US adults: A population-based cohort study. Lancet Public Health 2018, 3, e177–e184. [Google Scholar] [CrossRef]
- Goodman, M.; Laverda, N.; Clarke, C.; Foster, E.D.; Iannuzzi, J.; Mandel, J. Neurobehavioural testing in workers occupationally exposed to lead: Systematic review and meta-analysis of publications. Occup. Environ. Med. 2002, 59, 217–223. [Google Scholar] [CrossRef]
- Meyer-Baron, M.; Seeber, A. A meta-analysis for neurobehavioural results due to occupational lead exposure with blood lead concentrations. Arch. Toxicol. 2002, 76, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-L.; Thijs, L.; Saenen, N.; Melgarejo, J.D.; Wei, D.-M.; Yang, W.-Y.; Yu, C.-G.; Roels, H.A.; Nawrot, T.S.; Maestre, G.E.; et al. Two-year neurocognitive responses to first occupational lead exposure. Scand. J. Work. Environ. Health 2020, 47, 233–243. [Google Scholar] [CrossRef]
- Fenga, C.; Gangemi, S.; Alibrandi, A.; Costa, C.; Micali, E. Relationship between lead exposure and mild cognitive impairment. J. Prev. Med. Hyg. 2016, 57, E205–E210. [Google Scholar] [PubMed]
- Burghart, M.; Craig, J.; Radel, J.; Huisinga, J. Reliability and validity of a motion-based reaction time assessment using a mobile device. Appl. Neuropsychol. Adult 2019, 26, 558–563. [Google Scholar] [CrossRef]
- Laux, R.C.; Corazza, S.T. Improvement of reaction time after a workplace physical activity intervention. Rev. Bras. De Med. Do Esporte 2019, 25, 515–519. [Google Scholar] [CrossRef]
- Richardson, J.K.; Eckner, J.T.; Kim, H.; Ashton-Miller, J.A. A clinical method of evaluating simple reaction time and reaction accuracy is sensitive to a single dose of lorazepam. J. Psychopharmacol. 2020, 34, 920–925. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef] [Green Version]
- Gunn, C.; Mackus, M.; Griffin, C.; Munafò, M.R.; Adams, S. A systematic review of the next-day effects of heavy alcohol consumption on cognitive performance. Addiction 2018, 113, 2182–2193. [Google Scholar] [CrossRef]
- Edwards, L.; Ring, C.; McIntyre, D.; Carroll, D.; Martin, U. Psychomotor speed in hypertension: Effects of reaction time components, stimulus modality, and phase of the cardiac cycle. Psychophysiology 2007, 44, 459–468. [Google Scholar] [CrossRef]
- Forte, G.; De Pascalis, V.; Favieri, F.; Casagrande, M. Effects of Blood Pressure on Cognitive Performance: A Systematic Review. J. Clin. Med. 2019, 9, 34. [Google Scholar] [CrossRef] [Green Version]
- Muhil, M. Study of Auditory, Visual Reaction Time and Glycemic Control (HBA 1 C) in Chronic Type I I Diabetes Mellitus. J. Clin. Diagn. Res. 2014, 8, BC11–BC13. [Google Scholar] [CrossRef]
- Lauridsen, M.M.; Mikkelsen, S.; Svensson, T.; Holm, J.; Klüver, C.; Gram, J.; Vilstrup, H.; De Muckadell, O.B.S. The continuous reaction time test for minimal hepatic encephalopathy validated by a randomized controlled multi-modal intervention—A pilot study. PLoS ONE 2017, 12, e0185412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brodski, J.; Rossell, S.L.; Castle, D.J.; Tan, E.J. A Systematic Review of Cognitive Impairments Associated with Kidney Failure in Adults Before Natural Age-Related Changes. J. Int. Neuropsychol. Soc. 2018, 25, 101–114. [Google Scholar] [CrossRef]
- Leto, L.; Feola, M. Cognitive impairment in heart failure patients. J. Geriatr. Cardiol. 2014, 11, 316–328. [Google Scholar] [CrossRef]
- Kahlon, N.; Gandhi, A.; Mondal, S.; Narayan, S. Effect of iron deficiency anemia on audiovisual reaction time in adolescent girls. Indian J. Physiol. Pharmacol. 2012, 55, 53–59. [Google Scholar]
- Hultsch, D.F.; MacDonald, S.W.S.; Dixon, R.A. Variability in Reaction Time Performance of Younger and Older Adults. J. Gerontol. Ser. B 2002, 57, P101–P115. [Google Scholar] [CrossRef] [Green Version]
- Hastuti, P.; Sunarti, S.; Prasetyastuti, P.; Ngadikun, N.; Tasmini, T.; Rubi, D.S.; Sutarni, S.; Harahap, I.K.; Dananjoyo, K.; Suhartini, S.; et al. Hubungan timbal dan krom pada pemakaian pewarna batik dengan kadar hemoglobin dan jumlah sel darah pada pengrajin batik Kecamatan Lendah Kulon Progo. J. Community Empower. Health 2018, 1, 28–35. [Google Scholar] [CrossRef]
- Allen, J. The Online Reaction Time Test. 2002. Available online: https://faculty.washington.edu/chudler/java/redgreen.html (accessed on 5 February 2021).
