Impacts of Fluoride Neurotoxicity and Mitochondrial Dysfunction on Cognition and Mental Health: A Literature Review
Abstract
1. Introduction
2. Methods
3. Results
3.1. Fluoride and Neurodevelopment
3.1.1. Animal Studies: Cognitive Function
3.1.2. Animal Studies: Sex Differences
3.1.3. Animal Studies: Behavior
3.1.4. Human Studies: Cognitive Function and Previous Analyses
3.1.5. Human Studies: Mental Health and Neurobehavior
3.1.6. Human Studies: Sex Differences
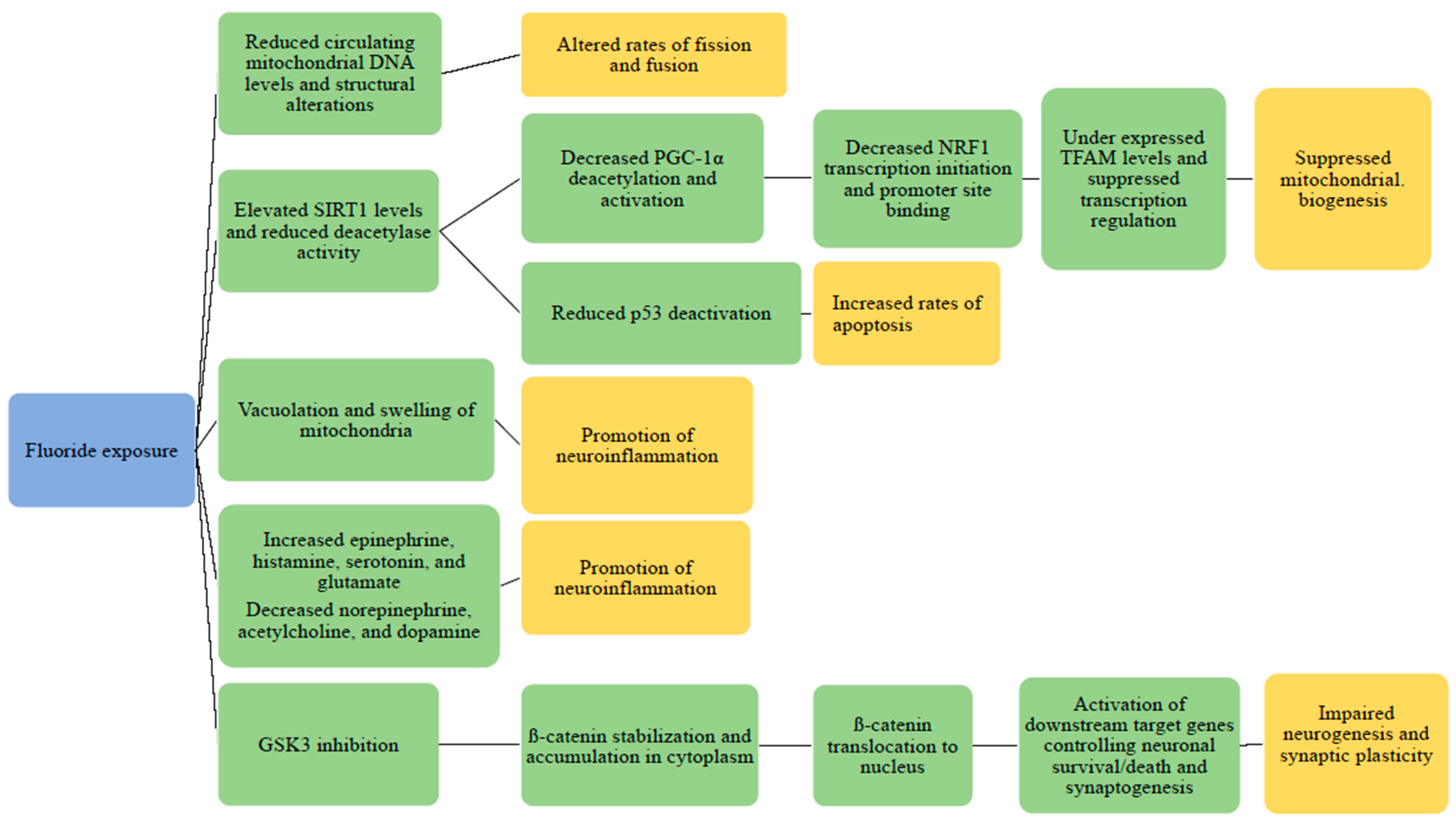
3.2. Mitochondrial Dysfunction and Other Potential Pathogenesis of Fluoride
3.2.1. Animal Studies: Fluoride and Mitochondrial Structure Changes
3.2.2. Animal Studies: Fluoride: Mitochondrial Damage and Neuroinflammation
3.2.3. Animal Studies: Fluoride, Neurotransmitters, and Signaling Pathways
3.2.4. Human Studies: Role of Mitochondrial function in Mental Health
Mitochondrial Volume
Mitochondrial Swelling, Autophagy, and Apoptosis
3.2.5. Human Studies: Areas with Limited Mitochondrial Research
3.3. Recent Toxicology Research
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- PubChem. Fluoride Ion. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/28179 (accessed on 23 November 2021).
