Abstract
The COVID-19 pandemic has had critical consequences for cancer care delivery, including altered treatment protocols and delayed services that may affect patients’ quality of life and long-term survival. Breast cancer patients from minoritized racial and ethnic groups already experience worse outcomes, which may have been exacerbated by treatment delays and social determinants of health (SDoH). This protocol details a mixed-methods study aimed at comparing cancer care disruption among a diverse sample of women (non-Hispanic White, non-Hispanic Black/African American, and Hispanic/Latina) and assessing how proximal, intermediate, and distal SDoH differentially contribute to care continuity and health-related quality of life. An embedded mixed-methods design will be implemented. Eligible participants will complete an online survey, followed by a semi-structured interview (with a subset of participants) to further understand factors that influence continuity of care, treatment decision-making, and self-reported engagement. The study will identify potentially modifiable factors to inform future models of care delivery and improve care transitions. These data will provide the necessary evidence to inform whether a subsequent, multilevel intervention is warranted to improve quality of care delivery in the COVID-19 aftermath. Additionally, results can be used to identify ways to leverage existing social resources to help manage and support patients’ outcomes.
1. Introduction
1.1. COVID-19 Is a Health Disparities Issue
The outbreak of Coronavirus Disease 2019 (COVID-19) has affected 230 million people worldwide, with 42 million cases registered in the USA and approximately 688,000 deaths as of summer 2021 [1,2]. COVID-19 disproportionately burdens minoritized racial and ethnic minorities [3], such as Black/African American and Hispanic/Latinx adults who have higher rates of COVID-19 infection than their non-Hispanic White (NHW) counterparts [3,4,5,6]. These minority groups are also overrepresented among hospitalized patients with the disease [7] and face proportionally higher mortality rates [4,8,9]. This is at least partially attributable to the exacerbation of pre-existing racial and health inequalities associated with social determinants of health (SDoH) [10,11,12], and rising rates of unemployment and loss of medical insurance [13,14,15] that have a greater effect on minority groups’ financial resources [16,17,18]. These same groups have lower incomes and higher proportions of poverty [19], public insurance or being uninsured [20,21] compared to NHWs.
Racial and ethnic minority groups share the unequal burden of being more likely to be low-income essential workers [22] and unemployed [23,24], forcing these populations to make economic cutbacks during the pandemic [22], which induces elevated stress and anxiety [22]. Residential racial segregation has been also consistently linked with a variety of adverse health outcomes, underlying health conditions, and affects minoritized groups more than NHWs [25,26,27]. Access to health services is contingent upon insurance and user-pay systems, which may disproportionately negatively affect those from minority groups [28]. Additionally, these groups may not have the health literacy skills necessary to fully respond to pandemic messaging [29] or to evaluate true versus misinformation on the virus. Consequently, racial and ethnic minority groups are likely to experience reduced life expectancies that are three to four times larger than in NHWs during the COVID-19 pandemic [30]. In sum, COVID-19 disparities in incidence and mortality should be situated in the context of multiple factors that affect health-related outcomes, such as material resources deprivation, access to social networks, and chronic stress due to structural discrimination [12].
1.2. COVID-19 and Cancer Care Delivery
Cancer patients have an increased risk of contracting COVID-19 [7,31], being hospitalized [7,31], and succumbing to the virus [32]. This vulnerability is due to being immunocompromised as a result of the malignancy itself and systemic anticancer therapies, resulting in higher susceptibility to severe infections and complications [32,33,34,35,36]. Thus, their long-term survival may be particularly affected by delays in surgery and administration of anticancer therapies [33,34,35,36]. Breast cancer patients in active treatments, compared to other groups, are the most likely to be infected with COVID-19 [7]. Notably, Black/African American breast cancer survivors are more likely to be diagnosed with COVID-19 compared to their NHW counterparts; the largest racial disparity for infection compared to other cancers [7].
The pandemic has had critical consequences for cancer care delivery [33,34,35,36,37,38,39,40,41,42,43,44,45,46] including a reduction in cancer screenings [47], diagnoses [47,48], and surgeries [47]. Recommendations and guidelines for triage, prioritization and altered treatment regimens [49,50,51,52,53,54] have also contributed to modifications to care protocols and the transition to tele-medicine services [8,13,35,36,37,38,39,40,41,47,48]. However, this adaptation of treatment pathways can have long-term implications for the timely detection of disease progression and complications that may affect cancer outcomes [13,39,40,55]. In May 2020, 79% of cancer patients in active treatment experienced some delay in their health care because of COVID-19 [56]; which may affect long-term survival because of suboptimal or delayed care [8,13,39,40,41,55,57,58,59]. Together, these trends can exacerbate pre-existing disparities in cancer morbidity and mortality experiences by Black/African American and Hispanic/Latinx adults [47,60,61,62].
In terms of breast cancer care, the pandemic has led to reductions in screening/diagnostic mammography [47,63,64], diagnoses [47,48,65], surgeries [47,66] including breast reconstruction [66,67] and genetic counseling/testing [63,64]. The reduction in the number of biopsies (71%) and diagnoses (51.8%) is higher in breast cancer compared to other cancers [47,48]. Additionally, registered breast cancer treatment delays [64,65,68,69,70,71,72], may be due to fear of COVID-19 infections [66,69,70,71,72,73]. Disruptions to oncology services negatively impact emotional well-being, anxiety and depression experienced by breast cancer patients, which have been associated with greater emotional vulnerability and poor cognitive function [68,69]. Breast cancer patients from racial and ethnic minority groups already experience significantly more cancer treatment delay [74,75,76,77,78], which may exacerbate COVID-19 related treatment delays and psychosocial outcomes in these groups [68,77].
1.3. Examining Breast Cancer Care and Minority Women’s Outcomes in the Context of Social Determinants of Health Is Necessary to Fully Understand COVID-19’s Impact on Health Disparities
The disproportionate burden of breast cancer is well documented, with Black/African American and Hispanic/Latina women reporting worse health outcomes than their NHW counterpart [79,80,81,82,83,84,85,86,87,88,89,90]. For instance, in Texas, where breast cancer is the leading cancer diagnosis, mortality is 44% higher for Black/African American than NHW women [79,91]. Disparities exist in incidence, mortality, and importantly, survivorship care [79,80,81,82,83,84,85,86,87,88,89,90]. Traditional explanations have included biologic differences in tumor characteristics [81], late-stage diagnosis due to lack of cancer screening [91,92,93], and socioeconomic factors [83,94]. However, a growing body of literature suggests that differences in selection and adherence to recommended treatments may play a major role in the maintenance of disparities in breast cancer outcomes [83,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109]. In the current oncologic practice, where timely engagement with treatment is crucial to prevent recurrence and reduce mortality [89,90], differences in treatment initiation and adherence to treatment have become increasingly relevant given the present recommendations for triage and modified delivery of cancer care [75,95,96,97,98,99]. Black/African American women are four times more likely to experience treatment delays and less likely to receive cancer-directed surgery; additionally, Black/African American and Hispanic/Latina women also fail to receive definitive local therapy, chemotherapy, and radiotherapy [99,100,101]. Minority breast cancer patients are disproportionally characterized by non-initiation, discontinuation, and non-adherence to adjuvant endocrine therapy [98,99,100,101,102,103,104], which leads to lower survival, shorter time to recurrence, increased medical costs, and lower quality of life [87,98,99,100,101,102,103,104,105]. Key determinants of disparities range from proximal factors (socio-demographic variables), to intermediate (social network characteristics), and distal factors (access to resources; healthcare system characteristics, and policy) [88,89,90,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109]. Although multiple levels of contextual influences affect behavior, currently available interventions to alleviate breast cancer disparities fail to comprehensively address these SDoH, or the interplay between them [106]. For minoritized racial and ethnic women receiving treatment for breast cancer, the effects of SDoH coupled with COVID-19 altered treatment protocols [54,110,111,112] may exacerbate the existing disproportionate burden of the disease [80,81,82,83,95,105,106]. More research is therefore needed to understand these potential effects and how to address them to achieve breast cancer health equity.
1.4. The Current Protocol
The present paper describes an NCI-funded mixed-methods study protocol investigating the impact the COVID-19 pandemic has on the receipt of optimal breast cancer care among a diverse sample of women (non-Hispanic Black/African American, Hispanic/Latina, and NHW). For the purpose of this investigation, groups were defined according to the classification from the U.S. Office of Management and Budget (OMB), which is used for the 2020 Census categorization of race and ethnicity. Individuals who identify with the ethnicity of Hispanic/Latinx may be of any race. This research project will fill a significant gap in current understanding of the long-term implications of the pandemic on cancer disparities by assessing differential rates of cancer care disruption and health-related quality of life among non-Hispanic Black/African American and Hispanic/Latina women (relative to non-Hispanic White women) diagnosed with early-stage breast cancer. The study will also examine how proximal, intermediate, and distal SDoH differentially contribute to these outcomes to inform future interventions able to sustain equitable models of care delivery.