- Kauranen, K.; Siira, P.; Vanharanta, H. Delayed-onset muscle soreness and motor performance of the upper extremity. Graefe’s Arch. Clin. Exp. Ophthalmol. 2001, 84, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Nene, A.S.; Pazare, P.A.; Sharma, K.D. A study of relation between body mass index and simple reaction time in healthy young females. Indian J. Physiol. Pharmacol. 2012, 55, 288–291. [Google Scholar]
- Sudheer, C.; Jagadeesan, S.; Kammar, F.K. Impact of BMI on Visual Reaction Time in Individuals with BMI in Normal Range. Int. J. Physiol. 2017, 5, 10. [Google Scholar] [CrossRef]
- Taimela, S.; Kujala, U.M. Reaction Times with Reference to Musculoskeletal Complaints in Adolescence. Percept. Mot. Ski. 1992, 75, 1075–1082. [Google Scholar] [CrossRef]
- Venna, S.; Hurri, H.; Alaranta, H. Correlation between neurological leg deficits and reaction time of upper limbs among low-back pain patients. Scand. J. Rehabil. Med. 1994, 26, 87–90. [Google Scholar]
- Yassin, M.; Talebian, S.; Ebrahimi-Takamjani, I.; Maroufi, N.; Ahmadi, A.; Sarrafzadeh, J.; Emrani, A. The effects of arm movement on reaction time in patients with latent and active upper trapezius myofascial trigger point. Med. J. Islam. Repub. Iran 2015, 29, 295. [Google Scholar]
- Wilson, J.; Corlett, E. Evaluation of human work: A practical ergonomics methodology. Appl. Ergon. 1991, 22, 58. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Bendel, R.B.; Afifi, A.A. Comparison of Stopping Rules in Forward “Stepwise” Regression. J. Am. Stat. Assoc. 1977, 72, 46. [Google Scholar] [CrossRef]
- Mickey, R.M.; Greenland, S. The impact of confounder selection criteria on effect estimation. Am. J. Epidemiol. 1989, 129, 125–137. [Google Scholar] [CrossRef]
- Blanca, M.J.; Alarcón, R.; Arnau, J. Non-normal data: Is ANOVA still a valid option? Psicothema 2017, 29, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, J. Digit Symbol Substitution Test. J. Clin. Psychopharmacol. 2018, 38, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic Effects and Biomarkers of Lead Exposure: A Review. Rev. Environ. Health 2009, 24, 15–46. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.S.; Stewart, W.F.; Bolla, K.I.; Simon, D.; Bandeen-Roche, K.; Gordon, B.; Links, J.M.; Todd, A.C. Past adult lead exposure is associated with longitudinal decline in cognitive function. Neurology 2000, 55, 1144–1150. [Google Scholar] [CrossRef] [Green Version]
