A Structural Equation Modelling Approach to Examine the Relationship between Socioeconomic Status, Diet Quality and Dyslipidaemia in South African Children and Adolescents, 6–18 Years
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Data Collection Tools
2.2.1. Dietary Assessment
2.2.2. Biochemical Measurements
2.2.3. Assessment of SES
2.3. Development of Latent Variables
2.4. Data Analysis
3. Results
Goodness-of-Fit of the Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jomaa, L.; Naja, F.; Cheaib, R.; Hwalla, N. Household food insecurity is associated with a higher burden of obesity and risk of dietary inadequacies among mothers in Beirut, Lebanon. BMC Public Health 2017, 17, 567. [Google Scholar] [CrossRef]
- Steyn, N.P.; Mchiza, Z.J. Obesity and the nutrition transition in Sub-Saharan Africa. Ann. N. Y. Acad. Sci. 2014, 1311, 88–101. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Shisana, O.; Labadarios, D.; Rehle, T.; Simbayi, L.; Zuma, K.; Dhansay, A.R.P.; Parker, W.; Hoosain, E.; Naidoo, P.; Hongoro, C.; et al. South African National Health and Nutrition Examination Survey (SANHANES-1); HSRC Press: Cape Town, South Africa, 2013. [Google Scholar]
- Vorster, H.H.; Kruger, A. Poverty, malnutrition, underdevelopment and cardiovascular disease: A South African perspective. Cardiovasc. J. Afr. 2007, 18, 321–324. [Google Scholar]
- Prentice, A.M. The Double Burden of Malnutrition in Countries Passing through the Economic Transition. Ann. Nutr. Metab. 2018, 72, 47–54. [Google Scholar] [CrossRef]
- Ochoa-Avilés, A.; Verstraeten, R.; Lachat, C.; Andrade, S.; Van Camp, J.; Donoso, S.; Kolsteren, P. Dietary intake practices associated with cardiovascular risk in urban and rural Ecuadorian adolescents: A cross-sectional study. BMC Public Health 2014, 14, 939. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rosengren, A.; Smyth, A.; Rangarajan, S.; Ramasundarahettige, C.; Bangdiwala, S.I.; Alhabib, K.F.; Avezum, A.; Boström, K.B.; Chifamba, J.; Gulec, S.; et al. Socioeconomic status and risk of cardiovascular disease in 20 low-income, middle-income, and high-income countries: The Prospective Urban Rural Epidemiologic (PURE) study. Lancet Glob. Health 2019, 7, e748–e760. [Google Scholar] [CrossRef]
- Quispe, R.; Benziger, C.P.; Bazo-Alvarez, J.C.; Howe, L.D.; Checkley, W.; Gilman, R.H.; Smeeth, L.; Bernabé-Ortiz, A.; Miranda, J.J.; Casas, J.P.; et al. The Relationship Between Socioeconomic Status and CV Risk Factors: The CRONICAS Cohort Study of Peruvian Adults. Glob. Heart 2016, 11, 121–130.e2. [Google Scholar] [CrossRef]
- Lopez, A.B.; Hooven, E.H.V.D.; Rueda-Clausen, C.; Serrano, N.; Ruiz, A.J.; Pereira, M.A.; Mueller, N. Socioeconomic status is positively associated with measures of adiposity and insulin resistance, but inversely associated with dyslipidaemia in Colombian children. J. Epidemiol. Community Health 2015, 69, 580–587. [Google Scholar] [CrossRef]
- Kalantari, S. Childhood cardiovascular risk factors, a predictor of late adolescent overweight. Adv. Biomed. Res. 2016, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Oldewage-Theron, W.; Egal, A.A.; Grobler, C. Lipid Profile, Hyperglycaemia, Systemic Inflammation and Anthropometry as Cardiovascular Risk Factors and Their Association with Dietary Intakes in Children from Rural Cofimvaba, Eastern Cape, South Africa. J. Consum. Sci. 2017, 2, 1–15. [Google Scholar]
