Qualitative Exploration of the ‘Rolling Unmasking Effect’ for Downwind Odor Dispersion from a Model Animal Source
Abstract
:1. Introduction
1.1. Is Solving the Downwind Odor Problem Possible without an Exhaustive List of Identified Compounds Emitted from a Source?
1.2. Proposed Solution—Based upon Simplification through Downwind Odorant Prioritization
1.3. Objective
1.4. Rationale
2. Background
2.1. Challenges to the Current State of the Art in Downwind Odor Assessment
2.2. Natural Examples of RUE
3. Materials and Methods
3.1. Odorant Prioritization Procedural Summary Outline
- Step 1—Downwind composite odor assessment—qualitative, at-site odor-character assessment by the panelist; in this case, (D.W.W.), a gas chromatography-olfactometry (GC-O) investigator with 20+ years of experience, odor troubleshooting for the industry. The goal of this stage is to observe recognizable odors that are consistent and perceived as characteristic of the source, at the downwind outer boundary and at the time of the at-site assessment.
- Step 2—GC-O-based odorant prioritization—qualitative, on-instrument assessment by the panelist (D.W.W.); attempting to make a connection between the observed downwind odor character and individual compounds that are perceived as character-defining for that odor.
- Step 3—First-pass odor-match validation of impact-priority hypothesis from Step 2—qualitative odor-match based confirmation by conference with associate GC-O investigator(s), where possible; in the case of the P.T. porcupine, an experienced GC-O investigator with 10+ years of experience (A.I.) odor troubleshooting for industry; generally involving on-instrument GC-O based crosscheck.