- Fluorine|F (Element)-PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/element/Fluorine (accessed on 24 November 2021).
- Water Fluoridation Basics|Community Water Fluoridation|Division of Oral Health|CDC. Available online: https://www.cdc.gov/fluoridation/basics/index.htm (accessed on 23 November 2021).
- Saeed, M.; Malik, R.N.; Kamal, A. Fluorosis and cognitive development among children (6–14 years of age) in the endemic areas of the world: A review and critical analysis. Environ. Sci. Pollut. Res. 2020, 27, 2566–2579. [Google Scholar] [CrossRef]
- Community Water Fluoridation|Division of Oral Health|CDC. Available online: https://www.cdc.gov/fluoridation/index.html (accessed on 23 November 2021).
- Surgeons General’s Statements on Community Water Fluoridation|Guidelines|Community Water Fluoridation|Oral Health|CDC. Available online: https://www.cdc.gov/fluoridation/guidelines/surgeons-general-statements.html (accessed on 23 November 2021).
- Water Fluoridation Additives|Engineering|Community Water Fluoridation|Division of Oral Health|CDC. Available online: https://www.cdc.gov/fluoridation/engineering/wfadditives.htm (accessed on 23 November 2021).
- ADA Applauds USPHS Final Recommendation on Optimal Fluoride Level in Drinking Water|American Dental Association. Available online: https://www.ada.org/resources/community-initiatives/fluoridation/ada-applauds-usphs-final-recommendation-on-optimal-fluoride-level-in-drinking-water (accessed on 28 November 2021).
- United States Environmental Protection Agency. Questions and Answers on Fluoride. United States Environmental Protection Agency. 2011; p. 10. Available online: https://www.epa.gov/sites/default/files/2015-10/documents/2011_fluoride_questionsanswers.pdf (accessed on 3 December 2021).
- Guth, S.; Hüser, S.; Roth, A.; Degen, G.; Diel, P.; Edlund, K.; Eisenbrand, G.; Engel, K.-H.; Epe, G.; Grune, T.; et al. Toxicity of fluoride: Critical evaluation of evidence for human developmental neurotoxicity in epidemiological studies, animal experiments and in vitro analyses. Arch. Toxicol. 2020, 94, 1375–1415. [Google Scholar] [CrossRef]
- Choi, A.L.; Sun, G.; Zhang, Y.; Grandjean, P. Developmental fluoride neurotoxicity: A systematic review and meta-analysis. Environ. Health Perspect. 2012, 120, 1362–1368. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef]
- Grandjean, P. Developmental fluoride neurotoxicity: An updated review. Environ. Health 2019, 18, 110. [Google Scholar] [CrossRef]
- Zhao, Q.; Niu, Q.; Chen, J.; Xia, T.; Zhou, G.; Li, P.; Dong, L.; Xu, C.; Tian, Z.; Luo, C.; et al. Roles of mitochondrial fission inhibition in developmental fluoride neurotoxicity: Mechanisms of action in vitro and associations with cognition in rats and children. Arch. Toxicol. 2019, 93, 709–726. [Google Scholar] [CrossRef]
- Zuo, H.; Chen, L.; Kong, L.; Qiu, L.; Lü, P.; Wu, P.; Yang, Y.; Chen, K. Toxic effects of fluoride on organisms. Life Sci. 2018, 198, 18–24. [Google Scholar] [CrossRef]
- Atig, R.K.-B.; Hsouna, S.; Beraud-Colomb, E.; Abdelhak, S. Mitochondrial DNA: Properties and applications. Arch Inst. Pasteur Tunis 2009, 86, 3–14. [Google Scholar]
- Schon, E.A.; DiMauro, S.; Hirano, M. Human mitochondrial DNA: Roles of inherited and somatic mutations. Nat. Rev. Genet. 2012, 13, 878–890. [Google Scholar] [CrossRef]
- Miranda, G.H.N.; Gomes, B.A.Q.; Bittencourt, L.O.; Aragão, W.; Nogueira, L.S.; Dionizio, A.; Buzalaf, M.A.R.; Monteiro, M.C.; Lima, R.R. Chronic Exposure to Sodium Fluoride Triggers Oxidative Biochemistry Misbalance in Mice: Effects on Peripheral Blood Circulation. Oxid Med. Cell. Longev. 2018, 2018, 8379123. [Google Scholar] [CrossRef]