- Overcash, F.M.; Reicks, M.; Ritter, A.; Leak, T.M.; Swenson, A.; Vickers, Z. Children Residing in Low-Income Households Like a Variety of Vegetables. Foods 2018, 7, 116. [Google Scholar] [CrossRef]
- Gerritsen, S.; Renker-Darby, A.; Harré, S.; Rees, D.; Raroa, D.A.; Eickstaedt, M.; Sushil, Z.; Allan, K.; Bartos, A.E.; Waterlander, W.; et al. Improving low fruit and vegetable intake in children: Findings from a system dynamics, community group model building study. PLoS ONE 2019, 14, e0221107. [Google Scholar] [CrossRef] [PubMed]
- Msambichaka, B.; Eze, I.C.; Abdul, R.; Abdulla, S.; Klatser, P.; Tanner, M.; Kaushik, R.; Geubbels, E.; Probst-Hensch, N. Insufficient Fruit and Vegetable Intake in a Low- and Middle-Income Setting: A Population-Based Survey in Semi-Urban Tanzania. Nutrients 2018, 10, 222. [Google Scholar] [CrossRef] [PubMed]
- Grimm, K.A.; Kim, S.A.; Yaroch, A.L.; Scanlon, K.S. Fruit and Vegetable Intake During Infancy and Early Childhood. Pediatrics 2014, 134 (Suppl. S1), S63–S69. [Google Scholar] [CrossRef]
- Di Noia, J.; Byrd-Bredbenner, C. Determinants of fruit and vegetable intake in low-income children and adolescents. Nutr. Rev. 2014, 72, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Tasevska, N.; DeLia, D.; Lorts, C.; Yedidia, M.; Ohri-Vachaspati, P. Determinants of Sugar-Sweetened Beverage Consumption among Low-Income Children: Are There Differences by Race/Ethnicity, Age, and Sex? J. Acad. Nutr. Diet. 2017, 117, 1900–1920. [Google Scholar] [CrossRef]
- Bruce, M.A.; Thorpe, R.J.; Beech, B.M.; Towns, T.; Odoms-Young, A. Sex, Race, Food Security, and Sugar Consumption Change Efficacy Among Low-Income Parents in an Urban Primary Care Setting. Fam. Community Health 2018, 41 (Suppl. S2), S25–S32. [Google Scholar] [CrossRef]
- Botelho, R.B.A.; Akutsu, R.D.C.; Zandonadi, R.P. Low-Income Population Sugar (Sucrose) Intake: A Cross-Sectional Study among Adults Assisted by a Brazilian Food Assistance Program. Nutrients 2019, 11, 798. [Google Scholar] [CrossRef]
- Department of Health (DoH). Strategic Plan for the Prevention and Control of Non-Communicable Diseases 2013-17; Department of Health Republic of South Africa: Pretoria, South Africa, 2013. [Google Scholar]
- Magnussen, C.G.; Venn, A.; Thomson, R.; Juonala, M.; Srinivasan, S.R.; Viikari, J.S.; Berenson, G.S.; Dwyer, T.; Raitakari, O.T. The Association of Pediatric Low- and High-Density Lipoprotein Cholesterol Dyslipidemia Classifications and Change in Dyslipidemia Status With Carotid Intima-Media Thickness in Adulthood: Evidence From the Cardiovascular Risk in Young Finns Study, the Bogalusa Heart Study, and the CDAH (Childhood Determinants of Adult Health) Study. J. Am. Coll. Cardiol. 2009, 53, 860–869. [Google Scholar] [CrossRef]
- Pletcher, M.J.; Vittinghoff, E.; Thanataveerat, A.; Bibbins-Domingo, K.; Moran, A.E. Young Adult Exposure to Cardiovascular Risk Factors and Risk of Events Later in Life: The Framingham Offspring Study. PLoS ONE 2016, 11, e0154288. [Google Scholar] [CrossRef]
- Kopin, L.; Lowenstein, C.J. Dyslipidemia. Ann. Intern. Med. 2017, 167, ITC81–ITC96. [Google Scholar] [CrossRef]
