Volatile Organic Compounds (VOCs) as Environmental Pollutants: Occurrence and Mitigation Using Nanomaterials
Abstract
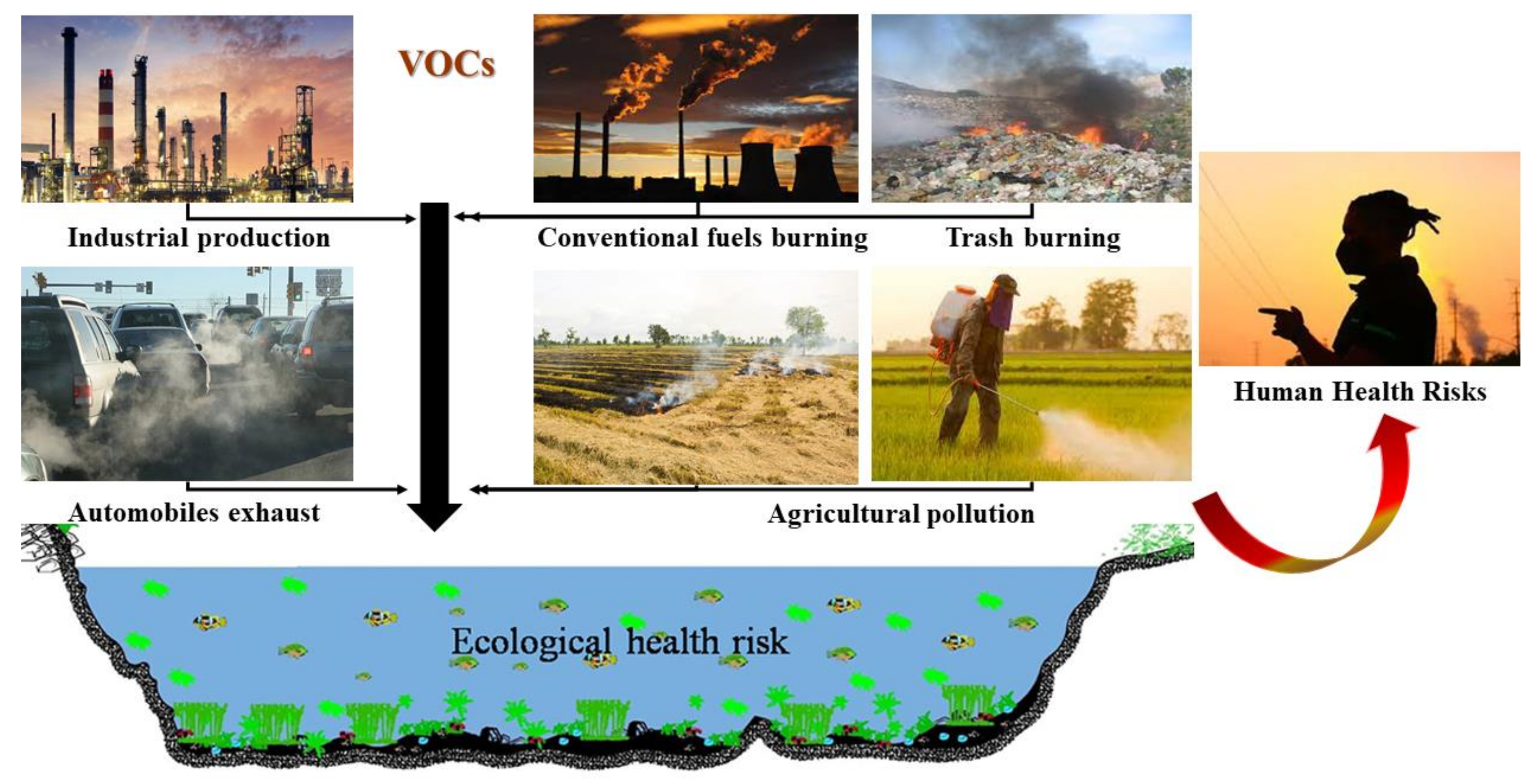
:1. Introduction
2. VOCs Classification
- (i)
- Exploitation and use of fossil fuels, e.g., incomplete burning of fossil fuels or their evaporation;
- (ii)
- Solvents used in paints and inks; approximately 12 billion litres of paints are produced annually and the usual solvents are aliphatic hydrocarbons, ethyl acetate, glycol ethers, acetone, etc. [27];
- (iii)
- The resulting products in the form of compressed aerosols, mainly butane and propane, contribute globally to 1.3 billion tonnes of VOC emissions per year [19];
- (iv)
- Use of biofuels, for example, cooking oils, bioethanol, bio-fuels;
- (v)
- Biomass combustion, especially from forests and agricultural wastes, although in principle the combustion results in carbon dioxide and water, incomplete combustion leads to a variety of VOCs;
- (vi)
- Toxic volatile organic compound released from metalworking fluids (MWFs);
- (vii)
- Incineration of household wastes and other sources.