2. Materials and Methods
2.1. Study Design
The study utilizes an embedded mixed methods design [113], chosen for the purpose of complementarity, in which the addition of qualitative data is used to enhance or elaborate upon the results of quantitative analyses. Each component will be used to capture overlapping, but distinct aspects of participants’ experiences [113], i.e., quantitative data will include psychosocial outcome measures, while qualitative data will focus on capturing the context of care such as women’s personal experiences of barriers and processes in accessing care. In this embedded design, quantitative and qualitative data will be collected concurrently, in which the second qualitative strand builds on the first quantitative strand and the qualitative interview participants are selected from among the survey respondents, integrating strands on the methods level through connection via the sampling frame [114]. Integration will also occur during the interpretation and reporting stages where the analyses from the two strands are integrated and compared through use of a joint display table. Integration will be achieved through using qualitative findings to elaborate or enhance upon quantitative results, yielding a more comprehensive view of participants’ experiences and barriers to care.
Study procedures will occur in two virtual “visits.” In the first visit, eligible patients complete an online survey assessing proximal, intermediate, and distal factors affecting care continuity and quality of life. In the second visit, a semi-structured interview is conducted with a selection of self-referred women to assess factors that influenced their access to and continuity of care.
Funding for the current protocol was obtained from the National Cancer Institute of the National Institute of Health (3P20CA221697-04S1), supported by grants P20CA221696 (to Lorna H. McNeill) and P20CA221697 (to Lorraine R. Reitzel).
2.1.1. Conceptual Framework
The conceptual framework of the study is based on the National Institute on Minority Health and Health Disparities (NIMHD) Research Framework [115,116] and the Centers for Population Health and Health Disparities (CPHHDs) [117,118] multilevel model, which are grounded in socio-ecological theory [119]. As outlined in Figure 1, the study targets (1) proximal, (2) intermediate, and (3) distal determinants of health disparities to identify which of them differentially influences cancer care receipt, patient-reported outcomes, and the interplay between them. Distal determinants include policies and organization/practice settings that affect the availability, receipt of, and quality of health care [115,117]. Intermediate determinants include social context, physical environment, and social relationships [115,117,120,121]. Social relationships refer to social networks, which are forms of social capital that suppress the negative effects of impoverished social environments. These negative effects can, in the absence of such networks, be increased by social isolation [122,123,124,125,126,127,128]. The physical environment includes availability and accessibility of local health care resources; transportation, quality air and water, healthy food; presence of crime; and neighborhood characteristics [129,130]. Finally, proximal determinants are embedded in the individual and include socioeconomic status, race/ethnicity, gender identity, and cultural beliefs; they also include engagement in risk behaviors [115,116,118]. The conceptual model of the study is informed by the taxonomy proposed by Taplin and Rodgers [131] about factors influencing the quality of cancer care and ultimately cancer-related health outcomes. This model also integrates recent evidence-based recommendations for the development of multilevel interventions to address racial/ethnic disparities [132,133,134].
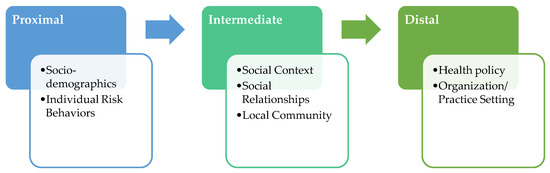
Figure 1.
Conceptual model of the study.
2.1.2. Study Aims and Hypotheses
Specific aims are to:
- Identify and compare rates of disruption in cancer care due to the COVID-19 pandemic and health-related quality of life among non-Hispanic Black/African American, Hispanic/Latina, and non-Hispanic White women diagnosed with early-stage breast cancer.
- Examine how proximal, intermediate, and distant determinants differentially predict cancer patients’ disruption of care and health-related quality of life during and after the acute phase of COVID-19 pandemic.
- Identify barriers and facilitators of cancer care receipt and health-related quality of life among participants who report high vs. low rates of cancer care disruptions during the COVID-19 pandemic.
It was hypothesized that: (1) differences in cancer care disruption and health-related quality of life will exist among non-Hispanic Black/African American, Hispanic/Latina, and non-Hispanic White women diagnosed with early-stage breast cancer; and that (2) higher rates of disruptions and more affected health-related quality of life will be experienced by participants who identify as non-Hispanic Black/African American or Hispanic/Latina, those with lower socioeconomic status, no insurance, and those reporting greater distress and more negative coping approaches. In addition, it was hypothesized that (3) higher rates of disruptions and more affected quality of life will be experienced by participants with lower social support and reduced social network size. Finally, (4) worse outcomes were expected to be reported by participants receiving care in institutions lacking patient navigation and psychoeducation, access to tele-medicine services, and limited community level resources.
2.1.3. Recruitment and Study Setting
Community-engaged approaches to survey development and recruitment strategies guide the research project. The investigative team presented the study materials (flyers, scripts and survey instruments) to a local Community Research Advisory Board (see: https://www.healthrcmi.com/crab, accessed on 29 November 2021) and an External Advisory Board (see: https://www.uhandpartnership.com/external-advisory-board, accessed on 29 November 2021), eliciting community-scientists feedback and making modifications accordingly. Participants will be recruited through a variety of strategies to maximize the likelihood of reaching the expected sample size, placing particular emphasis on equal representation among the three groups. Breast cancer survivors will be recruited from local and national community-based organizations and advocacy groups for women’s health and breast cancer prevention, in collaboration with patient advocates and community health workers. In addition, recruitment scripts and flyers will be disseminated via social media, during breast cancer-related community events, and through postings on community bulletin boards in Black/Latinx communities and churches. Study recruitment efforts will be conducted also in target clinic-sites serving women with breast cancer from minority groups, including targeted emails to participants of previous studies who have expressed interest in being contacted for future research.
2.1.4. Participants
The target sample will comprise 120 breast cancer patients equally divided in groups of 40 non-Hispanic Black/African American, 40 Hispanic/Latina and 40 NHW women. Additionally, purposive sampling will be used to select a subset of 30 women, stratified by race/ethnicity and low vs. high rates of disruption in care, to complete an individual semi-structured interview to further understand factors influencing continuity of care and treatment decision-making. Initially, women will be selected for interviews on a first-come, first-served basis, with attention to ensuring equal representation by race/ethnicity until n = 30 is reached. Once data analysis has commenced, theoretical sampling will also guide selection of interviewees in keeping with grounded theory.
Inclusion criteria for the present study are:
- Self-identify as non-Hispanic Black/African American, Hispanic/Latina, or NHW woman;
- Having been diagnosed with early stage (I–III) breast cancer in January 2020 or later;
- Receiving care for breast cancer at time of enrollment;
- Being 18 years of age or older;
- Having access to a computer, smartphone, tablet, or other devices allowing the capability to complete internet-based survey and interview;
- Having the ability to speak and read English.
The exclusion criteria are:
- Having a cognitive impairment or severe mental illness;
- Being unable to consent;
- Being in prison/custody; or
- Being diagnosed with metastatic disease.
Potential participants are presented with a list of exclusionary criteria and asked to select out of the study if any inclusion criteria are not met or if any exclusion criteria are applicable.
2.2. Data Collection
2.2.1. Quantitative Questionnaire—Measures
Demographic/medical characteristics: Participants will be asked sociodemographic information including age, sex at birth, gender identity, race/ethnicity, marital status, duration of current relationship (if applicable), religious affiliation, number of children, occupational status, education, insurance status, income, and residential street address. Medical information will include cancer diagnosis (first diagnosis vs. recurrence), stage of the disease, time since diagnosis, and treatment type.
Proximal determinants: Psychological distress is assessed with the Perceived Stress Scale [135,136], consisting of 10 items that examine the degree to which situations in one’s life are appraised as stressful. Coping is measured with the Brief COPE [137], a multidimensional measure assessing 14 coping dimensions. The Brief COPE has been shown to be reliable, valid, and it has been extensively used with breast cancer patients [138,139,140,141]. The Cancer Behavior Inventory-Brief Version (CBI-B) [142], assesses respondents’ self-efficacy in managing the illness. Health literacy is measured with the single health literacy screening measure [143]. Individual risk factors such as smoking, alcohol and substance use, physical activity, and diet (cups of fruits and vegetables consumed) will be assessed with single items [144]. Depression and anxiety will be assessed with the Patient Health Questionnaire-8 [145,146,147] and the GAD-7 [148].
Intermediate determinants: Social support will be measured with the MOS Social Support Scale [149], a 19-item questionnaire assessing tangible, emotional/informational, affectionate support, and positive social interaction. The PROMIS Social Isolation Scale [150,151,152], 4 items, will assess social isolation. In addition, the Social Network Index (12 items) [153] will examine characteristics of the social network of the individual. Emotional intimacy with a partner will be measured with six items from the Personal Assessment of Intimacy in Relationships (PAIR) Inventory [154]. Local community level resources and characteristics will be examined through GIS data based on geocoding each participant’s residential address [155]. Participants also complete two five-item brief measures of Social Cohesion and Trust and Informal Social Control [156], as well as a 10-item measure of Neighborhood Problems [157].