- Zhao, Q.; Tian, Z.; Zhou, G.; Niu, Q.; Chen, J.; Li, P.; Dong, L.; Xia, T.; Zhang, S.; Wang, A. SIRT1-dependent mitochondrial biogenesis supports therapeutic effects of resveratrol against neurodevelopment damage by fluoride. Theranostics 2020, 10, 4822–4838. [Google Scholar] [CrossRef]
- Reddy, Y.P.; Tiwari, S.; Tomar, L.K.; Desai, N.; Sharma, V.K. Fluoride-Induced Expression of Neuroinflammatory Markers and Neurophysiological Regulation in the Brain of Wistar Rat Model. Biol. Trace Elem. Res. 2021, 199, 2621–2626. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ning, H.; Yin, Z.; Song, X.; Feng, Y.; Qin, H.; Li, Y.; Wang, J.; Ge, Y.; Wang, W. The effects of fluoride on neuronal function occurs via cytoskeleton damage and decreased signal transmission. Chemosphere 2017, 185, 589–594. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.R.; Jardim, F.R.; Setzer, W.N.; Nabavi, S.M.; Nabavi, S.F. Curcumin, mitochondrial biogenesis, and mitophagy: Exploring recent data and indicating future needs. Biotechnol. Adv. 2016, 34, 813–826. [Google Scholar] [CrossRef]
- Liang, H.; Ward, W.F. PGC-1alpha: A key regulator of energy metabolism. Adv. Physiol. Educ. 2006, 30, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Litonin, D.; Sologub, M.; Shi, Y.; Savkina, M.; Anikin, M.; Falkenberg, M.; Gustafsson, C.M.; Temiakov, D. Human mitochondrial transcription revisited: Only TFAM and TFB2M are required for transcription of the mitochondrial genes in vitro. J. Biol. Chem. 2010, 285, 18129–18133. [Google Scholar] [CrossRef]
- Deng, H.; Fujiwara, N.; Cui, H.; Whitford, G.M.; Bartlett, J.D.; Suzuki, M. Histone acetyltransferase promotes fluoride toxicity in LS8 cells. Chemosphere 2020, 247, 125825. [Google Scholar] [CrossRef]
- Fridman, J.S.; Lowe, S.W. Control of apoptosis by p53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef]
- Suzuki, M.; Ikeda, A.; Bartlett, J.D. Sirt1 Overexpression Suppresses Fluoride-induced p53 Acetylation to Alleviate Fluoride Toxicity in Ameloblasts Responsible for Enamel Formation. Arch. Toxicol. 2018, 92, 1283–1293. [Google Scholar] [CrossRef]
- Bansal, Y.; Kuhad, A. Mitochondrial Dysfunction in Depression. Curr. Neuropharmacol. 2016, 14, 610–618. [Google Scholar] [CrossRef]
- Kramer, P.; Bressan, P. Our (Mother’s) Mitochondria and Our Mind. Perspect. Psychol. Sci. 2018, 13, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.W.; Na Kwon, B.; Kim, H.J.; Han, S.H.; Lee, N.R.; Lim, M.H.; Kwon, H.J.; Jin, H.J. Assessment of associations between mitochondrial DNA haplogroups and attention deficit and hyperactivity disorder in Korean children. Mitochondrion 2019, 47, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Singh, A.; Nthenge-Ngumbau, D.N.; Rajamma, U.; Sinha, S.; Mukhopadhyay, K.; Mohanakumar, K.P. Attention deficit-hyperactivity disorder suffers from mitochondrial dysfunction. BBA Clin. 2016, 6, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Yang, L.; Luo, C.; Liu, H.; Li, P.; Cui, Y.; Liu, L.; Yu, X.; Zeng, Q.; Chen, J.; et al. Low-to-moderate fluoride exposure, relative mitochondrial DNA levels, and dental fluorosis in Chinese children. Environ. Int. 2019, 127, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Rubenstein, J.; Popoli, R.; Capulong, R.; Till, C. Sex-specific neurotoxic effects of early-life exposure to fluoride: A review of the epidemiologic and animal literature. Curr. Epidemiol. Rep. 2020, 7, 263–273. [Google Scholar] [CrossRef]