3. VOCs Impact
4. VOCs Mitigation by Nanomaterials Use
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Faroon, O.; Taylor, J.; Roney, N.; Fransen, M.E.; Bogaczyk, S.; Diamond, G. Toxicological Profile for Carbon Tetrachloride; Department of Health and Human Services, Public Health Service Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2005.
- Chowdhury, S.; Champagne, P. Risk from exposure to trihalomethanes during shower: Probabilistic assessment and control. Sci. Total Environ. 2009, 407, 1570–1578. [Google Scholar] [CrossRef]
- Pandey, P.; Yadav, R. A Review on Volatile Organic Compounds (VOCs) as Environmental Pollutants: Fate and Distribution. Int. J. Plant Environ. 2018, 4, 14–26. [Google Scholar] [CrossRef]
- Liang, H.-M.; Liao, C.-M. Modeling VOC-odor exposure risk in livestock buildings. Chemosphere 2007, 68, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, M.E.; Abu Hassan, M.A.; Noor, Z.Z.; Ibrahim, R.K.R. Application of a packed column air stripper in the removal of volatile organic compounds from wastewater. Rev. Chem. Eng. 2014, 30, 431–451. [Google Scholar] [CrossRef]
- Spengler, J.D.; Yan, C.Q. Indoor air quality factors in designing a healthy building. Annu. Rev. Energy Environ. 2002, 25, 567–601. [Google Scholar] [CrossRef] [Green Version]
- Diduch, M.; Polkowska, Z.; Namiesnik, J. Chemical Quality of Bottled Waters: A Review. J. Food Sci. 2011, 76, R178–R196. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.; Dott, W. Relevance of airborne fungi and their secondary metabolites for environmental, occupational and indoor hygiene. Arch. Microbiol. 2003, 179, 75–82. [Google Scholar] [CrossRef]
- Komilis, D.P.; Ham, R.K.; Park, J.K. Emission of volatile organic compounds during composting of municipal solid wastes. Water Res. 2004, 38, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Escudero, L.B.; Grijalba, A.C.; Martinis, E.M.; Wuilloud, R.G. Bioanalytical separation and preconcentration using ionic liquids. Anal. Bioanal. Chem. 2013, 405, 7597–7613. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency USEPA. Available online: https://www.epa.gov/saferchoice (accessed on 2 November 2021).
- Lelieveld, J.; Hoor, P.; Jöckel, P.; Pozzer, A.; Hadjinicolaou, P.; Cammas, J.-P.; Beirle, S. Atmospheric Chemistry and Physics Severe Ozone Air Pollution in the Persian Gulf Region. Atmos. Chem. Phys. 2009, 9, 1393–1406. [Google Scholar] [CrossRef] [Green Version]
- Murrells, T. Climate Change Consequences of VOC Emission Controls. Report to the Department for Environment, Food and Rural Affairs, Welsh Assembly Government, the Scottish Executive and the Department of the Environment for Northern Ireland. AEA Energy Environ. 2007, 9, 1–19. [Google Scholar]
- Grodowska, K.; Parczewski, A. Organic solvents in the pharmaceutical industry. Acta Pol. Pharm. Drug Res. 2010, 67, 3–12. [Google Scholar]
- Attia, M.F.; Swasy, M.I.; Ateia, M.; Alexis, F.; Whitehead, D.C. Periodic mesoporous organosilica nanomaterials for rapid capture of VOCs. Chem. Commun. 2019, 56, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Roman, P.; Bijmans, M.F.M.; Janssen, A.J.H. Influence of methanethiol on biological sulphide oxidation in gas treatment system. Environ. Technol. 2016, 37, 1693–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimotakis, E.D.; Cal, M.P.; Economy, J.; Rood, M.J.; Larson, S.M. Chemicallv Treated Activated Carbon Cloths for Removal of Volatile Organic Carbons from Gas Streams: Evidence for Enhanced Physical Adsorption. Environ. Sci. Technol. 1995, 29, 1876–1880. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.D.; Attia, M.F.; Whitehead, D.C.; Alexis, F. Nanotechnology for Environmental Remediation: Materials and Applications. Molecules 2018, 23, 1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.; Koppmann, R. Volatile Organic Compounds in the Atmosphere: An Overview. Environ. Chem. 2007, 1–32. [Google Scholar] [CrossRef]