Distal determinants: Participants will complete questions, developed by the investigators, about the healthcare setting where they have been receiving care and will be invited to report whether patient navigation, education and supportive services are offered, and whether these services have transitioned to telehealth because of the pandemic. Additionally, women’s experience with health care services and medical mistrust will be investigated with Short-Form Patient Satisfaction Questionnaire (PSQ-18) [158], and the Group-Based Medical Mistrust Scale [159], a 12-item instrument designed to assess race-based medical mistrust from health care systems/professionals and the treatment provided to individuals of the patient’s ethnic or racial group.
Disruption in Cancer Care: Cancer care disruptions will be measured with a series of questions investigating the impact of the COVID-19 outbreak on access to health care services (such as the type of health care services that have been impacted and the reason for the change in care). Questions have been adapted from the ACS CAN COVID-19 Impact on Cancer Patients and Survivors survey with permission [160].
Health-Related Quality of Life: Health-related quality of life will be assessed with the Functional Assessment of Cancer Therapy-Breast (FACT-B) Scale [161], a 37-item instrument measuring physical, social/family, emotional, and functional well-being, along with breast cancer-specific concerns. In addition, four items from the Behavioral Risk Factor Surveillance System survey [162] have been included. Items assess (a) self-rated health, (b) poor physical health days, (c) poor mental health days, and (d) activity limited days due to poor physical or mental health. Self-rated health is assessed with a single item with which participants rate their health on a 5-point scale from excellent (1) to poor (5). Finally, participants will be invited to report the number of days in the previous 30 days in which poor physical or mental health limited their ability to perform usual activities [162].
2.2.2. Qualitative Interviews
At the end of the online survey, participants will be invited to express their interest in participating in a qualitative interview (n = 30). The goal of the interview is to assess factors that influence women’s access to and continuity of care, treatment decision-making, self-reported engagement in the process of care, and overall satisfaction. A semi-structured topic guide will be used to focus interviews; however, it will remain open and flexible to change to respond to participants’ experiences and concerns. In addition, women will be asked about how their social networks facilitated or impaired their cancer treatment management. Those who express interest will be contacted by the research team via e-mail/phone. Members of the research team will share information about the topics covered in the interviews, how interviews will be conducted and how data will be collected. Participants will be orally consented at the beginning of the interview. Instructions will be provided to participants to access the HIPAA—compliant online platform selected for the interview. Online interviews were chosen to reduce the risk of exposure to COVID-19.
2.3. Analysis
2.3.1. Sample Size Calculation
Power and sample size requirements were based on (1) Aim 1: one-way analysis of variance (ANOVA) with one between subjects factor (i.e., three racial/ethnic groups), and (2) Aim 2: regression analyses with maximum of eight predictors when examining the effect of differential factors (e.g., proximal, intermediate, distal, and their sub measures). Power analysis showed that there is 80% power to detect a moderate (0.25) to large (0.40) effect size of 0.287 (f) for Aim 1 and 0.067 (f2) for Aim 2 [163]. Given one between subjects factor design (Aim 1) and regression analyses (Aim 2), two-sided significant level, an alpha of 0.05, and 120 participants (40 participant in each racial/ethnic group). Additionally, with the sample size of 120, it will be possible to detect as small as w of 0.28 for chi-square tests, and correlation of |r| of 0.25 or larger when assessing the correlation between two continuous variables.
2.3.2. Data Analysis
Descriptive analyses will be computed to characterize the distributional nature of all variables. ANCOVAs controlling for covariates (e.g., age) will explore mean differences between racial/ethnic groups, whereas chi-square tests will compare participants and those who withdrew on sociodemographic demographic and medical factors. Adjusted means of outcomes of interest for each racial/ethnic group will be reported and pairwise comparisons will be conducted when significant group differences are found. In addition, the association between proximal, intermediate, and distant factors, cancer care disruptions, and health-related quality of life will be examined with bivariate correlations. Then, a series of hierarchical multiple regression analysis will investigate the contribution of the predictors on these outcomes in two steps where variables will be sequentially added and retained: (1) participant characteristics (e.g., age and race/ethnicity); and (2) the addition of proximal, intermediate, or distant factors (step 2). The increase in total explained outcome variance (∆R2) will be examined. A final model including significant predictors from hierarchical multiple regression analyses will be examined. To investigate whether the model differs by race, an interaction term of race and the identified significant predictors will be introduced. Post hoc analyses will be conducted if significant interaction effect is found. p-values < 0.05 will be considered statistically significant. Statistical analyses will be performed using SAS 9.4 [164].
2.3.3. Qualitative Analysis
Interview recordings will be transcribed verbatim. Barriers and facilitators experienced by women who report high vs. low rates of cancer care disruption across the three groups will be assessed using a grounded theory approach [165]. The constant comparison method will be used to distinguish similarities and differences across individual transcripts. Iterative data collection and analysis will be used, such that emerging results will guide subsequent data collection and refining of analytic concepts [166]. Researcher triangulation will ensure rigor in data analysis, in which two or more researchers will independently code transcripts, and meet to discuss any discrepancies in coding until agreement is reached. Transcripts of the semi-structured interviews (n = 30) will be analyzed completing three phases of coding to identify the emerging theoretical framework. The first phase, (open coding), entails intense line-by-line coding of early data to explore the dimensions and properties of emerging concepts and key processes. Subsequently, these initial codes and concepts will be condensed and organized around a single coding category (axial coding) [165]. They will be compared until a condition of theoretical saturation is reached and no new codes are identified. Finally, information will be arranged in a diagram (selective coding) synthesizing the emerging theoretical model [165,166,167]. Codes will be inductively derived from the data rather than being determined a priori. Atlas.ti software (ATLAS.ti Scientific Software Development GmbH, Berlin, Germany) will be used to manage and organize qualitative data [168].
3. Discussion
Despite growing evidence that supports adverse consequences of COVID-19 on clinical and psychosocial outcomes of cancer patients, a limited number of studies have investigated cancer care disruption and patient-reported outcomes among racial and ethnic minoritized women diagnosed with breast cancer. Given the disproportionate burden of the disease, assessing the impact of the pandemic and associated SDoH among a tri-racial and ethnic sample is critical to inform best-practices for future models of care delivery and to improve care transitions [169,170,171,172]. Specifically, these data will provide the necessary evidence to inform whether a subsequent, multilevel intervention addressing these factors is warranted to improve quality of care delivery during and after the COVID-19 pandemic. The present work will yield information about “at risk” patients that can be used to alert healthcare professionals about patient-level factors to consider when creating plans to improve care transitions. The study will also identify ways to leverage existing social resources (i.e., family caregivers, social network members, community agencies/organizations) to help manage and support patients’ outcomes.
4. Conclusions
This study will add to the cancer health disparities evidence base about the: (1) impact of COVID-19 pandemic on cancer care among non-Hispanic Black/African American and Hispanic/Latina women, relative to non-Hispanic White breast cancer survivors in the U.S., and (2) the differential influence of proximal, intermediate, and distal factors on cancer care receipt and health-related quality of life of women—and specifically vulnerable groups—undergoing treatment for breast cancer during a public health crisis. Ultimately, we seek to mitigate disparities in care and outcomes for women whose health may be disproportionately affected by the effects of the pandemic and its sequelae.
Author Contributions
Conceptualization, C.A. and L.R.R.; methodology, C.A., L.R.R., T.A.C., I.M.L.; software, T.A.C., I.M.L.; formal analysis, T.A.C., I.M.L., A.R., C.A.; investigation, C.A., L.R.R., T.A.C., I.M.L. and S.K.C.; data curation, T.A.C., I.M.L.; writing—original draft preparation, C.A.; writing—review and editing, C.A., L.R.R., L.H.M., S.K.C., T.A.C., I.M.L., A.A.H., A.R., S.R.; project administration, C.A., L.R.R. and A.A.H.; funding acquisition, L.R.R. and C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Cancer Institute of the National Institutes of Health, through grants P20CA221697-04S1 (to Chiara Acquati), supported by grants P20CA221697 (to Lorraine R. Reitzel) and P20CA221696 (to Lorna H. McNeill). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the sponsoring organization.
Institutional Review Board Statement
The study protocol has received ethical and governance approvals from the University of Houston, Institutional Review Board, ref. number: STUDY00002665.
Informed Consent Statement
Informed consent will be obtained from all subjects involved in the study, for both quantitative and qualitative study components. All participants are free to withdraw at any time.
Data Availability Statement
Not applicable.