- Sporisević, L.; Krzelj, V.; Bajraktarević, A.; Jahić, E. Evaluation of Cardiovascular Risk in School Children. Bosn. J. Basic Med. Sci. 2009, 9, 182–186. [Google Scholar] [CrossRef]
- Hartwell, M.L.; Khojasteh, J.; Wetherill, M.S.; Croff, J.M.; Wheeler, D. Using Structural Equation Modeling to Examine the Influence of Social, Behavioral, and Nutritional Variables on Health Outcomes Based on NHANES Data: Addressing Complex Design, Nonnormally Distributed Variables, and Missing Information. Curr. Dev. Nutr. 2019, 3, nzz010. [Google Scholar] [CrossRef]
- Duncan, G.J.; Daly, M.C.; McDonough, P.; Williams, D.R. Optimal Indicators of Socioeconomic Status for Health Research. Am. J. Public Health 2002, 92, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Darin-Mattsson, A.; Fors, S.; Kåreholt, I. Different indicators of socioeconomic status and their relative importance as determinants of health in old age. Int. J. Equity Health 2017, 16, 173. [Google Scholar] [CrossRef]
- Galobardes, B.; Shaw, M.; Lawlor, D.A.; Lynch, J.W.; Davey Smith, G. Indicators of socioeconomic position (part 1). J. Epidemiol. Commun. Health 2006, 60, 7–12. [Google Scholar] [CrossRef]
- Widmer, R.J.; Flammer, A.J.; Lerman, L.O.; Lerman, A. The Mediterranean Diet, its Components, and Cardiovascular Disease. Am. J. Med. 2015, 128, 229–238. [Google Scholar] [CrossRef]
- Wang, D.D.; Hu, F.B. Dietary Fat and Risk of Cardiovascular Disease: Recent Controversies and Advances. Annu. Rev. Nutr. 2017, 37, 423–446. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Bo, Y.; Liu, Y. Dietary total fat, fatty acids intake, and risk of cardiovascular disease: A dose-response meta-analysis of cohort studies. Lipids Health Dis. 2019, 18, 91. [Google Scholar] [CrossRef] [PubMed]
- Boodai, S.; Reilly, J.; Cherry, L.; Naveed, S. Prevalence of cardiometabolic risk factors and metabolic syndrome in obese Kuwaiti adolescents. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 505–511. [Google Scholar] [CrossRef] [PubMed]
- INDDEX Project. Data4Diets: Building Blocks for Diet-Related Food Security Analysis. Available online: https://inddex.nutrition.tufts.edu/data4diets (accessed on 5 March 2020).
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 10 September 2019).
- Stephenson, M.T.; Holbert, R.L.; Zimmerman, R.S. On the Use of Structural Equation Modeling in Health Communication Research. Health Commun. 2006, 20, 159–167. [Google Scholar] [CrossRef]
- Little, T.D. Longitudinal Structural Equation Modeling, 1st ed.; Guilford Press: New York, NY, USA, 2013. [Google Scholar]
- Rosseel, Y. Lavaan: An R Package for Structural Equation Modeling. J. Stat. Softw. 2012, 48, 1–36. [Google Scholar] [CrossRef]
- WHO. Promoting Fruit and Vegetable Consumption around the World. Available online: https://www.who.int/dietphysicalactivity/fruit/index1.html (accessed on 5 March 2020).
- AHA. Dietary Recommendations for Healthy Children|American Heart Association. Available online: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/dietary-recommendations-for-healthy-children (accessed on 2 February 2020).
- Medline. High Cholesterol in Children and Teens. Available online: https://medlineplus.gov/highcholesterolinchildrenandteens.html (accessed on 23 January 2020).