- Winneke, G.; Lilienthal, H.; Krämer, U. The Neurobehavioural Toxicology and Teratology of Lead. Arch. Toxicol. 1996, 18, 57–70. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Naghizadeh, B.; López-Larrubia, P.; Cauli, O. Behavioral deficits induced by lead exposure are accompanied by serotonergic and cholinergic alterations in the prefrontal cortex. Neurochem. Int. 2013, 62, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, G.W. Evidence that lead acts as a calcium substitute in second messenger metabolism. Neurotoxicology 1993, 14, 97–101. [Google Scholar]
- Simons, T.J.B. Cellular interactions between lead and calcium. Br. Med. Bull. 1986, 42, 431–434. [Google Scholar] [CrossRef]
- Brookes, P.; Yoon, Y.; Robotham, J.L.; Anders, M.W.; Sheu, S.-S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Physiol. 2004, 287, C817–C833. [Google Scholar] [CrossRef]
- Latash, M.L. Neurophysiological Basis of Movement, 3rd ed.; Human Kinetics: Champaign, IL, USA, 2008. [Google Scholar]
- Pocock, S.J.; Smith, M.; Baghurst, P. Environmental lead and children’s intelligence: A systematic review of the epidemiological evidence. BMJ 1994, 309, 1189–1197. [Google Scholar] [CrossRef] [Green Version]
- Silbergeld, E.K. Mechanisms of lead neurotoxicity, or looking beyond the lamppost. FASEB J. 1992, 6, 3201–3206. [Google Scholar] [CrossRef]
- Bressler, J.P.; Goldstein, G.W. Mechanisms of lead neurotoxicity. Biochem. Pharmacol. 1991, 41, 479–484. [Google Scholar] [CrossRef]
- Cookman, G.R.; King, W.; Regan, C.M. Chronic Low-Level Lead Exposure Impairs Embryonic to Adult Conversion of the Neural Cell Adhesion Molecule. J. Neurochem. 1987, 49, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Singh, V.; Sobolewski, M.; Cory-Slechta, D.A.; Schneider, J.S. Sex-Dependent Effects of Developmental Lead Exposure on the Brain. Front. Genet. 2018, 9, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonberg, N.; Pesch, B.; Ulrich, N.; Moebus, S.; Eisele, L.; Marr, A.; Arendt, M.; Jöckel, K.