Acknowledgments
Recruitment is supported by the leading investigators and research staff within the UHAND (University of Houston/MD Anderson) Partnership to Eliminate Cancer Disparities, and the Health Research Institute and the Center for Addictions Research and Cancer Prevention (U54MD015946) at the University of Houston, as well as community partners. We thank the External Advisory Board of the UHAND Program (P20CA221697/ P20CA221696) for input into study design and development. The study investigators are supplemented by an External Advisory Board comprising national experts who provide direction to the underlying research, education, and training grant. For more information, please see Haq, A.A.; Reitzel, L.R.; Chen, T.A.; Chang, S.; Escoto, K.H.; Solari Williams, K.D.; Roberson, C.; Koshy, L.; McNeill, L.H. “UHAND”- A National Cancer Institute Funded Partnership to Advance Cancer Health Equity through Scholar Training. Int. J. Environ. Res. Public Health 2021, 18, 5054. https://doi.org/10.3390/ijerph18105054, accessed on 23 June 2021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Johns Hopkins Coronavirus Resource Center. COVID-19 Map. Available online: https://coronavirus.jhu.edu/map.html (accessed on 26 September 2021).
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 26 September 2021).
- Boserup, B.; McKenney, M.; Elkbuli, A. Disproportionate impact of COVID-19 pandemic on racial and ethnic minorities. Am Surg. 2020, 86, 1615–1622. [Google Scholar] [CrossRef]
- Tirupathi, R.; Muradova, V.; Shekhar, R.; Salim, S.A.; Al-Tawfiq, J.A.; Palabindala, V. COVID-19 disparity among racial and ethnic minorities in the US: A cross sectional analysis. Travel Med. Infect. Dis. 2020, 38, 101904. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.L.; Bakouny, Z.; Bhalla, S.; Steinharter, J.A.; Tremblay, D.A.; Awad, M.M.; Kessler, A.J.; Haddad, R.I.; Evans, M.; Busser, F. Cancer care disparities during the COVID-19 pandemic: COVID-19 and cancer outcomes study. Cancer Cell 2020, 38, 769–770. [Google Scholar] [CrossRef] [PubMed]
- Kullar, R.; Marcelin, J.R.; Swartz, T.H.; Piggott, D.A.; Macias Gil, R.; Mathew, T.A.; Tan, T. Racial disparity of Coronavirus Disease 2019 in African American communities. J. Infect. Dis. 2020, 222, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Berger, N.A.; Xu, R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021, 7, 220–227. [Google Scholar] [CrossRef]
- Viale, G.; Licata, L.; Sica, L.; Zambelli, S.; Zucchinelli, P.; Rognone, A.; Aldrighetti, D.; Di Micco, R.; Zuber, V.; Pasetti, M.; et al. Personalized risk-benefit ratio adaptation of breast cancer care at the epicenter of COVID-19 outbreak. Oncologist 2020, 25, e1013–e1020. [Google Scholar] [CrossRef] [PubMed]
- Price-Haywood, E.G.; Burton, J.; Fort, D.; Seoane, L. Hospitalization and mortality among Black patients and White patients with COVID-19. N. Engl. J. Med. 2020, 382, 2534–2543. [Google Scholar] [CrossRef] [PubMed]
- Milam, A.J.; Furr-Holden, D.; Edwards-Johnson, J.; Webb, B.; Patton, J.W.; Ezekwemba, N.C.; Porter, L.; Davis, T.; Chukwurah, M.; Webb, A.J.; et al. Are clinicians contributing to excess African American COVID-19 deaths? Unbeknownst to them, they may be. Health Equity 2020, 4, 139–141. [Google Scholar] [CrossRef]
- Van Dorn, A.; Cooney, R.E.; Sabin, M.L. COVID-19 exacerbating inequalities in the US. Lancet 2020, 395, 1243–1244. [Google Scholar] [CrossRef]
- Chowkwanyun, M.; Reed, A.L. Racial health disparities and Covid-19—Caution and context. N. Engl. J. Med. 2020, 383, 201–203. [Google Scholar] [CrossRef]
- Chan, A.; Ashbury, F.; Fitch, M.I.; Koczwara, B.; Chan, R.J. Cancer survivorship care during COVID-19—perspectives and recommendations from the MASCC Survivorship Study Group. Support Care Cancer 2020, 28, 3485–3488. [Google Scholar] [CrossRef] [PubMed]
- Poteat, T.; Millett, G.A.; Nelson, L.E.; Beyrer, C. Understanding COVID-19 risks and vulnerabilities among Black communities in America: The lethal force of syndemics. Ann. Epidemiol. 2020, 47, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bureau, Labor Statistics. Employment Situation News Release. Available online: https://www.bls.gov/news.release/archives/empsit_07022021.htm (accessed on 21 June 2021).
- Kayman, H.; Ablorh-Odjidja, A. Revisiting public health preparedness: Incorporating social justice principles into pandemic preparedness planning for influenza. J. Public Health Manag. Pract. 2006, 12, 373–380. [Google Scholar] [CrossRef]
- Hutchins, S.S.; Fiscella, K.; Levine, R.S.; Ompad, D.C.; McDonald, M. Protection of racial/ethnic minority populations during an influenza pandemic. Am. J. Public Health 2009, 99 (Suppl. S2), S261–S270. [Google Scholar] [CrossRef] [PubMed]
- Blumenshine, P.; Reingold, A.L.; Egerter, S.; Mockenhaupt, R.; Braveman, P.; Marks, J. Pandemic influenza planning in the United States from a health disparities perspective. Emerg Infect Dis. 2008, 14, 709. Available online: https://wwwnc.cdc.gov/eid/article/14/5/07-1301_article (accessed on 15 June 2021). [CrossRef] [PubMed]
- U.S. Census Bureau. Income and Poverty in the United States: 2019. Available online: https://www.census.gov/library/visualizations/2020/demo/p60-270.html (accessed on 15 June 2021).
- U.S. Census Bureau. Health Insurance Coverage in the United States: 2019. Available online: https://www.census.gov/library/publications/2020/demo/p60-271.html (accessed on 15 June 2021).
- U.S. Census Bureau. Measuring Household Experiences during the Coronavirus Pandemic. Available online: https://www.census.gov/householdpulsedata (accessed on 15 June 2021).
- Hibel, L.C.; Boyer, C.J.; Buhler-Wassmann, A.C.; Shaw, B.J. The psychological and economic toll of the COVID-19 pandemic on Latina mothers in primarily low-income essential worker families. Traumatology 2021, 27, 40–47. [Google Scholar] [CrossRef]
- Couch, K.A.; Fairlie, R.W.; Xu, H. Early evidence of the impacts of COVID-19 on minority unemployment. J. Public Econ. 2020, 192, 104287. [Google Scholar] [CrossRef]
- Gemelas, J.; Davison, J.; Keltner, C.; Ing, S. Inequities in employment by race, ethnicity, and sector during COVID-19. J. Racial Ethn. Health Disparities 2021, 15, 1–6. [Google Scholar] [CrossRef]
- Landrine, H.; Corral, I.; Lee, J.G.L.; Efird, J.T.; Hall, M.B.; Bess, J.J. Residential segregation and racial cancer disparities: A systematic review. J. Racial Ethn. Health Disparities 2017, 4, 1195–1205. [Google Scholar] [CrossRef]
- Pruitt, S.L.; Lee, S.J.C.; Tiro, J.A.; Xuan, L.; Ruiz, J.M.; Inrig, S. Residential racial segregation and mortality among Black, White, and Hispanic urban breast cancer patients in Texas, 1995 to 2009. Cancer 2015, 121, 1845–1855. [Google Scholar] [CrossRef] [Green Version]
- Jackson, S.A.; Anderson, R.T.; Johnson, N.J.; Sorlie, P.D. The relation of residential segregation to all-cause mortality: A study in Black and White. Am. J. Public Health 2000, 90, 615–617. [Google Scholar]
- Lagarde, M.; Palmer, N. The impact of user fees on access to health services in Low- and Middle-Income Countries. Cochrane Database Syst. Rev. 2011, 4, CD009094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan, E.; Tinker, T. Effective health risk communication about pandemic influenza for vulnerable populations. Am. J. Public Health 2009, 99 (Suppl. S2), S324–S332. [Google Scholar] [CrossRef]