- Step 4—Development of a synthetic formulationfor final odor-match-based validation—the panelist (D.W.W.) attempts to develop a formulation, in low odor, food-grade propylene glycol carrier, which reflects a high-fidelity odor-match to that of the targeted environment downwind. This formulation can range from very simple single odorants to multi-odorant blends, matching the odorant concentration ratios existing in the targeted environments downwind.
- Step 5—Final odor-match validation of impact-priority hypothesis from Steps 1 and 2—qualitative or quantitative odor-match-based validation by conference with volunteer sensory panelists drawn from (a) downwind citizenry; (b) other community stakeholders or (c) professional sensory panel.
- (as required) Step 6—Analytical method development targeting impact-priority odorants defined and validated; Steps 1 through 5—quantitative method development for follow-on odor investigation, monitoring, and mitigation strategy focusing.
- (as required) Step 7—Instrument-based environmental odor monitoring based upon impact-priority odorants—quantitative monitoring for correlating downwind environmental impact and upwind source prioritization.
3.2. PT Porcupine Urine Sampling
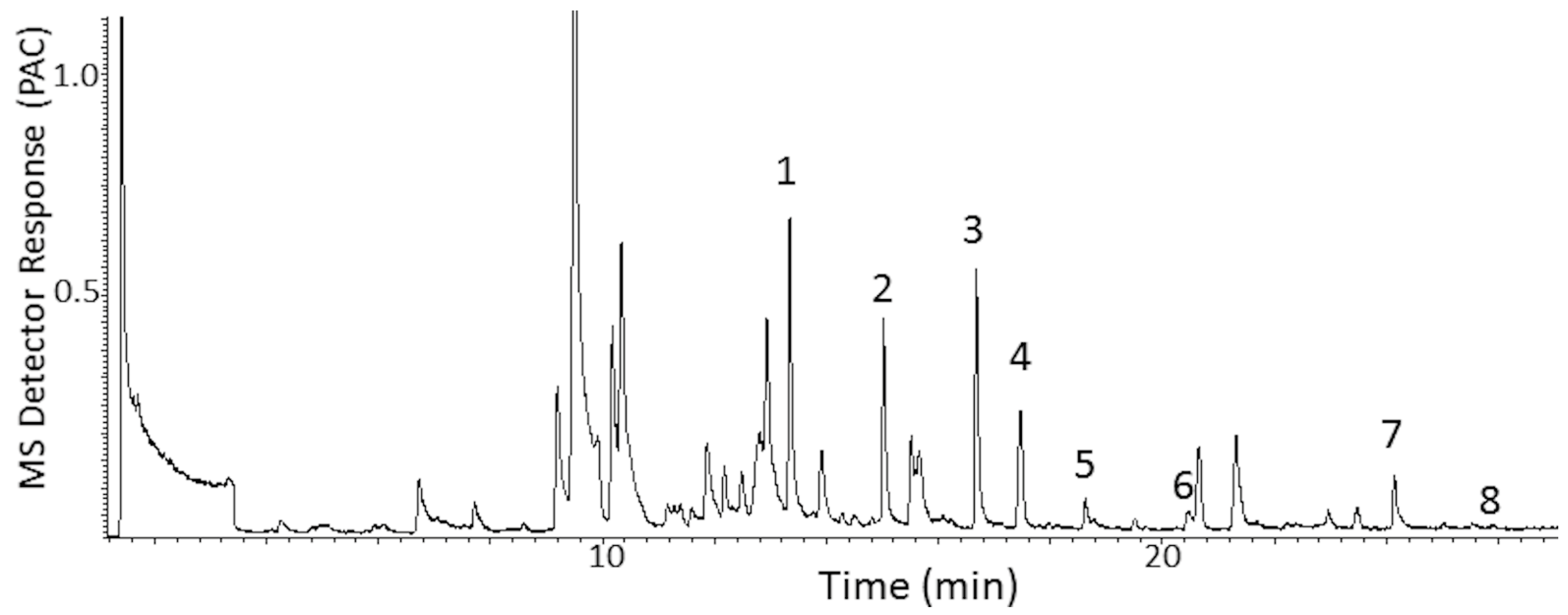
3.3. PT Porcupine Exhibit—Downwind Air Sample Collections with SPME
3.4. Multidimensional Gas Chromatography-Mass Spectrometry-Olfactometry
3.5. MDGC Parameters for Compound Isolation with Heart-Cutting
3.6. Chemical Identification
4. Results
4.1. Case Study: Prehensile-Tailed Porcupine
4.1.1. Initial Odor Assessment at the Source and Downwind
- (1)
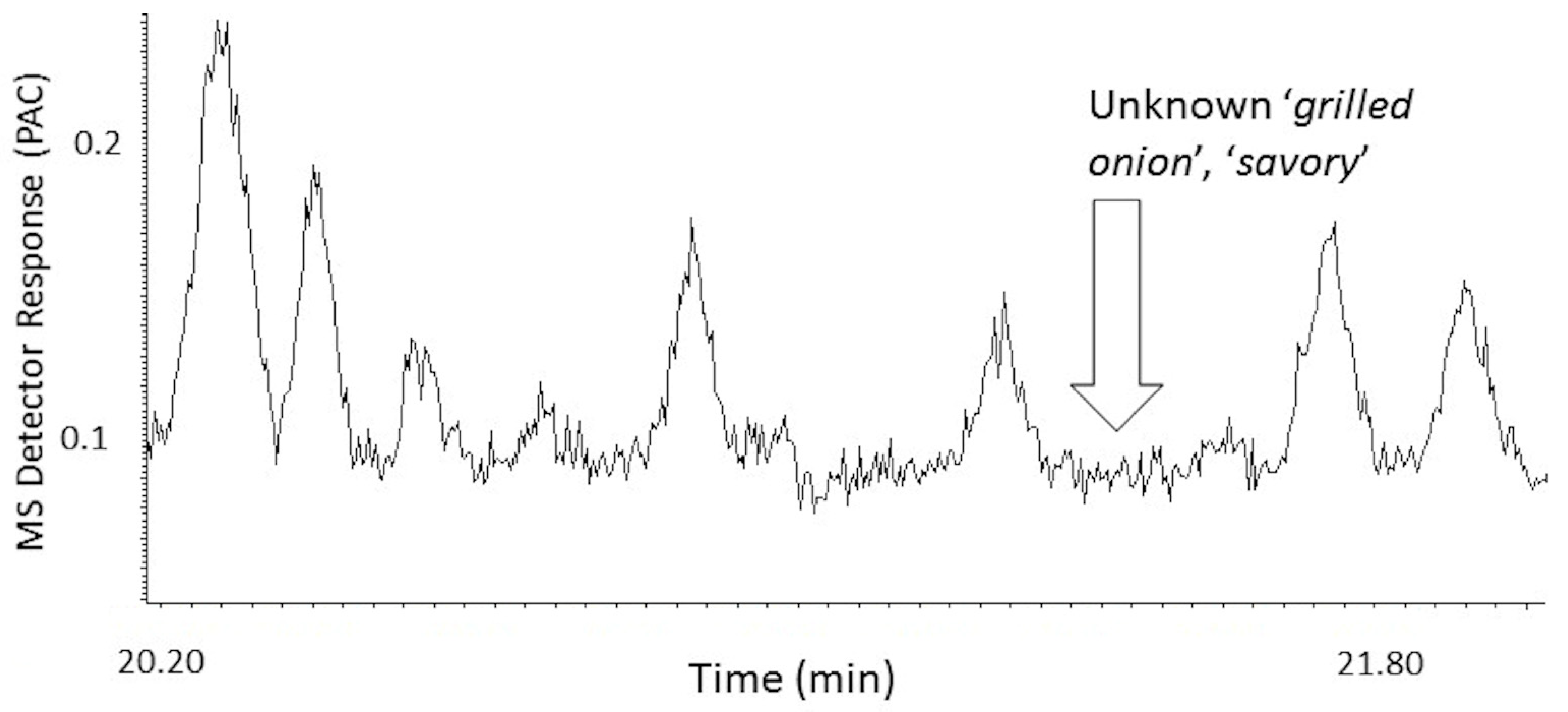
- due to the remarkable similarity in downwind odor characteristics, there is assumed to be some chemical compound commonality between the priority odorant subsets for the P.T. porcupine and typical ‘grilled onion’ odors. Likewise,
- (2)
- due to the remarkable dis-similarity in downwind odor characteristics, there is assumed to be some chemical compound disconnect between the priority odorant subsets for the P.T. porcupine and typical swine CAFO odors.