-H.; Brüning, T.; Weiss, T. The distribution of blood concentrations of lead (Pb), cadmium (Cd), chromium (Cr) and manganese (Mn) in residents of the German Ruhr area and its potential association with occupational exposure in metal industry and/or other risk factors. Int. J. Hyg. Environ. Health 2017, 220, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Attridge, N.; Keogh, E.; Eccleston, C. The effect of pain on task switching: Pain reduces accuracy and increases reaction times across multiple switching paradigms. Pain 2016, 157, 2179–2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ploner, M.; Gross, J.; Timmermann, L.; Schnitzler, A. Pain Processing Is Faster than Tactile Processing in the Human Brain. J. Neurosci. 2006, 26, 10879–10882. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention. Lead in Blood and Urine 8003. 1994. Available online: https://www.cdc.gov/niosh/docs/2003-154/pdfs/8003.pdf (accessed on 1 October 2021).
- Yang, T.; Sun, C.; Hsu, H.; Liou, S.; Wu, D.; Chu, S.; Tung, H.; Chen, Y.; Chen, J. Stability of blood lead levels in stored specimens: Effects of storage time and temperature. J. Med. Sci. 2006, 26, 211. [Google Scholar]
- Barbosa, F., Jr.; Tanus-Santos, J.E.; Gerlach, R.F.; Parsons, P.J. A critical review of biomarkers used for monitoring human exposure to lead: Advantages, limitations and future needs|uma avaliação crítica sobre biomarcadores utilizados para o monitoramento biológico de exposição ao chumbo: Vantagens, limitações e pers. Cienc. E Saude Coletiva 2006, 11, 229–241. [Google Scholar] [CrossRef]
- Shih, R.A.; Hu, H.; Weisskopf, M.G.; Schwartz, B.S. Cumulative Lead Dose and Cognitive Function in Adults: A Review of Studies That Measured Both Blood Lead and Bone Lead. Environ. Health Perspect. 2007, 115, 483–492. [Google Scholar] [CrossRef] [Green Version]
- Juliani, A. Heavy metal characteristics of wastewater from batik industry in yogyakarta area, indonesia. Int. J. Geomate 2021, 20, 59–67. [Google Scholar] [CrossRef]
- Morgan, S.F.; Pickens, R.W. Reaction time performance as a function of cigarette smoking procedure. Psychopharmacology 1982, 77, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.; Fenoll, R.; Macià, D.; Martínez-Vilavella, G.; Alvarez-Pedrerol, M.; Rivas, I.; Forns, J.; Deus, J.; Blanco-Hinojo, L.; Querol, X.; et al. Airborne copper exposure in school environments associated with poorer motor performance and altered basal ganglia. Brain Behav. 