- Andrasfay, T.; Goldman, N. Reductions in 2020 US life expectancy due to COVID-19 and the disproportionate impact on the Black and Latino populations. Proc. Natl. Acad. Sci. USA 2021, 118. Available online: https://www.pnas.org/content/118/5/e2014746118 (accessed on 16 June 2021). [CrossRef] [PubMed]
- Lee, K.A.; Ma, W.; Sikavi, D.R.; Drew, D.A.; Nguyen, L.H.; Bowyer, R.C.E.; Cardoso, M.J.; Fall, T.; Freidin, M.B.; Gomez, M.; et al. Cancer and risk of COVID-19 through a general community survey. Oncologist 2021, 26, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Goel, S.; Kabarriti, R.; Cole, D.; Goldfinger, M.; Acuna-Villaorduna, A.; Pradhan, K.; Thota, R.; Reissman, S.; Sparano, J.A.; et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020, 10, 935–941. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.Y.; Desai, A.; de Lima Lopes, G.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 20, 1907–1918. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020, 21, e181. [Google Scholar] [CrossRef]
- El-Shakankery, K.H.; Kefas, J.; Crusz, S.M. Caring for our cancer patients in the wake of COVID-19. Br. J. Cancer 2020, 123, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Shankar, A.; Saini, D.; Roy, S.; Mosavi Jarrahi, A.; Chakraborty, A.; Bharti, S.J.; Taghizadeh-Hesary, F. Cancer care delivery challenges amidst Coronavirus Disease-19 (COVID-19) outbreak: Specific precautions for cancer patients and cancer care providers to prevent spread. Asian Pac. J. Cancer Prev. 2020, 21, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Al-Shamsi, H.O.; Alhazzani, W.; Alhuraiji, A.; Coomes, E.A.; Chemaly, R.F.; Almuhanna, M.; Wolff, R.A.; Ibrahim, N.K.; Chua, M.; Hotte, S.J.; et al. A practical approach to the management of cancer patients during the novel Coronavirus Disease 2019 (COVID-19) pandemic: An international collaborative group. Oncologist 2020, 25, e936–e945. [Google Scholar] [CrossRef] [Green Version]
- Al-Quteimat, O.M.; Amer, A.M. The impact of the COVID-19 pandemic on cancer patients. Am. J. Clin. Oncol. 2020, 43, 452–455. [Google Scholar] [CrossRef]
- Richards, M.; Anderson, M.; Carter, P.; Ebert, B.L.; Mossialos, E. The impact of the COVID-19 pandemic on cancer care. Nat. Cancer 2020, 1, 565–567. [Google Scholar] [CrossRef]
- Raymond, E.; Thieblemont, C.; Alran, S.; Faivre, S. Impact of the COVID-19 outbreak on the management of patients with cancer. Target. Oncol. 2020, 15, 249–259. [Google Scholar] [CrossRef] [PubMed]
- van de Haar, J.; Hoes, L.R.; Coles, C.E.; Seamon, K.; Fröhling, S.; Jäger, D.; Valenza, F.; de Braud, F.; De Petris, L.; Bergh, J.; et al. Caring for patients with cancer in the COVID-19 era. Nat. Med. 2020, 26, 665–671. [Google Scholar] [CrossRef] [Green Version]
- COVID-19 More Frequent, Severe in Cancer Patients. Cancer Discov. 2020, 10, OF1. Available online: https://doi.org/10.1158/2159-8290.CD-NB2020-032 (accessed on 29 November 2021). [CrossRef]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Oh, W.K. COVID-19 infection in cancer patients: Early observations and unanswered questions. Ann. Oncol. 2020, 31, 838–839. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, F.; Xie, L.; Wang, C.; Wang, J.; Chen, R.; Jia, P.; Guan, H.Q.; Peng, L.; Chen, Y.; et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020, 31, 894–901. [Google Scholar] [CrossRef]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with cancer appear more vulnerable to SARS-CoV-2: A multicenter study during the COVID-19 Outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [PubMed]
- Patt, D.; Gordan, L.; Diaz, M.; Okon, T.; Grady, L.; Harmison, M.; Markward, N.; Sullivan, M.; Peng, J.; Zhou, A. Impact of COVID-19 on cancer care: How the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin. Cancer Inform. 2020, 4, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.W.; Chen, Z.; Niles, J.; Fesko, Y. Changes in the number of US patients with newly identified cancer before and during the Coronavirus Disease 2019 (COVID-19) pandemic. JAMA Netw. Open 2020, 3, e2017267. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7403918/ (accessed on 18 June 2021).
- ESMO. Cancer Patient Management During the COVID-19 Pandemic. Available online: https://www.esmo.org/guidelines/cancer-patient-management-during-the-COVID-19-pandemic (accessed on 22 June 2021).
- ASCO. COVID-19 Patient Care Information. Available online: https://www.asco.org/asco-coronavirus-information/care-individuals-cancer-during-covid-19 (accessed on 22 June 2021).
- NCCN. COVID-19 Resources. Available online: https://www.nccn.org/covid-19 (accessed on 22 June 2021).
- CDC. Coronavirus Disease (COVID-19). Centers for Disease Control and Prevention. 2021. Available online: https://www.cdc.gov/media/dpk/diseases-and-conditions/coronavirus/coronavirus-2020.html (accessed on 22 June 2021).
- de Azambuja, E.; Trapani, D.; Loibl, S.; Delaloge, S.; Senkus, E.; Criscitiello, C.; Poortman, P.; Gnant, M.; Di Cosimo, S.; Cortes, J.; et al. ESMO Management and treatment adapted recommendations in the COVID-19 era: Breast Cancer. ESMO Open 2020, 5, e000793. [Google Scholar] [CrossRef] [PubMed]
- Dietz, J.R.; Moran, M.S.; Isakoff, S.J.; Kurtzman, S.H.; Willey, S.C.; Burstein, H.J.; Bleicher, R.J.; Lyons, J.A.; Sarantou, T.; Baron, P.L.; et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. The COVID-19 pandemic breast cancer consortium. Breast Cancer Res. Treat. 2020, 181, 487–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nekhlyudov, L.; Duijts, S.; Hudson, S.V.; Jones, J.M.; Keogh, J.; Love, B.; Lustberg, M.; Smith, K.C.; Tevaarwerk, A.; Yu, X.; et al. Addressing the needs of cancer survivors during the COVID-19 pandemic. J. Cancer Surviv. 2020, 14, 601–606. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society Cancer Action Network. COVID-19 Pandemic Ongoing Impact on the Cancer Community: May 2020. Available online: https://www.fightcancer.org/policy-resources/covid-19-pandemic-ongoing-impact-cancer-community-may-2020 (accessed on 22 June 2021).
- Spiess, P.E.; Greene, J.; Keenan, R.J.; Paculdo, D.; Letson, G.D.; Peabody, J.W. Meeting the challenge of the 2019 Novel Coronavirus Disease in patients with cancer. Cancer 2020, 126, 3174–3175. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.A.; Cronin, K.A.; Plevritis, S.K.; Fryback, D.G.; Clarke, L.; Zelen, M.; Mandelblatt, J.S.; Yakovlev, A.Y.; Habbema, J.D.; Feuer, E.J. Cancer Intervention and Surveillance Modeling Network (CISNET) Collaborators. Effect of screening and adjuvant therapy on mortality from breast cancer. N. Engl. J. Med. 2005, 353, 1784–1792. [Google Scholar] [CrossRef]
- Barton, M.K. Earlier adjuvant therapy is beneficial in patients with breast and colon cancer. CA Cancer J. Clin. 2016, 66, 3–5. [Google Scholar] [CrossRef]
- Ginsburg, K.B.; Curtis, G.L.; Patel, D.N.; Chen, W.M.; Strother, M.C.; Kutikov, A.; Derweesh, I.H.; Cher, M.L. Association of surgical delay and overall survival in patients with T2 renal masses: Implications for critical clinical decision-making during the COVID-19 pandemic. Urology 2021, 147, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.G.; Pasea, L.; Banerjee, A.; Denaxas, S.; Katsoulis, M.; Chang, W.H.; Williams, B.; Pillay, D.; Noursadeghi, M.; Linch, D.; et al. Estimating excess mortality in people with cancer and multimorbidity in the COVID-19 emergency. MedRxiv 2020. [Google Scholar] [CrossRef]