- Peñalvo, J.L.; Oliva, B.; Sotos-Prieto, M.; Uzhova, I.; Moreno-Franco, B.; León-Latre, M.; Ordovás, J.M. Greater Adherence to a Mediterranean Dietary Pattern Is Associated With Improved Plasma Lipid Profile: The Aragon Health Workers Study Cohort. Rev. Española Cardiol. (Engl. Ed.) 2015, 68, 290–297. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J. Association between Dietary Pattern and Incidence of Cholesterolemia in Korean Adults: The Korean Genome and Epidemiology Study. Nutrients 2018, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Sonego, M.; Sagrado, M.J.; Escobar, G.; Lazzerini, M.; Rivas, E.; Martín-Cañavate, R.; de López, E.P.; Ayala, S.; Castaneda, L.; Aparicio, P.; et al. Dyslipidemia, Diet and Physical Exercise in Children on Treatment With Antiretroviral Medication in El Salvador: A Cross-Sectional Study. Pediatr. Infect. Dis. J. 2016, 35, 1111–1116. [Google Scholar] [CrossRef][Green Version]
| Variable | Mean | Standard Deviation | Reference Values | Classification |
|---|---|---|---|---|
| Fruit FGDS a | 2.257 (32 g/day) | ±1.79 | 400 g/day of fruit and vegetables [39] | Low |
| Vegetable FGDS a | 3.046 (24 g/day) | ±1.98 | Low | |
| Added Sugar (grams) | 36.74 | ±31.52 | 12–25 g/day [40] | High |
| Total Fat (g) and Total Energy (TE) | 48.11 g (25.4% TE) | ±33.22 | 25–35% TE depending on age [40] | Moderate |
| Dietary Diversity Score (DDS) | 7.932 | ±1.17 | 7–9 food groups [34] | High |
| Total Serum Cholesterol (mg/dL) | 127.02 | ±26.52 | <170 mg/dL [41] | Good |
| Serum LDL-Cholesterol (mg/dL) b | 68.12 | ±24.61 | <100 mg/dL [41] | Good |
| Latent Variable | Indicator | Factor Loading | Significance (p-Value) |
|---|---|---|---|
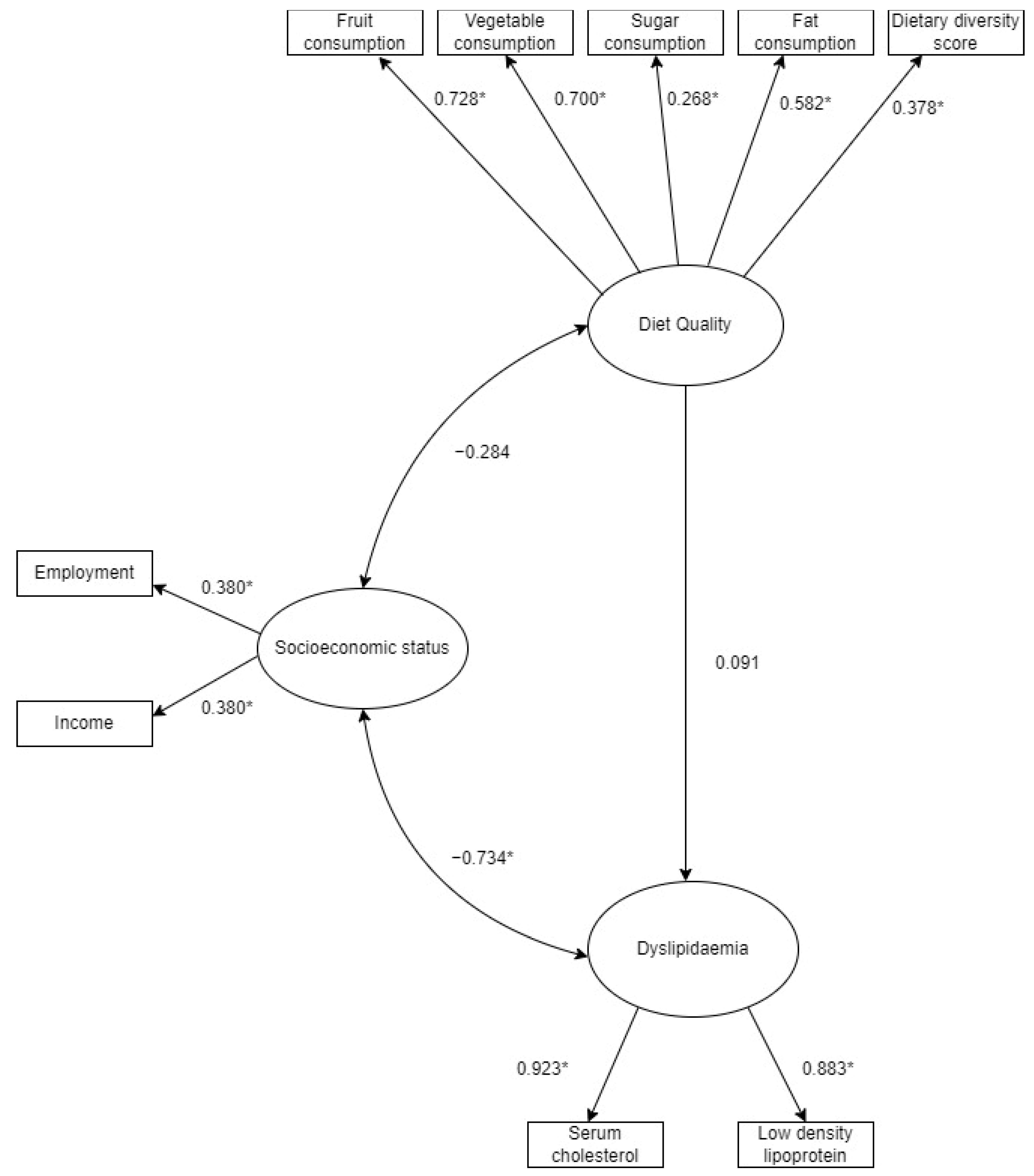
| SES | Employment | 0.380 | 0.026 * |
| SES | Income | 0.380 | 0.026 * |
| Diet Quality | Fruit FGDS a | 0.728 | <0.001 * |
| Diet Quality | Vegetable FGDS a | 0.700 | <0.001 * |
| Diet Quality | Added Sugar | 0.268 | <0.001 * |
| Diet Quality | Dietary Diversity Score | 0.582 | <0.001 * |
| Diet Quality | Total Fat Intake | 0.373 | <0.001 * |
| Dyslipidaemia | Total Serum Cholesterol | 0.923 | <0.001 * |