- Bartos, M.; Gumilar, F.; Gallegos, C.E.; Bras, C.; Dominguez, S.; Cancela, L.M.; Minetti, A. Effects of Perinatal Fluoride Exposure on Short- and Long-Term Memory, Brain Antioxidant Status, and Glutamate Metabolism of Young Rat Pups. Int. J. Toxicol. 2019, 38, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Ibarluzea, J.; Gallastegi, M.; Santa-Marina, L.; Zabala, A.J.; Arranz, E.; Molinuevo, A.; Lopez-Espinosa, M.-J.; Ballester, F.; Villanueva, C.M.; Riano, I.; et al. Prenatal exposure to fluoride and neuropsychological development in early childhood: 1-to 4 years old children. Environ. Res. 2021, 112181. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Hu, H.; Till, C.; Green, R.; Bashash, M.; Flora, D.; Tellez-Rojo, M.M.; Song, P.X.; Lanphear, B.; Budtz-Jørgensen, E. A Benchmark Dose Analysis for Maternal Pregnancy Urine-Fluoride and IQ in Children. Risk Anal. 2020, 20221374. [Google Scholar] [CrossRef] [PubMed]
- Farmus, L.; Till, C.; Green, R.; Hornung, R.; Mier, E.A.M.; Ayotte, P.; Muckle, G.; Lanphear, B.P.; Flora, D.B. Critical windows of fluoride neurotoxicity in Canadian children. Environ. Res. 2021, 200, 111315. [Google Scholar] [CrossRef] [PubMed]
- Adkins, E.A.; Yolton, K.; Strawn, J.R.; Lippert, F.; Ryan, P.H.; Brunst, K.J. Fluoride exposure during early adolescence and its association with internalizing symptoms. Environ. Res. 2021, 204, 112296. [Google Scholar] [CrossRef]
- Mullenix, P.J.; Denbesten, P.K.; Schunior, A.; Kernan, W.J. Neurotoxicity of sodium fluoride in rats. Neurotoxicology Teratol. 1995, 17, 169–177. [Google Scholar] [CrossRef]
- Liu, F.; Ma, J.; Zhang, H.; Liu, P.; Liu, Y.-P.; Xing, B.; Dang, Y.-H. Fluoride exposure during development affects both cognition and emotion in mice. Physiol. Behav. 2014, 124, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bartos, M.; Gumilar, F.; Bras, C.; Gallegos, C.E.; Giannuzzi, L.; Cancela, L.M.; Minetti, A. Neurobehavioural effects of exposure to fluoride in the earliest stages of rat development. Physiol. Behav. 2015, 147, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.K.M.; Aragão, W.A.B.; Bittencourt, L.O.; Puty, B.; Dionizio, A.; de Souza, M.P.C.; Buzalaf, M.A.R.; de Oliveira, E.H.; Crespo-Lopez, M.E.; Lima, R.R. Fluoride exposure during pregnancy and lactation triggers oxidative stress and molecular changes in hippocampus of offspring rats. Ecotoxicol. Environ. Saf. 2021, 208, 111437. [Google Scholar] [CrossRef]
- Dec, K.; Łukomska, A.; Skonieczna-Żydecka, K.; Jakubczyk, K.; Tarnowski, M.; Lubkowska, A.; Baranowska-Bosiacka, I.; Styburski, D.; Skórka-Majewicz, M.; Maciejewska, D.; et al. Chronic Exposure to Fluoride Affects GSH Level and NOX4 Expression in Rat Model of This Element of Neurotoxicity. Biomolecules 2020, 10, 422. [Google Scholar] [CrossRef] [PubMed]
- Bera, I.; Sabatini, R.; Auteri, P.; Flace, P.; Sisto, G.; Montagnani, M.; Potenza, M.A.; Marasciulo, F.L.; Carratu, M.R.; Coluccia, A.; et al. Neurofunctional effects of developmental sodium fluoride exposure in rats. Eur. Rev. Med. Pharmacol. Sci. 2007, 11, 211–224. [Google Scholar] [PubMed]
- Li, X.; Zhang, J.; Niu, R.; Manthari, R.K.; Yang, K.; Wang, J. Effect of fluoride exposure on anxiety- and depression-like behavior in mouse. Chemosphere 2019, 215, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Zhang, Y.; Trivedi, A.; Jiang, X.; Chandra, D.; Zheng, J.; Nakano, Y.; Uyghurturk, D.A.; Jalai, R.; Onur, S.G.; et al. Fluoride related changes in behavioral outcomes may relate to increased serotonin. Physiol. Behav. 2019, 206, 76–83. [Google Scholar] [CrossRef]
- Young, S.N. How to increase serotonin in the human brain without drugs. J. Psychiatry. Neurosci. 2007, 32, 394–399. [Google Scholar]