- Cancino, R.S.; Su, Z.; Mesa, R.; Tomlinson, G.E.; Wang, J. The impact of COVID-19 on cancer screening: Challenges and opportunities. JMIR Cancer 2020, 6, e21697. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Singh, P.; Drohan, B.; Hughes, K.S. Breast imaging, breast surgery, and cancer genetics in the age of COVID-19. Cancer 2020, 126, 4466–4472. [Google Scholar] [CrossRef] [PubMed]
- Papautsky, E.L.; Hamlish, T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res. Treat. 2020, 184, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Gathani, T.; Clayton, G.; MacInnes, E.; Horgan, K. The COVID-19 pandemic and impact on breast cancer diagnoses: What happened in England in the first half of 2020. Br. J. Cancer 2021, 124, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Fancellu, A.; Sanna, V.; Rubino, C.; Ariu, M.L.; Piredda, C.; Piana, G.Q.; Piana, G.Q.; Cottu, P.; Spanu, A.; Cossu, A.; et al. The COVID-19 outbreak may be associated to a reduced level of care for breast cancer. A comparative study with the Pre-COVID era in an Italian breast unit. Healthcare 2020, 8, 474. [Google Scholar] [CrossRef]
- Jallali, N.; Hunter, J.E.; Henry, F.P.; Wood, S.H.; Hogben, K.; Almufti, R.; Hadjiminas, D.; Dunne, J.; Thiruchelvam, P.; Leff, D.R. The feasibility and safety of immediate breast reconstruction in the COVID-19 era. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Swainston, J.; Chapman, B.; Grunfeld, E.A.; Derakshan, N. COVID-19 lockdown and its adverse impact on psychological health in breast cancer. Front. Psychol. 2020, 11. Available online: https://www.frontiersin.org/articles/10.3389/fpsyg.2020.02033/full (accessed on 22 June 2021). [CrossRef]
- Alipour, S.; Moini, A.; Orouji, M.; Saberi, A.; Motamedi, M.; Eskandari, A. COVID-19 outbreak and consequent delays in schedules of the breast clinic: Effects on patients’ breast and emotional symptoms. Eur. J. Breast Health 2020, 16, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Shinan-Altman, S.; Levkovich, I.; Tavori, G. Healthcare utilization among breast cancer patients during the COVID-19 outbreak. Palliat. Support. Care 2020, 18, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Lou, E.; Teoh, D.; Brown, K.; Blaes, A.; Holtan, S.G.; Jewett, P.; Parsons, H.; Mburu, E.W.; Thomaier, L.; Hui, J.; et al. Perspectives of cancer patients and their health during the COVID-19 pandemic. PLoS ONE 2020, 15, e0241741. [Google Scholar] [CrossRef] [PubMed]
- Guven, D.C.; Sahin, T.K.; Aktepe, O.H.; Yildirim, H.C.; Aksoy, S.; Kilickap, S. Perspectives, knowledge, and fears of cancer patients about COVID-19. Front. Oncol. 2020, 10, 1553. Available online: https://www.frontiersin.org/articles/10.3389/fonc.2020.01553/full (accessed on 22 June 2021). [CrossRef] [PubMed]
- Juanjuan, L.; Santa-Maria, C.A.; Hongfang, F.; Lingcheng, W.; Pengcheng, Z.; Yuanbing, X.; Yuyan, T.; Zhongchun, L.; Bo, D.; Meng, L.; et al. Patient-reported outcomes of patients with breast cancer during the COVID-19 outbreak in the epicenter of China: A cross-sectional survey study. Clin. Breast Cancer 2020, 20, e651–e662. [Google Scholar] [CrossRef]
- Bleicher, R.J.; Ruth, K.; Sigurdson, E.R.; Beck, J.R.; Ross, E.; Wong, Y.-N.; Patel, S.A.; Boraas, M.; Chang, E.I.; Topham, N.S.; et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016, 2, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, S.A.; Edge, S.B.; Stewart, A.K.; Halpern, M.T.; Marlow, N.M.; Ward, E.M. Race and ethnicity are associated with delays in breast cancer treatment (2003–2006). J. Health Care Poor Underserved 2011, 22, 128–141. [Google Scholar] [PubMed]
- George, P.; Chandwani, S.; Gabel, M.; Ambrosone, C.B.; Rhoads, G.; Bandera, E.V.; Demissie, K. Diagnosis and surgical delays in African American and White women with early-stage breast cancer. J. Womens Health 2015, 24, 209–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obeng-Gyasi, S.; Oppong, B.; Paskett, E.D.; Lustberg, M. Purposeful surgical delay and the coronavirus pandemic: How will Black breast cancer patients fare? Breast Cancer Res. Treat. 2020, 182, 527–530. [Google Scholar] [CrossRef]
- Garg, S. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed Coronavirus Disease 2019—COVID-NET, 14 States, 1–30 March 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 458–464. Available online: https://www.cdc.gov/mmwr/volumes/69/wr/mm6915e3.htm (accessed on 23 June 2021). [CrossRef] [PubMed]
- TCR|Breast Cancer. Texas Department of State Health Services. Available online: https://dshs.state.tx.us/tcr/data/breast-cancer.aspx (accessed on 23 June 2021).
- American Cancer Society. Cancer Facts & Figures 2019; American Cancer Society: Atlanta, Georgia, 2019; Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html (accessed on 23 June 2021).
- American Cancer Society. Cancer Facts & Figures 2020; American Cancer Society: Atlanta, Georgia, 2020; Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2020.html (accessed on 23 June 2021).
- American Cancer Society. Breast Cancer Facts & Figures 2019–2020; American Cancer Society: Atlanta, Georgia, 2019; Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf (accessed on 23 June 2021).
- Reeder-Hayes, K.E.; Wheeler, S.B.; Mayer, D.K. Health disparities across the breast cancer continuum. Semin. Oncol. Nurs. 2015, 31, 170–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, Y.; Thompson, B.; Espinoza, N.; Ceballos, R. Breast cancer interventions serving US-based Latinas: Current approaches and directions. Womens Health 2013, 9, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Yanez, B.; Thompson, E.H.; Stanton, A.L. Quality of life among Latina breast cancer patients: A systematic review of the literature. J. Cancer Surviv. 2011, 5, 191–207. [Google Scholar] [CrossRef] [Green Version]
- Ashing-Giwa, K.T.; Padilla, G.V.; Bohórquez, D.E.; Tejero, J.S.; Garcia, M. Understanding the breast cancer experience of Latina women. J. Psychosoc. Oncol. 2006, 24, 19–52. [Google Scholar] [CrossRef]
- Matthews, A.K.; Tejeda, S.; Johnson, T.P.; Berbaum, M.L.; Manfredi, C. Correlates of quality of life among African American and White cancer survivors. Cancer Nurs. 2012, 35, 355–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejeda, S.; Stolley, M.R.; Vijayasiri, G.; Campbell, R.T.; Ferrans, C.E.; Warnecke, R.B.; Rauscher, G.H. Negative psychological consequences of breast cancer among recently diagnosed ethnically diverse women. Psychooncology 2017, 26, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.C.; Samuel, C.A.; Reeder-Hayes, K.E.; Wheeler, S.B.; Olshan, A.F.; Reeve, B.B. Understanding racial differences in health-related quality of life in a population-based cohort of breast cancer survivors. Breast Cancer Res. Treat. 2016, 159, 535–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danforth, D.N., Jr. Disparities in breast cancer outcomes between Caucasian and African American women: A model for describing the relationship of biological and nonbiological factors. Breast Cancer Res. 2013, 15, 208. [Google Scholar] [CrossRef]
- TCR|Cancer Disparities. Texas Department of State Health Services. Available online: https://dshs.texas.gov/tcr/data/cancer-disparities.aspx (accessed on 23 June 2021).