- Are there common character-impact odorants to both the P.T. porcupine and typical ‘grilled onion’ sources that account for the striking similarity in composite odor at their respective odor frontal boundaries?
- Are there character-impact odorants for P.T. porcupine and swine CAFO sources that account for the striking difference in composite odor character at their respective odor frontal boundaries?
- What is the overall agreement between the P.T. porcupine and swine CAFOs when comparing their minimum priority odorant subsets downwind with their full (at source) odorant and underlying VOC profiles?
4.1.2. Odorant Prioritization
4.1.3. Contrasting Downwind Odorant Prioritizations—The P.T. Porcupine vs. a Swine-Barn CAFO
5. Discussion
5.1. Implications of the Rolling Unmasking Effect and Odorant Prioritization for Environmental Odor Mitigation and Monitoring Strategy Development
5.2. Counter-Intuitive Odor Masking
5.3. Implications of the RUE for Community Environmental Odor Issues
5.4. DoubleHeart-Cut Isolation of High-Impact Odorants from Crude Source Materials
6. Conclusions
- the potential for focusing on odor monitoring strategy development to that most technologically appropriate for the impact-priority subset of odorants. Understanding that one of the two character-defining odorants was a semi-volatile (i.e., with limited volatility) we know that long-term storage of whole-air samples in plastic bags is not an option.
- the focusing of odor mitigation strategy development to the impact-priority subset of odorants. Understanding that one of the two character-defining odorants was a semi-volatile (i.e., with limited volatility), we know that activated carbon adsorption-based mitigation strategy becomes a more economically viable option.