- Baldwin, D.; Rudge, S. The role of serotonin in depression and anxiety. Int. Clin. Psychopharmacol. 1995, 9, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.N. Neuropharmacologic specificity of a simple animal model for the behavioral actions of benzodiazepines. Pharmacol. Biochem. Behav. 1981, 15, 695–699. [Google Scholar] [CrossRef]
- File, S.E. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav. Brain Res. 2001, 125, 151–157. [Google Scholar] [CrossRef]
- Choi, A.L.; Grandjean, P.; Sun, G.; Zhang, Y. Developmental fluoride neurotoxicity: Choi et al. Respond. Environ. Health Perspect. 2013, 121, A70. [Google Scholar] [CrossRef]
- Sabour, S.; Ghorbani, Z. Developmental fluoride neurotoxicity: Clinical importance versus statistical significance. Environ. Health Perspect. 2013, 121, A70. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, K.; An, N.; Huang, H.; Duan, L.; Ma, J.; Ding, J.; He, T.; Zhu, J.; Li, Z.; Cheng, X.; et al. Fluoride exposure and intelligence in school-age children: Evidence from different windows of exposure susceptibility. BMC Public Health 2020, 20, 1657. [Google Scholar] [CrossRef]
- Riddell, J.K.; Malin, A.J.; Flora, D.; McCague, H.; Till, C. Association of water fluoride and urinary fluoride concentrations with attention deficit hyperactivity disorder in Canadian youth. Environ. Int. 2019, 133, 105190. [Google Scholar] [CrossRef] [PubMed]
- Bashash, M.; Marchand, M.; Hu, H.; Till, C.; Martinez-Mier, E.A.; Sanchez, B.N.; Basu, N.; Peterson, K.E.; Green, R.; Schnaas, L.; et al. Prenatal fluoride exposure and attention deficit hyperactivity disorder (ADHD) symptoms in children at 6–12 years of age in Mexico City. Environ. Int. 2018, 121, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Lanphear, B.; Hornung, R.; Flora, D.; Martinez-Mier, E.A.; Neufeld, R.; Ayotte, P.; Muckle, G.; Till, C. Association Between Maternal Fluoride Exposure During Pregnancy and IQ Scores in Offspring in Canada. JAMA Pediatr. 2019, 173, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Cantoral, A.; Téllez-Rojo, M.M.; Malin, A.J.; Schnaas, L.; Osorio-Valencia, E.; Mercado, A.; Martínez-Mier, E. Ángeles; Wright, R.O.; Till, C. Dietary fluoride intake during pregnancy and neurodevelopment in toddlers: A prospective study in the progress cohort. Neurotoxicology 2021, 87, 86–93. [Google Scholar] [CrossRef]
- Malin, A.J.; Till, C. Exposure to fluoridated water and attention deficit hyperactivity disorder prevalence among children and adolescents in the United States: An ecological association. Environ. Health 2015, 14, 17. [Google Scholar] [CrossRef]
- Dec, K.; Łukomska, A.; Skonieczna-Żydecka, K.; Kolasa-Wołosiuk, A.; Tarnowski, M.; Baranowska-Bosiacka, I.; Gutowska, I. Long-term exposure to fluoride as a factor promoting changes in the expression and activity of cyclooxygenases (COX1 and COX2) in various rat brain structures. NeuroToxicology 2019, 74, 81–90. [Google Scholar] [CrossRef]
- Jiang, P.; Li, G.; Zhou, X.; Wang, C.; Qiao, Y.; Liao, D.; Shi, D. Chronic fluoride exposure induces neuronal apoptosis and impairs neurogenesis and synaptic plasticity: Role of GSK-3β/β-catenin pathway. Chemosphere 2019, 214, 430–435. [Google Scholar] [CrossRef]