- Ko, N.Y.; Hong, S.; Winn, R.A.; Calip, G.S. Association of insurance status and racial disparities with the detection of early-stage breast cancer. JAMA Oncol. 2020, 6, 385–392. [Google Scholar] [CrossRef]
- Hill, D.A.; Prossnitz, E.R.; Royce, M.; Nibbe, A. Temporal trends in breast cancer survival by race and ethnicity: A population-based cohort study. PLoS ONE 2019, 14, e0224064. [Google Scholar] [CrossRef]
- Yedjou, C.G.; Tchounwou, P.B.; Payton, M.; Miele, L.; Fonseca, D.D.; Lowe, L.; Alo, R.A. Assessing the racial and ethnic disparities in breast cancer mortality in the United States. Int. J. Environ. Res. Public Health 2017, 14, 486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foy, K.C.; Fisher, J.L.; Lustberg, M.B.; Gray, D.M.; DeGraffinreid, C.R.; Paskett, E.D. Disparities in breast cancer tumor characteristics, treatment, time to treatment, and survival probability among African American and White women. NPJ Breast Cancer 2018, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tariq, K.; Rana, F. TNBC vs. Non-TNBC: A five-year retrospective review of differences in mean age, family history, smoking history, and stage at diagnosis at an inner-city university program. World J. Oncol. 2014, 4, 241–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.L.; Fang, S.; Meyer, T.E. Impact of treatment and socioeconomic status on racial disparities in survival among older women with breast cancer. Am. J. Clin. Oncol. 2008, 31, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Rajan, S.S.; Du, X.L.; Franzini, L.; Giordano, S.H.; Morgan, R.O. Association between financial burden and adjuvant hormonal therapy adherence and persistent use for privately insured women aged 18–64 years in BCBS of Texas. Breast Cancer Res. Treat. 2018, 169, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Farias, A.J.; Hansen, R.N.; Zeliadt, S.B.; Ornelas, I.J.; Li, C.I.; Thompson, B. The association between out-of-pocket costs and adherence to adjuvant endocrine therapy among newly diagnosed breast cancer patients. Am. J. Clin. Oncol. 2018, 41, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Farias, A.J.; Du, X.L. Association between out-of-pocket costs, race/ethnicity, and adjuvant endocrine therapy adherence among Medicare patients with breast cancer. JCO 2017, 35, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Farias, A.J.; Hansen, R.N.; Zeliadt, S.B.; Ornelas, I.J.; Li, C.I.; Thompson, B. Factors associated with adherence to adjuvant endocrine therapy among privately insured and newly diagnosed breast cancer patients: A quantile regression analysis. J. Manag. Care Spec. Pharm. 2016, 22, 969–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, M.C.; Wheeler, S.B.; Reeder-Hayes, K. Racial/Ethnic and socioeconomic disparities in endocrine therapy adherence in breast cancer: A systematic review. Am. J. Public Health 2015, 105 (Suppl. S3), e4–e15. [Google Scholar] [CrossRef]
- Sheppard, V.B.; He, J.; Sutton, A.; Cromwell, L.; Adunlin, G.; Salgado, T.M.; Tolsma, D.; Trout, M.; Robinson, B.E.; Edmonds, M.C.; et al. Adherence to adjuvant endocrine therapy in insured Black and White breast cancer survivors: Exploring adherence measures in patient data. J. Manag. Care Spec. Pharm. 2019, 25, 578–586. [Google Scholar] [CrossRef] [PubMed]
- McCue, D.A.; Lohr, L.K.; Pick, A.M. Improving adherence to oral cancer therapy in clinical practice. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2014, 34, 481–494. [Google Scholar] [CrossRef]
- Green, A.K.; Aviki, E.M.; Matsoukas, K.; Patil, S.; Korenstein, D.; Blinder, V. Racial disparities in chemotherapy administration for early-stage breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2018, 172, 247–263. [Google Scholar] [CrossRef]
- Wheeler, S.B.; Reeder-Hayes, K.E.; Carey, L.A. Disparities in breast cancer treatment and outcomes: Biological, social, and health system determinants and opportunities for research. Oncologist 2013, 18, 986–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burstein, H.J.; Temin, S.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.E.; Giordano, S.H.; Hudis, C.A.; Rowden, D.; Solky, A.J.; et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update. JCO 2014, 32, 2255–2269. [Google Scholar] [CrossRef] [PubMed]
- McCowan, C.; Wang, S.; Thompson, A.M.; Makubate, B.; Petrie, D.J. The value of high adherence to tamoxifen in women with breast cancer: A community-based cohort study. Br. J. Cancer 2013, 109, 1172–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farias, A.J.; Wu, W.-H.; Du, X.L. Racial and geographic disparities in adherence and discontinuation to adjuvant endocrine therapy in Texas Medicaid-insured patients with breast cancer. Med. Oncol. 2018, 35, 113. [Google Scholar] [CrossRef]
- Liu, B.L.; Ma, F.; Wang, J.N.; Fan, Y.; Mo, H.N.; Xu, B.H. Health management of breast cancer patients outside the hospital during the outbreak of 2019 novel Coronavirus Disease. Zhonghua Zhong Liu Za Zhi 2020, 42, 288–291. [Google Scholar] [PubMed]
- Braunstein, L.Z.; Gillespie, E.F.; Hong, L.; Xu, A.; Bakhoum, S.F.; Cuaron, J.; Mueller, B.; McCormick, B.; Cahlon, O.; Powell, S.; et al. Breast radiation therapy under COVID-19 pandemic resource constraints—Approaches to defer or shorten treatment from a comprehensive cancer center in the United States. Adv. Radiat. Oncol. 2020, 5, 582–588. [Google Scholar] [CrossRef]
- Coles, C.E.; Aristei, C.; Bliss, J.; Boersma, L.; Brunt, A.M.; Chatterjee, S.; Hanna, G.; Jagsi, R.; Kaidar Person, O.; Kirby, A.; et al. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin. Oncol. (R Coll Radiol.) 2020, 32, 279–281. [Google Scholar] [CrossRef]
- Greene, J.C.; Caracelli, V.J.; Graham, W.F. Toward a conceptual framework for mixed-method evaluation designs. Educ. Eval. Policy Anal. 1989, 11, 255–274. [Google Scholar] [CrossRef]
- Fetters, M.D.; Curry, L.A.; Creswell, J.W. Achieving integration in mixed methods designs-principles and practices. Health Serv. Res. 2013, 48 Pt 2, 2134–2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taplin, S.H.; Anhang Price, R.; Edwards, H.M.; Foster, M.K.; Breslau, E.S.; Chollette, V.; Prabhu Das, I.; Clauser, S.B.; Fennell, M.L.; Zapka, J. Introduction: Understanding and influencing multilevel factors across the cancer care continuum. JNCI Monogr. 2012, 2012, 2–10. [Google Scholar] [CrossRef]
- Alvidrez, J.; Castille, D.; Laude-Sharp, M.; Rosario, A.; Tabor, D. The National Institute on Minority Health and Health Disparities Research Framework. Am. J. Public Health 2019, 109 (Suppl. S1), S16–S20. [Google Scholar] [CrossRef]
- Diez-Roux, A.V.; Nieto, F.J.; Muntaner, C.; Tyroler, H.A.; Comstock, G.W.; Shahar, E.; Cooper, L.S.; Watson, R.L.; Szklo, M. Neighborhood environments and coronary heart disease: A multilevel analysis. Am. J. Epidemiol. 1997, 146, 48–63. [Google Scholar] [CrossRef]
- Warnecke, R.B.; Oh, A.; Breen, N.; Gehlert, S.; Paskett, E.; Tucker, K.L.; Lurie, N.; Rebbeck, T.; Goodwin, J.; Flack, J.; et al. Approaching health disparities from a population perspective: The National Institutes of Health Centers for Population Health and Health Disparities. Am. J. Public Health 2008, 98, 1608–1615. [Google Scholar] [CrossRef]
- Bronfenbrenner, U. Toward an experimental ecology of human development. Am. Psychol. 1977, 32, 513–531. [Google Scholar] [CrossRef]
- Sampson, R.J. Neighborhood-Level Context and Health: Lessons from Sociology. In Neighborhoods and Health; Oxford University Press: Oxford, UK, 2003; Available online: https://oxford.universitypressscholarship.com/view/10.1093/acprof:oso/9780195138382.001.0001/acprof-9780195138382-chapter-6 (accessed on 23 June 2021).
- Jones, K.; Duncan, C. Individuals and their ecologies: Analysing the geography of chronic illness within a multilevel modelling framework. Health Place 1995, 1, 27–40. [Google Scholar] [CrossRef]
- Lochner, K.A.; Kawachi, I.; Brennan, R.T.; Buka, S.L. Social capital and neighborhood mortality rates in Chicago. Soc. Sci. Med. 2003, 56, 1797–1805. [Google Scholar] [CrossRef]
- Seeman, T.E. Social ties and health: The benefits of social integration. Ann. Epidemiol. 1996, 6, 442–451. [Google Scholar] [CrossRef]
- Subramanian, S.V.; Lochner, K.A.; Kawachi, I. Neighborhood differences in social capital: A compositional artifact or a contextual construct? Health Place 2003, 9, 33–44. [Google Scholar] [CrossRef]
- Szreter, S.; Woolcock, M. Health by association? Social capital, social theory, and the political economy of public health. Int. J. Epidemiol. 2004, 33, 650–667. [Google Scholar] [CrossRef] [PubMed]
- Thoits, P.A. Stress, coping, and social support processes: Where are we? What next? J. Health Soc. Behav. 1995, 53–79. [Google Scholar] [CrossRef] [Green Version]
- Kawachi, I.; Berkman, L.F. Social Capital, Social Cohesion, and Health. In Social Epidemiology; Oxford University Press: Oxford, UK, 2015; Available online: https://oxfordmedicine.com/view/10.1093/med/9780195377903.001.0001/med-9780195377903-chapter-8 (accessed on 14 July 2021).
- Glanz, K.; Rimer, B.K.; Viswanath, K. Theory, Research, and Practice; Wiley: Hoboken, NJ, USA.