2016, 6, e00467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciesielski, T.; Bellinger, D.C.; Schwartz, J.; Hauser, R.; Wright, R. Associations between cadmium exposure and neurocognitive test scores in a cross-sectional study of US adults. Environ. Health 2013, 12, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tun, P.A.; Lachman, M.E. Age differences in reaction time and attention in a national telephone sample of adults: Education, sex, and task complexity matter. Dev. Psychol. 2008, 44, 1421–1429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
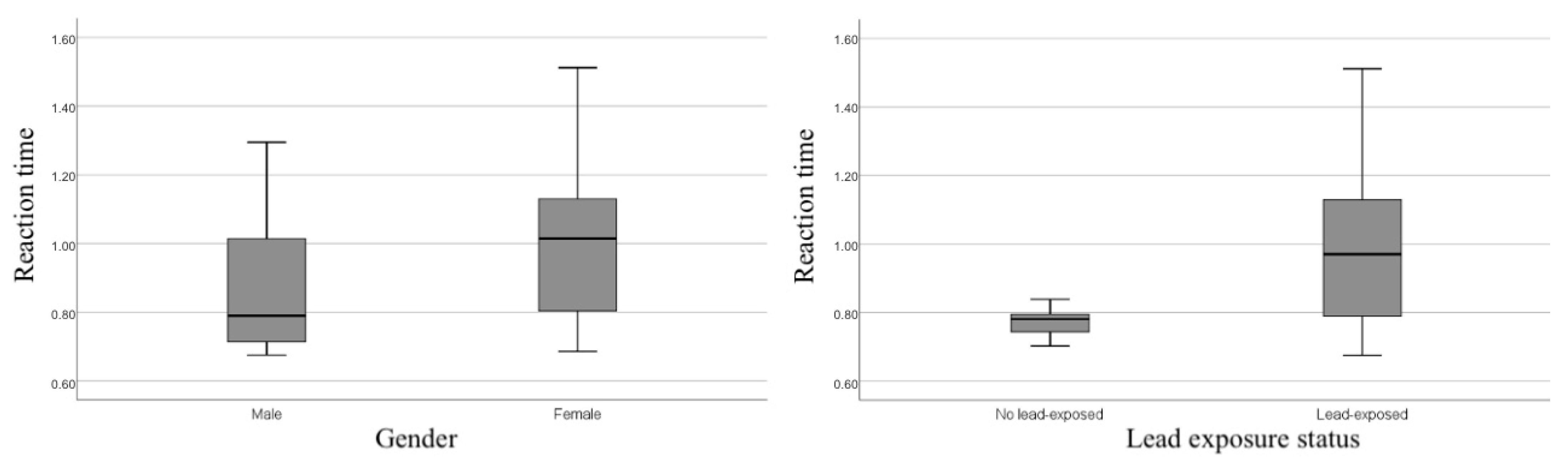
| Total (M (SD)/Mdn (Min–Max)) N = 59 | Male (M (SD)/Mdn (Min–Max)) N = 21 | Female (M (SD)/Mdn (Min–Max)) N = 38 | Difference (M Diff (p)/Mdn Diff (p)) | Lead-Exposed (M (SD)/Mdn (Min–Max)) N = 52 | No Lead-Exposed (M (SD)/Mdn (Min–Max)) N = 7 | Difference (M Diff (p)/Mdn Diff (p)) | |
|---|---|---|---|---|---|---|---|
| Age (years) | 40 (18–59) | 25 (18–55) | 44 (21–59) | 19.5 (p = 0.01) | 41 (18–59) | 36 (24–45) | −1.5 (p = 0.506) |
| BMI (kg/m2) | 22.45 (4.09) | 20.75 (3.16) | 23.64 (4.29) | 2.89 (p = 0.012) | 22.31 (4.18) | 23.68 (3.17) | 1.36 (p = 0.48) |
| Nordic score neck complaints | 0 (0–4) | 0 (0–3) | 0 (0–4) | 0 (p = 0.019) | 0 (0–4) | 0 (0–2) | 0 (p = 0.615) |
| Nordic score shoulder complaints | 0 (0–4) | 1 (0–3) | 0 (0–4) | −1 (p = 0.022) | 0 (0–4) | 2 (0–3) | 2 (p = 0.206) |
| Nordic score back complaints | 1 (0–8) | 2 (0–8) | 0.5 (0–8) | −1.5 (p = 0.052) | 1 (0–8) | 0 (0–3) | −1 (p = 0.313) |
| Nordic score right upper limb complaints | 0 (0–10) | 2 (0–10) | 0 (0–9) | −2 (p = 0.001) | 0 (0–10) | 0 (0–4) | 0 (p = 0.708) |
| Nordic score left upper limb complaints | 0 (0–7) | 0 (0–7) | 0 (0–6) | 0 (p = 0.194) | 0 (0–7) | 0 (0–0) | 0 (p = 0.253) |
| Nordic score right lower limb complaints | 3 (0–14) | 0 (0–12) | 0 (0–14) | 0 (p = 0.185) | 0 (0–14) | 0 (0–12) | 0 (p = 0.841) |
| Nordic score left lower limb complaints | 0 (0–14) | 0 (0–8) | 0 (0–14) | 0 (p = 0.145) | 0 (0–14) | 0 (0–8) | 0 (p = 0.824) |
| Years of service (months) | 60 (2–360) | 60 (2–144) | 84 (2–360) | 24 (p = 0.053) | 73 (2–360) | 60 (5–144) | −13 (p = 0.634) |
| Correlation Coefficient (p) | |
|---|---|
| Age | 0.47 (p < 0.001) |
| BMI | 0.25 (p = 0.06) |
| Nordic score neck complaints | −0.092 (p = 0.52) |
| Nordic score shoulder complaints | −0.003 (p = 0.98) |
| Nordic score back complaints | 0.059 (p = 0.68) |