- Daviu, N.; Bruchas, M.R.; Moghaddam, B.; Sandi, C.; Beyeler, A. Neurobiological links between stress and anxiety. Neurobiol. Stress 2019, 11, 100191. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Liu, Y.; Liu, S.; Cao, S.; Wang, F.; Wang, Z.; Xi, S. Fluoride-Induced Neuron Apoptosis and Expressions of Inflammatory Factors by Activating Microglia in Rat Brain. Mol. Neurobiol. 2016, 53, 4449–4460. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Zhang, Q.; Liu, Y.; Han, L.; Wang, Q.; Chen, P.; Zhang, S.; Wang, A.; Zhou, X. Fluoride induces apoptosis via inhibiting SIRT1 activity to activate mitochondrial p53 pathway in human neuroblastoma SH-SY5Y cells. Toxicol. Appl. Pharmacol. 2018, 347, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Bashash, M.; Thomas, D.; Hu, H.; Martinez-Mier, E.A.; Sanchez, B.N.; Basu, N.; Peterson, K.E.; Ettinger, A.S.; Wright, R.; Zhang, Z.; et al. Prenatal fluoride exposure and cognitive outcomes in children at 4 and 6–12 years of age in Mexico. Environ. Health Perspect. 2017, 125, 097017. [Google Scholar] [CrossRef] [PubMed]
- Whitford, G.M.; Sampaio, F.C.; Pinto, C.S.; Maria, A.G.; Cardoso, V.E.S.; Buzalaf, M.a.R. Pharmacokinetics of ingested fluoride: Lack of effect of chemical compound. Arch. Oral. Biol. 2008, 53, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.R. Low Prenatal Exposures to Fluoride: Are There Neurotoxic Risks for Children? Env. Health Perspect. 2017, 125, 104002. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program (NTP). NTP Research Report on Systematic Literature Review on the Effects of Fluoride on Learning and Memory in Animal Studies; National Toxicology Program: Research Triangle Park, NC, USA, 2016. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Review of the Revised NTP Monograph on the Systematic Review of Fluoride Exposure and Neurodevelopmental and Cognitive Health Effects: A Letter Report; The National Academies Press: Washington, DC, USA, 2021. [Google Scholar] [CrossRef]
- Committee to Review the NTP Monograph on the Systematic Review of Fluoride, Exposure and Neurodevelopmental and Cognitive Health Effects, Board on Environmental Studies and Toxicology, Division on Earth and Life Studies, and National Academies of Sciences, Engineering, and Medicine. In Review of the Draft NTP Monograph: Systematic Review of Fluoride Exposure and Neurodevelopmental and Cognitive Health Effects; National Academies Press: Washington, DC, USA, 2020; p. 25715. [CrossRef]
- NASEM Strongly Recommends Revisions to NTP’s Second Draft Monograph on Fluoride Exposure Hazards. Available online: https://www.agd.org/constituent/news/2021/02/18/nasem-strongly-recommends-revisions-to-ntp-s-second-draft-monograph-on-fluoride-exposure-hazards (accessed on 29 November 2021).
- Seddek, A.-L.; Ghallab, A. Fluoride: No evidence of developmental neurotoxicity due to current exposure levels in Europe. Arch. Toxicol. 2020, 94, 2543–2544. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adkins, E.A.; Brunst, K.J. Impacts of Fluoride Neurotoxicity and Mitochondrial Dysfunction on Cognition and Mental Health: A Literature Review. Int. J. Environ. Res. Public Health 2021, 18, 12884. https://doi.org/10.3390/ijerph182412884
Adkins EA, Brunst KJ. Impacts of Fluoride Neurotoxicity and Mitochondrial Dysfunction on Cognition and Mental Health: A Literature Review. International Journal of Environmental Research and Public Health. 2021; 18(24):12884. https://doi.org/10.3390/ijerph182412884
Chicago/Turabian StyleAdkins, Emily A., and Kelly J. Brunst. 2021. "Impacts of Fluoride Neurotoxicity and Mitochondrial Dysfunction on Cognition and Mental Health: A Literature Review" International Journal of Environmental Research and Public Health 18, no. 24: 12884. https://doi.org/10.3390/ijerph182412884
APA StyleAdkins, E. A., & Brunst, K. J. (2021). Impacts of Fluoride Neurotoxicity and Mitochondrial Dysfunction on Cognition and Mental Health: A Literature Review. International Journal of Environmental Research and Public Health, 18(24), 12884. https://doi.org/10.3390/ijerph182412884