- making possible the integration of odor-matching as a reconciling tool for improving communication, among stakeholders, regarding community odor issues.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGorrin, R.J. Character-impact flavor compounds. Chapter 9. In Sensory Directed Flavor Analysis; Marsili, R., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Maurer, D.L.; Koziel, J.A.; Harmon, J.D.; Hoff, S.J.; Rieck-Hinz, A.M.; Andersen, D.S. Summary of performance data for technologies to control gaseous, odor, and particulate emissions from livestock operations: Air Management Practices Assessment Tool (AMPAT). Data Brief 2016, 7, 1413–1429. [Google Scholar] [CrossRef] [Green Version]
- Parker, D.; Rhoades, M.B.; Schuster, G.; Koziel, J.A.; Perschbacher-Buser, Z. Odor characterization at open-lot cattle feedyards using triangular forced-choice olfactometry. Trans. ASABE 2005, 48, 1527–1535. [Google Scholar] [CrossRef]
- Jacobson, L.D.; Hetchler, B.P.; Schmidt, D.R.; Nicolai, R.E.; Heber, A.J.; Ni, J.; Hoff, S.J.; Koziel, J.A.; Zhang, Y.; Beasley, D.B.; et al. Quality Assured Measurements of Animal Building Emissions: Odor Concentrations. J. Air Waste Manag. Assoc. 2008, 58, 806–811. [Google Scholar] [CrossRef] [Green Version]
- Akdeniz, N.; Jacobson, L.D.; Hetchler, B.P.; Bereznicki, S.D.; Heber, A.J.; Koziel, J.A.; Cai, L.; Zhang, S.; Parker, D.B. Odor and odorous chemical emissions from animal buildings: Part 2—odor emissions. Trans. ASABE 2012, 55, 2335–2345. [Google Scholar] [CrossRef]
- Bokowa, A.; Diaz, C.; Koziel, J.A.; McGinley, M.; Barclay, J.; Schauberger, G.; Guillot, J.M.; Sneath, R.; Capelli, L.; Zorich, V.; et al. Summary and overview of odour regulations worldwide. Atmosphere 2021, 12, 206. [Google Scholar] [CrossRef]
- Brancher, M.; Griffiths, K.D.; Franco, D.; de Melo Lisboa, H. A review of odour impact criteria in selected countries around the world. Chemosphere 2017, 168, 1531–1570. [Google Scholar] [CrossRef]
- Koziel, J.A.; Spinhirne, J.P.; Lloyd, J.; Parker, D.; Wright, D.; Kuhrt, F. Evaluation of sample recoveries of malodorous gases for odor bags, SPME, air sampling canisters, and sorbent tubes. J. Air Waste Manag. Assoc. 2005, 55, 1147–1157. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Koziel, J.A.; Cai, L.; Wright, D.; Kuhrt, F. Testing odorants recovery from a novel metalized fluorinated ethylene propylene gas sampling bag. J. Air Waste Manag. Assoc. 2015, 65, 1434–1445. [Google Scholar] [CrossRef] [Green Version]
- Akdeniz, N.; Jacobson, L.D.; Hetchler, B.P.; Bereznicki, S.D.; Heber, A.J.; Koziel, J.A.; Cai, L.; Zhang, S.; Parker, D.B. Odor and odorous chemical emissions from animal buildings: Part 4—Correlations between sensory and chemical concentrations. Trans. ASABE 2012, 55, 2347–2356. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, L.; Koziel, J.A.; Hoff, S.J.; Heathcote, K.Y.; Jacobson, L.D.; Akdeniz, N.; Hetchler, B.; Parker, D.B.; Caraway, E.A.; et al. Odor and odorous chemical emissions from animal buildings: Part 5—Correlations between odor intensities and chemical concentrations (GC-MS/O). Trans. ASABE 2015, 58, 1349–1359. [Google Scholar] [CrossRef]
- Caraway, E.A.; Parker, D.B.; Ruby, M.; Green, R.; Spears, J.; Buser, Z.; Rhoades, M.B.; Mark, J.O. Identification of Malodorous Compounds from a Fish Meal Plant. In Proceedings of the International Symposium on Air Quality and Waste Management for Agriculture, Broomfield, CO, USA, 16–19 September 2007. [Google Scholar]
- Luo, J.; Vanoostrom, A. Biofilters for controlling animal rendering odour; a pilot plant study. Pure Appl. Chem. 1997, 69, 2403–2410. [Google Scholar] [CrossRef]
- Koziel, J.A.; Guenther, A.; Byers, M.; Iwasinska, A.; Parker, D.; Wright, D. Update on the Development of a New ASTM Standard for Environmental Odor Assessment. Plenary Lecture at the NOSE2020. In Proceedings of the 7th International Conference on Environmental Odour Monitoring and Control, Virtual Conference, Online, 18–21 April 2021. [Google Scholar]
- Long., E. Byers Scientific, Iowa State University, and Odor Experts Identify the Volatile Chemical Compound Responsible for Cannabis Odor Complaints; Business Wire (a Berkshire Hathaway Company): Bloomington, IN, USA, 22 March 2021; Available online: https://www.businesswire.com/news/home/20210322005837/en/Byers-Scientific-Iowa-State-University-and-Odor-Experts-Identify-the-Volatile-Chemical-Compound-Responsible-for-Cannabis-Odor-Complaints (accessed on 29 November 2021).