| Dyslipidaemia | Serum LDL-Cholesterol b | 0.883 | <0.001 * |
| Pathway | Association | Significance (p-Value) |
|---|---|---|
| a Socioeconomic status and Dyslipidaemia | −0.734 | 0.029 * |
| a Socioeconomic status and diet quality | −0.284 | 0.208 |
| b Dyslipidaemia and diet quality | 0.091 | 0.159 |
| Model | χ2 | df | RMSEA | RMSEA 90% CI | SRMR | CFI | TLI |
|---|---|---|---|---|---|---|---|
| SEM | 53.740 (p = 0.009) | 32 | 0.062 | 0.031–0.090 | 0.065 | 0.903 | 0.891 |
| Acceptable | Acceptable | Good | Mediocre/OK | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyo, G.; Montenegro-Montenegro, E.; Stickley, Z.; Egal, A.; Oldewage-Theron, W. A Structural Equation Modelling Approach to Examine the Relationship between Socioeconomic Status, Diet Quality and Dyslipidaemia in South African Children and Adolescents, 6–18 Years. Int. J. Environ. Res. Public Health 2021, 18, 12825. https://doi.org/10.3390/ijerph182312825
Moyo G, Montenegro-Montenegro E, Stickley Z, Egal A, Oldewage-Theron W. A Structural Equation Modelling Approach to Examine the Relationship between Socioeconomic Status, Diet Quality and Dyslipidaemia in South African Children and Adolescents, 6–18 Years. International Journal of Environmental Research and Public Health. 2021; 18(23):12825. https://doi.org/10.3390/ijerph182312825
Chicago/Turabian StyleMoyo, Gugulethu, Esteban Montenegro-Montenegro, Zachary Stickley, Abdulkadir Egal, and Wilna Oldewage-Theron. 2021. "A Structural Equation Modelling Approach to Examine the Relationship between Socioeconomic Status, Diet Quality and Dyslipidaemia in South African Children and Adolescents, 6–18 Years" International Journal of Environmental Research and Public Health 18, no. 23: 12825. https://doi.org/10.3390/ijerph182312825
APA StyleMoyo, G., Montenegro-Montenegro, E., Stickley, Z., Egal, A., & Oldewage-Theron, W. (2021). A Structural Equation Modelling Approach to Examine the Relationship between Socioeconomic Status, Diet Quality and Dyslipidaemia in South African Children and Adolescents, 6–18 Years. International Journal of Environmental Research and Public Health, 18(23), 12825. https://doi.org/10.3390/ijerph182312825