| Nordic score right upper limb complaints | −0.189 (p = 0.18) |
| Nordic score left upper limb complaints | −0.055 (p = 0.70) |
| Nordic score right lower limb complaints | −0.018 (p = 0.89) |
| Nordic score left lower limb complaints | −0.027 (p = 0.85) |
| Years of service | 0.158 (p = 0.27) |
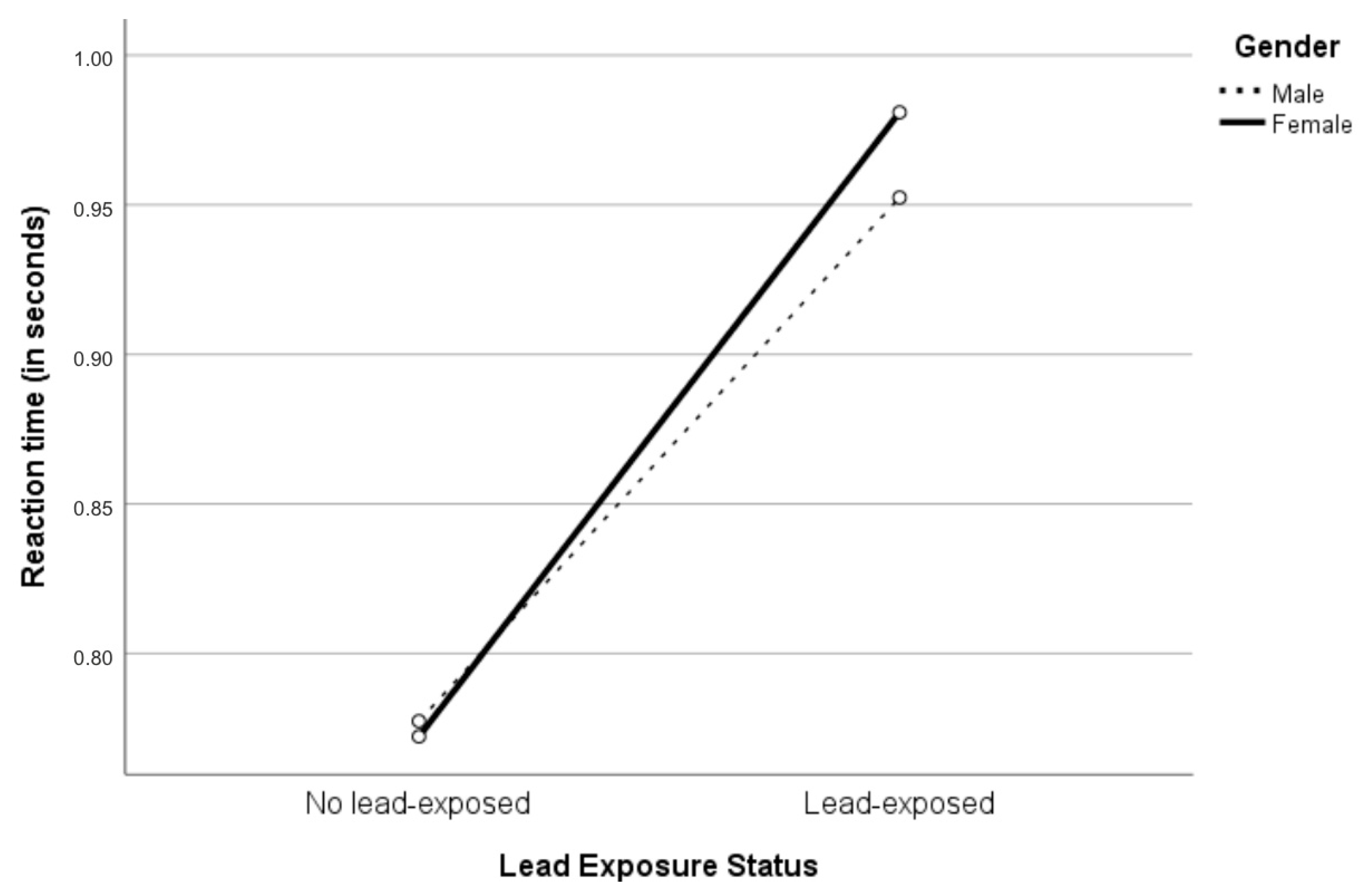
| Reaction Time | No Lead-Exposed | Lead-Exposed | ||||
|---|---|---|---|---|---|---|
| All (N = 7) | Male (N = 3) | Female (N = 4) | All (N = 52) | Male (N = 18) | Female (N = 34) | |
| M | 0.772 | 0.774 | 0.769 | 0.970 *** | 0.888 | 1.023 + |
| (SD) | 0.052 | 0.068 | 0.036 | 0.206 | 0.192 | 0.201 |
| Madj a | 0.775 | 0.777 | 0.772 | 0.967 * | 0.952 | 0.981 |
| (SE) | 0.083 | 0.103 | 0.129 | 0.027 | 0.047 | 0.037 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agustiningsih, D.; Sofyana, M.; Budiharjo, S.; Febriana, S.A.; Nurokhmanti, H.; Suhartini, S.; Priyambodo, D.Y.; Nugrahaningsih, D.A.A.; Roto, R.; Wibowo, R.A. Reaction Times among Batik Workers: The Influence of Gender and Occupational Lead Exposure. Int. J. Environ. Res. Public Health 2021, 18, 12605. https://doi.org/10.3390/ijerph182312605
Agustiningsih D, Sofyana M, Budiharjo S, Febriana SA, Nurokhmanti H, Suhartini S, Priyambodo DY, Nugrahaningsih DAA, Roto R, Wibowo RA. Reaction Times among Batik Workers: The Influence of Gender and Occupational Lead Exposure. International Journal of Environmental Research and Public Health. 2021; 18(23):12605. https://doi.org/10.3390/ijerph182312605
Chicago/Turabian StyleAgustiningsih, Denny, Meida Sofyana, Santosa Budiharjo, Sri Awalia Febriana, Hikmawati Nurokhmanti, Suhartini Suhartini, Dewanto Yusuf Priyambodo, Dwi Aris Agung Nugrahaningsih, Roto Roto, and Rakhmat Ari Wibowo. 2021. "Reaction Times among Batik Workers: The Influence of Gender and Occupational Lead Exposure" International Journal of Environmental Research and Public Health 18, no. 23: 12605. https://doi.org/10.3390/ijerph182312605
APA StyleAgustiningsih, D., Sofyana, M., Budiharjo, S., Febriana, S. A., Nurokhmanti, H., Suhartini, S., Priyambodo, D. Y., Nugrahaningsih, D. A. A., Roto, R., & Wibowo, R. A. (2021). Reaction Times among Batik Workers: The Influence of Gender and Occupational Lead Exposure. International Journal of Environmental Research and Public Health, 18(23), 12605. https://doi.org/10.3390/ijerph182312605