- Parker, D.B.; Koziel, J.A.; Cai, L.; Jacobson, L.D.; Akdeniz, N.; Lim, T.T.; Caraway, E.A.; Zhang, S.; Hoff, S.J.; Heber, A.J.; et al. Odor and odorous chemical emissions from animal buildings: Part 6—odor activity value. Trans. ASABE 2012, 55, 2357–2368. [Google Scholar] [CrossRef]
- Rice, S.; Koziel, J.A. Characterizing the smell of marijuana by odor impact of volatile compounds: An application of simultaneous chemical and sensory analysis. PLoS ONE 2015, 10, e0144160. [Google Scholar] [CrossRef] [Green Version]
- Rice, S.; Koziel, J.A. Odor impact of volatiles emitted from marijuana, cocaine, heroin and their surrogate scents. Data Brief 2015, 5, 653–706. [Google Scholar] [CrossRef] [Green Version]
- Rice, S.; Koziel, J.A. The relationship between chemical concentration and odor activity value explains the inconsistency in making a comprehensive surrogate scent training tool representative of illicit drugs. Forensic Sci. Int. 2015, 257, 257–270. [Google Scholar] [CrossRef] [Green Version]
- Bulliner, E.A., IV; Koziel, J.A.; Cai, L.; Wright, D. Characterization of livestock odors using steel plates, solid phase microextraction, and multidimensional-gas chromatography-mass spectrometry-olfactometry. J. Air Waste Manag. Assoc. 2007, 56, 1391–1403. [Google Scholar] [CrossRef] [Green Version]
- Laor, Y.; Koziel, J.A.; Cai, L.; Ravid, U. Chemical-sensory characterization of dairy manure odor by headspace solid phase microextraction and multidimensional gas chromatography–mass spectrometry-olfactometry. J. Air Waste Manag. Assoc. 2008, 58, 1187–1197. [Google Scholar] [CrossRef] [Green Version]
- Lo, Y.M.; Koziel, J.A.; Cai, L.; Hoff, S.J.; Jenks, W.S.; Xin, H. Simultaneous chemical and sensory characterization of VOCs and semi-VOCs emitted from swine manure using SPME and multidimensional gas chromatography-mass spectrometry-olfactometry system. J. Environ. Qual. 2008, 37, 521–534. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, L.; Koziel, J.A.; Hoff, S.J.; Clanton, C.J.; Schmidt, D.R.; Jacobson, L.D.; Parker, D.B.; Heber, A.J. Field air sampling and simultaneous chemical and sensory analysis of livestock odorants with sorbent tube GC-MS/Olfactometry. Sens. Actuators B 2010, 146, 427–432. [Google Scholar] [CrossRef]
- Wright, D.W.; Eaton, D.K.; Nielsen, L.T.; Kuhrt, F.W.; Koziel, J.A.; Parker, D.B. Improved, GC-olfactometry based, malodor assessment of swine CAFOs utilizing novel air sampling technologies. In Proceedings of the Air & Waste Management Association Symposium, Minneapolis, MN, USA, 21–24 June 2005. [Google Scholar]
- Koziel, J.A.; Cai, L.; Wright, D.; Hoff, S. Solid phase microextraction as a novel air sampling technology for improved, GC-Olfactometry-based, assessment of livestock odors. J. Chromatogr. Sci. 2006, 44, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Wright, D.W.; Eaton, D.K.; Nielsen, L.T.; Kuhrt, F.W.; Koziel, J.A.; Cai, L.; Lo, Y.; Parker, D.B.; Buser, Z. Synthetic CAFO odor formulation; an effective technique for validation of odorant prioritizations. In Proceedings of the Ecological Society of America Conference, Washington, DC, USA, 5–8 June 2006. [Google Scholar]
- Wright, D.W.; Nielsen, L.; Eaton, D.; Kuhrt, F.; Koziel, J.A.; Cai, L.; Parker, D.B. Animal Odor Assessment—Chickens, Pigs, Bats or Cats; it is Still Analytical Chemistry. In Proceedings of the National Poultry Waste Management Symposium, Springdale, AK, USA, 23–25 October 2006. [Google Scholar]