- Sloggett, A.; Joshi, H. Higher mortality in deprived areas: Community or personal disadvantage? BMJ 1994, 309, 1470–1474. [Google Scholar] [CrossRef] [Green Version]
- Waitzman, N.J.; Smith, K.R. Phantom of the area: Poverty-area residence and mortality in the United States. Am. J. Public Health 1998, 88, 973–976. [Google Scholar] [CrossRef] [Green Version]
- Taplin, S.H.; Rodgers, A.B. Toward Improving the quality of cancer care: Addressing the interfaces of primary and oncology-related subspecialty care. JNCI Monogr. 2010, 2010, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Purnell, T.S.; Calhoun, E.A.; Golden, S.H.; Halladay, J.R.; Krok-Schoen, J.L.; Appelhans, B.M.; Cooper, L.A. Achieving health equity: Closing the gaps in health care disparities, interventions, and research. Health Aff. 2016, 35, 1410–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, A.F.; Ma, G.X.; Miranda, J.; Eng, E.; Castille, D.; Brockie, T.; Jones, P.; Airhihenbuwa, C.O.; Farhat, T.; Zhu, L.; et al. Structural interventions to reduce and eliminate health disparities. Am. J. Public Health 2019, 109 (Suppl. S1), S72–S78. [Google Scholar] [CrossRef]
- Agurs-Collins, T.; Persky, S.; Paskett, E.D.; Barkin, S.L.; Meissner, H.I.; Nansel, T.R.; Arteaga, S.S.; Zhang, X.; Das, R.; Farhat, T. Designing and assessing multilevel interventions to improve minority health and reduce health disparities. Am. J. Public Health 2019, 109 (Suppl. S1), S86–S93. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Bright, E.E.; Petrie, K.J.; Partridge, A.H.; Stanton, A.L. Barriers to and facilitative processes of endocrine therapy adherence among women with breast cancer. Breast Cancer Res. Treat. 2016, 158, 243–251. [Google Scholar] [CrossRef]
- Carver, C.S. You want to measure coping but your protocol’s too long: Consider the brief COPE. Int. J. Behav. Med. 1997, 4, 92–100. [Google Scholar] [CrossRef]
- Dougall, A.L.; Smith, A.W.; Somers, T.J.; Posluszny, D.M.; Rubinstein, W.S.; Baum, A. Coping with genetic testing for breast cancer susceptibility. Psychosom. Med. 2009, 71, 98–105. [Google Scholar] [CrossRef] [PubMed]
- David, D.; Montgomery, G.H.; Bovbjerg, D.H. Relations between coping responses and optimism-pessimism in predicting anticipatory psychological distress in surgical breast cancer patients. Pers. Individ. Dif. 2006, 40, 203–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luszczynska, A.; Gerstorf, D.; Boehmer, S.; Knoll, N.; Schwarzer, R. Patients’ coping profiles and partners’ support provision. Psychol. Health 2007, 22, 749–764. [Google Scholar] [CrossRef]
- Scrignaro, M.; Barni, S.; Magrin, M.E. The combined contribution of social support and coping strategies in predicting post-traumatic growth: A longitudinal study on cancer patients. Psychooncology 2011, 20, 823–831. [Google Scholar] [CrossRef]
- Heitzmann, C.A.; Merluzzi, T.V.; Jean-Pierre, P.; Roscoe, J.A.; Kirsh, K.L.; Passik, S.D. Assessing self-efficacy for coping with cancer: Development and psychometric analysis of the brief version of the Cancer Behavior Inventory (CBI-B). Psychooncology 2011, 20, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Rice, L.J.; Halbert, C.H. Social Networks across common cancer types: The evidence, gaps, and areas of potential impact. Adv. Cancer Res. 2017, 133, 95–128. [Google Scholar] [PubMed]
- Yaroch, A.L.; Tooze, J.; Thompson, F.E.; Blanck, H.M.; Thompson, O.M.; Colón-Ramos, U.; Shaikh, A.R.; McNutt, S.; Nebeling, L.C. Evaluation of three short dietary instruments to assess fruit and vegetable intake: The National Cancer Institute’s food attitudes and behaviors survey. J. Acad. Nutr. Diet 2012, 112, 1570–1577. [Google Scholar] [CrossRef] [Green Version]
- Kroenke, K.; Strine, T.W.; Spitzer, R.L.; Williams, J.B.W.; Berry, J.T.; Mokdad, A.H. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord. 2009, 114, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Razykov, I.; Ziegelstein, R.C.; Whooley, M.A.; Thombs, B.D. The PHQ-9 versus the PHQ-8—Is item 9 useful for assessing suicide risk in coronary artery disease patients? Data from the Heart and Soul Study. J. Psychosom. Res. 2012, 73, 163–168. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr. Ann. 2002, 32, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Spitzer, R.L.; Kroenke, K.; Williams, J.B.W.; Löwe, B. A brief measure for assessing Generalized Anxiety Disorder: The GAD-7. Arch. Intern. Med. 2006, 166, 1092–1097. [Google Scholar] [CrossRef] [Green Version]
- Sherbourne, C.D.; Stewart, A.L. The MOS social support survey. Soc. Sci. Med. 1991, 32, 705–714. [Google Scholar] [CrossRef]
- Cella, D.; Gershon, R.; Lai, J.-S.; Choi, S. The future of outcomes measurement: Item banking, tailored short-forms, and computerized adaptive assessment. Qual. Life Res. 2007, 16, 133–141. [Google Scholar] [CrossRef]
- Hahn, E.A.; DeWalt, D.A.; Bode, R.K.; Garcia, S.F.; DeVellis, R.F.; Correia, H.; Cella, D.; PROMIS Cooperative Group. New English and Spanish social health measures will facilitate evaluating health determinants. Health Psychol. 2014, 33, 490–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carle, A.C.; Riley, W.; Hays, R.D.; Cella, D. Confirmatory Factor Analysis of the Patient Reported Outcomes Measurement Information System (PROMIS) Adult Domain Framework using Item Response Theory scores. Med. Care 2015, 53, 894–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, S.; Doyle, W.J.; Skoner, D.P.; Rabin, B.S.; Gwaltney, J.M. Social ties and susceptibility to the common cold. JAMA 1997, 277, 1940–1944. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, M.T.; Olson, D.H. Assessing Intimacy: The Pair Inventory. J. Marital. Fam. Ther. 1981, 7, 47–60. [Google Scholar] [CrossRef]
- Musa, G.J.; Chiang, P.-H.; Sylk, T.; Bavley, R.; Keating, W.; Lakew, B.; Tsou, H.C.; Hoven, C.W. Use of GIS mapping as a public health tool-from cholera to cancer. Health Serv. Insights 2013, 6, 111–116. [Google Scholar] [CrossRef]
- Sampson, R.J.; Raudenbush, S.W.; Earls, F. Neighborhoods and Violent Crime: A multilevel study of collective efficacy. Science 1997, 277, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Steptoe, A.; Feldman, P.J. Neighborhood problems as sources of chronic stress: Development of a measure of neighborhood problems, and associations with socioeconomic status and health. Ann. Behav. Med. 2001, 23, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.N.; Hays, R.D. The Patient Satisfaction Questionnaire Short Form (PSQ-18). 31 December 1993. Available online: https://www.rand.org/pubs/papers/P7865.html (accessed on 7 July 2021).
- Thompson, H.S.; Valdimarsdottir, H.B.; Winkel, G.; Jandorf, L.; Redd, W. The Group-Based Medical Mistrust Scale: Psychometric properties and association with breast cancer screening. Prev. Med. 2004, 38, 209–218. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society Cancer Action Network. COVID-19 Pandemic Impact on Cancer Patients and Survivors. Survey Findings Summary. American Cancer Society Cancer Action Network (ACS CAN). Available online: https://www.fightcancer.org/sites/default/files/National%20Documents/Survivor%20Views.COVID19%20Polling%20Memo.Final_.pdf (accessed on 14 July 2021).
- Brady, M.J.; Cella, D.F.; Mo, F.; Bonomi, A.E.; Tulsky, D.S.; Lloyd, S.R.; Deasy, S.; Cobleigh, M.; Shiomoto, G. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. J. Clin. Oncol. 1997, 15, 974–986. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Survey Questionnaire; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2020. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Hillsdale, N.J., Ed.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988; 567p. [Google Scholar]
- SAS Institute Inc. SAS (Version 9.4) [Computer Software]; SAS Institute, Inc.: Cary, NC, USA, 2014. [Google Scholar]
- Corbin, J.; Strauss, A. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory, 4th ed.; SAGE Publications, Inc.: Los Angeles, CA, USA, 2014. [Google Scholar]
- Bryant, A.; Charmaz, K. The SAGE Handbook of Grounded Theory; SAGE Publications Ltd.: London, UK, 2007. [Google Scholar]
- Creswell, J.W.; Poth, C.N. Qualitative Inquiry and Research Design; SAGE Publications Inc.: London, UK, 2017. [Google Scholar]
- ATLAS.ti Scientific Software Development GmbH. Version 9. Available online: https://atlasti.com/ (accessed on 29 November 2021).
- Pennell, N.A.; Dillmon, M.; Levit, L.A.; Moushey, E.A.; Alva, A.S.; Blau, S.; Cannon, T.L.; Dickson, N.R.; Diehn, M.; Gonen, M.; et al. American Society of Clinical Oncology Road to Recovery Report: Learning from the COVID-19 experience to improve clinical research and cancer care. JCO 2021, 39, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Babatunde, O.A.; Eberth, J.M.; Felder, T.; Moran, R.; Truman, S.; Hebert, J.R.; Zhang, J.; Adams, S.A. Social determinants of racial disparities in breast cancer mortality among Black and White Women. J. Racial. Ethn. Health Disparities 2021, 8, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Siker, M.L.; Deville, C.; Suneja, G.; Winkfield, K. Lessons from COVID-19: Addressing health equity in cancer care. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Behrman, P.; Dulin, A.; Baskin, M.L.; Buscemi, J.; Alcaraz, K.I.; Goldstein, C.M.; Carson, T.L.; Shen, M.; Fitzgibbon, M. Addressing inequities in COVID-19 morbidity and mortality: Research and policy recommendations. Transl. Behav. Med. 2020, 10, 516–519. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).