- Wright, D.W.; Nielsen, L.; Eaton, D.; Kuhrt, F.; Koziel, J.A.; Spinhirne, J.P.; Parker, D.B. Multidimensional GC-MS-olfactometry for identification and prioritization of malodors from confined animal feeding operations. J. Agric. Food Chem. 2005, 53, 8663–8672. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, L.T.; Eaton, D.K.; Wright, D.W.; Schmidt-French, B. Characteristic odors of Tadarida braziliensis Mexicana chiroptera: Molossidae. J. Cave Karst Stud. 2006, 68, 27–31. [Google Scholar]
- Wright, D.W.; Mahler, K.O.; Ballard, L.B. The Application of an Expanded Multidimensional G.C. System to Complex Fragrance Evaluations. J. Chromatogr. Sci. 1986, 24, 60–65. [Google Scholar] [CrossRef]
- Bleiler, R.; Iwasinska, A.; Kuhrt, F.; Wright, D. Formulations: A stable chemical/sensory equivalent to natural products for permeation/package testing. In Proceedings of the Society of Sensory Professionals Meeting, Tucson, AZ, USA, 17–19 September 2014. [Google Scholar]
- Yu, T.-H.; Wu, C.-M.; Rosen, R.T.; Hartman, T.G.; Ho, C.-T. Volatile compounds generated from thermal degradation of alliin and deoxyalliin in an aqueous solution. J. Agric. Food Chem. 1994, 42, 146–153. [Google Scholar] [CrossRef]
- Roze, U.; Leung, K.T.; Nix, E.; Burton, G.; Chapman, D.M. Microanatomy and bacterial flora of the perineal glands of the North American porcupine. Can. J. Zool. 2010, 88, 59–68. [Google Scholar] [CrossRef]
- Soso, S.B.; Koziel, J.A.; Johnson, A.; Lee, Y.J.; Fairbanks, W.S. Analytical methods for chemical and sensory characterization of scent-markings in large wild mammals: A review. Sensors 2014, 14, 4428–4465. [Google Scholar] [CrossRef] [Green Version]
- Soso, S.B.; Koziel, J.A. Analysis of odorants in marking fluid of Siberian tiger (Panthera tigris altaica) using simultaneous sensory and chemical analysis with headspace solid-phase microextraction and multidimensional gas chromatography-mass spectrometry-olfactometry. Molecules 2016, 21, 834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soso, S.B.; Koziel, J.A. Characterizing the scent and chemical composition of Panthera leo marking fluid using solid-phase microextraction and multidimensional gas chromatography-mass spectrometry-olfactometry. Sci. Rep. 2017, 7, 5137. [Google Scholar] [CrossRef]
- Boelens, M.H.; van Gemert, L.J. Volatile character-impact sulfur compounds and their sensory properties. Perf. Flav. 1993, 18, 29. [Google Scholar]
- May-Chien, K.; Mingjien, C.; Chi-Tang, H. Novel polysulfides identified in the volatile components from Welsh onions and scallions. J. Agric. Food Chem. 1990, 38, 1378–1381. [Google Scholar]
- Merck Index. An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th ed.; Merck & Co., Inc.: Whitehouse Station, NJ, USA, 2001. [Google Scholar]
- Park, N.; Lee, Y.; Lee, S.; Cho, J. Removal of taste and odor model compound (2,4,6-trichloroanisole) by tight ultrafiltration membranes. Desalination 2007, 212, 28–36. [Google Scholar] [CrossRef]
- Mallert, L.; Bruchet, A. A taste and odour episode caused by 2,4,6-tribromoanisole. J. Am. Water Work. Assoc. 2002, 94, 84–95. [Google Scholar] [CrossRef]
- Torrice, M. (adapted from Suffet, M.). The scientists who sniff water. Chem. Eng. News 2017, 95, 16–19. [Google Scholar]
- Harbison, M. (adapted from Dredge, M.). Daily infographics; beer edition: The beer flavor and aroma wheel. Pop. Sci. (Web Ed.) 2013, 10. Available online: https://www.popsci.com/science/article/2013-01/infographic-day-beersci-edition-beer-flavor-wheel/ (accessed on 9 December 2021).
- Wright, D.W. MDGC-MS-Olfactometry Based Device and Strategy for Preparation of Odor-Match Blends from Crude Odor Source Materials. U.S. Patent Application No. 62/605,374, 18 July 2017. [Google Scholar]
- Wright, D. Final Summary Report for Bridgeton Landfill Downwind Odor Assessment and Odorant Prioritization for the Missouri Attorney General’s Office; Missouri Department of Natural Resources: Jefferson City, MO, USA, August 2015. Available online: https://dnrservices.mo.gov/bridgeton/docs/bridgetonodorassessment.pdf (accessed on 9 December 2021).








| Steps | Case Study: PT Porcupine |
|---|---|
| (1) Downwind composite odor assessment | X (Figures S3 and S4) |
| (2) GC-O based odorant prioritization | X |
| (3) First-pass odor-match validation—GC-O | X |
| (4) Development of a synthetic odor-match formulation | X |
| (5) Final odor-match validation | X |
| (6) Analytical method development | (optional, not required) |
| (7) Instrument based environmental odor monitoring | (optional, not required) |
| Prehensile Porcupine VOCs & Odorants * | Common Priority Odorants * | Swine Barn VOCs & Odorants * |
|---|---|---|
| odor character = ‘grilled onion’ | odor character = ‘barnyard’ | |
| unknown ‘onion’ @13.9 min | ||
| unknown ‘onion’ @20.6 min | ||
| p-cresol | p-cresol | p-cresol |
| butyric acid | butyric acid | butyric acid |
| isovaleric acid | isovaleric acid | isovaleric acid |
| 2-amino acetophenone | ||
| 4-ethyl phenol | 4-ethyl phenol | 4-ethyl phenol |
| -quinazoline | ||
| skatole | skatole | skatole |
| indole | indole | indole |
| Sulfides | Sulfides | |
| dimethyl trisulfide | dimethyl trisulfide | dimethyl trisulfide |
| methyl mercaptan | methyl mercaptan | methyl mercaptan |
| dimethyl sulfide | dimethyl sulfide | dimethyl sulfide |
| propyl mercaptan | propyl mercaptan | |
| dimethyl disulfide | dimethyl disulfide | dimethyl disulfide |
| hydrogen sulfide | hydrogen sulfide | hydrogen sulfide |
| Fatty Acids | Fatty Acids | |
| valeric acid | valeric acid | valeric acid |
| hexanoic acid | hexanoic acid | hexanoic acid |
| propanoic acid | propanoic acid | propanoic acid |
| acetic acid | acetic acid | acetic acid |
| heptanoic acid | heptanoic acid | heptanoic acid |
| Amines | Amines | |
| trimethylamine | trimethylamine | |
| diethylamine | diethylamine | |
| 1-pyrroline | 1-pyrroline | |
| Aromatics | Aromatics | |
| guaiacol | guaiacol | guaiacol |
| benzaldehyde | benzaldehyde | benzaldehyde |
| 4-ethyl phenol | 4-ethyl phenol | 4-ethyl phenol |
| phenol | phenol | phenol |
| 4-methyl-2-nitrophenol | 4-methyl-2-nitrophenol | |
| para-vinyl phenol | para-vinyl phenol | |
| benzoic acid | benzoic acid | |
| phenyl acetic acid | phenyl acetic acid | |
| benzyl alcohol | benzyl alcohol | benzyl alcohol |
| Ketones | Ketones | |
| 2-octanone | 2-octanone | |
| 6-methyl-5-heptene-2-one | ||
| 2-undecanone | ||
| pentadecanone | ||
| diacetyl | diacetyl | diacetyl |
| acetone | acetone | acetone |
| Aldehydes | Aldehydes | |
| hexanal | hexanal | hexanal |
| nonanal | nonanal | nonanal |
| methional | methional | methional |
| undecanal | ||
| Alcohols | Alcohols | |
| 1-octene-3-ol | ||
| 3-octanol | ||
| 1-heptene-3-ol | ||
| trans-farnesol | ||
| maltol | ||
| geosmin | ||
| Miscellaneous | Miscellaneous | |
| 2-methyl furan | 2-methyl furan | 2-methyl furan |
| 1,3-pentadiene | 2-pentyl furan | |
| dimethyl pyrazine | dimethyl pyrazine | |
| 4,8-dimethyl-1,3,7-nonatriene | acetamide | |
| 1-methoxy-1,3,5-cycloheptatriene | 4-methyl pyridine | |
| tridecane | propanamide | |
| 6-heptyltetrahydro-2H-pyran-2-one | ||
| butanamide | ||
| 3-methyl-phenyl acetate | ||
| phenyl ethyl alcohol | ||
| pentamide | ||
| 2-pyrrolidinone | ||
| hexadecane | ||
| valerolactam | ||
| 5-methyl-2,4-imidazolidinedione |
| Panelist | Designation | Odor Descriptor | Odor-Match? | Match-Source | Odor-Match Components |
|---|---|---|---|---|---|
| #1 | lead investigator (D.W.W.) | ‘grilled onion’/‘hamburger joint’ | yes | environment + urine headspace + GC-O odorant isolate | environment + GC-O + GC-O odorant isolate |
| #2 | investigator #2 (A.I.) | ‘grilled onion’ | yes | urine headspace + GC-O odorant isolate | urine headspace + GC-O + GC-O odorant isolate |
| #3 | associate #1 | ‘grilled onion’ | yes | GC-O odorant isolate | odor character description |
| #4 | associate #2 | ‘grilled onion’ | yes | GC-O odorant isolate | odor character description |
| #5 | associate #3 | - | no | GC-O odorant isolate | odor character description |
| #6 | zookeeper | ‘savory sauce’ | yes | environment + GC-O odorant isolate | environment + GC-O odorant isolate |
| #7 | curator (P.K.) | ‘stale onion’ | - | environment | not available for survey |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wright, D.W.; Koziel, J.A.; Parker, D.B.; Iwasinska, A.; Hartman, T.G.; Kolvig, P.; Wahe, L. Qualitative Exploration of the ‘Rolling Unmasking Effect’ for Downwind Odor Dispersion from a Model Animal Source. Int. J. Environ. Res. Public Health 2021, 18, 13085. https://doi.org/10.3390/ijerph182413085
Wright DW, Koziel JA, Parker DB, Iwasinska A, Hartman TG, Kolvig P, Wahe L. Qualitative Exploration of the ‘Rolling Unmasking Effect’ for Downwind Odor Dispersion from a Model Animal Source. International Journal of Environmental Research and Public Health. 2021; 18(24):13085. https://doi.org/10.3390/ijerph182413085
Chicago/Turabian StyleWright, Donald W., Jacek A. Koziel, David B. Parker, Anna Iwasinska, Thomas G. Hartman, Paula Kolvig, and Landon Wahe. 2021. "Qualitative Exploration of the ‘Rolling Unmasking Effect’ for Downwind Odor Dispersion from a Model Animal Source" International Journal of Environmental Research and Public Health 18, no. 24: 13085. https://doi.org/10.3390/ijerph182413085
APA StyleWright, D. W., Koziel, J. A., Parker, D. B., Iwasinska, A., Hartman, T. G., Kolvig, P., & Wahe, L. (2021). Qualitative Exploration of the ‘Rolling Unmasking Effect’ for Downwind Odor Dispersion from a Model Animal Source. International Journal of Environmental Research and Public Health, 18(24), 13085. https://doi.org/10.3390/ijerph182413085