- Pichersky, E.; Gershenzon, J. The formation and function of plant volatiles: Perfumes for pollinator attraction and defense. Curr. Opin. Plant. Biol. 2002, 5, 237–243. [Google Scholar] [CrossRef]
- Sindelarova, K.; Granier, C.; Bouarar, I.; Guenther, A.; Tilmes, S.; Stavrakou, T.; Müller, J.-F.; Kuhn, U.; Stefani, P.; Knorr, W. Global data set of biogenic VOC emissions calculated by the MEGAN model over the last 30 years. Atmos. Chem. Phys. Discuss. 2014, 14, 9317–9341. [Google Scholar] [CrossRef] [Green Version]
- Reimann, S.; Lewis, A.C. Anthropogenic VOCs. In Volatile Organic Compounds in the Atmosphere; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Yeoman, A.M.; Lewis, A.C. Global emissions of VOCs from compressed aerosol products. Elem. Sci. Anth. 2021, 9, 117. [Google Scholar] [CrossRef]
- Holøs, S.B.; Yang, A.; Lind, M.; Thunshelle, K.; Schild, P.; Mysen, M. VOC emission rates in newly built and renovated buildings, and the influence of ventilation—A review and meta-analysis. Int. J. Vent. 2018, 18, 153–166. [Google Scholar] [CrossRef]
- Heeley-Hill, A.C.; Grange, S.K.; Ward, M.W.; Lewis, A.C.; Owen, N.; Jordan, C.; Hodgson, G.; Adamson, G. Frequency of use of household products containing VOCs and indoor atmospheric concentrations in homes. Environ. Sci. Process. Impacts 2021, 23, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.M.; Lawal, O.; Nijsen, T.M.; Goodacre, R.; Fowler, S.J. Exhaled Volatile Organic Compounds of Infection: A Systematic Review. ACS Infect. Dis. 2017, 3, 695–710. [Google Scholar] [CrossRef]
- Stoye, D.; Funke, W.; Hoppe, L.; Hasselkus, U.; Hoehne, K.; Zech, H.-J.; Heiling, P.; Yamabe, M.; Schupp, H.; Schmitthenner, M.; et al. Paints and Coatings. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000; pp. 1–200. [Google Scholar] [CrossRef]
- Wang, S.; Ang, H.M.; Tade, M.O. Volatile organic compounds in indoor environment and photocatalytic oxidation: State of the art. Environ. Int. 2007, 33, 694–705. [Google Scholar] [CrossRef]
- Langer, S.; de Wit, C.A.; Giovanoulis, G.; Fäldt, J.; Karlson, L. The effect of reduction measures on concentrations of hazardous semivolatile organic compounds in indoor air and dust of Swedish preschools. Indoor Air 2021, 31, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Yli-Juuti, T.; Mohr, C.; Riipinen, I. Open questions on atmospheric nanoparticle growth. Commun. Chem. 2020, 3, 1–4. [Google Scholar] [CrossRef]
- Guo, S.; Hu, M.; Peng, J.; Wu, Z.; Zamora, M.L.; Shang, D.; Du, Z.; Zheng, J.; Fang, X.; Tang, R.; et al. Remarkable nucleation and growth of ultrafine particles from vehicular exhaust. Proc. Natl. Acad. Sci. USA 2020, 117, 3427–3432. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Chen, C.; Nagatsu, M.; Wang, X. Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem. Eng. J. 2011, 170, 395–410. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-Layered Graphene Oxide Nanosheets As Superior Sorbents for Heavy Metal Ion Pollution Management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Fang, Z.; Yan, X.; Gu, F.; Jiang, F. Emergency remediation of simulated chromium (VI)-polluted river by nanoscale zero-valent iron: Laboratory study and numerical simulation. Chem. Eng. J. 2012, 193–194, 358–365. [Google Scholar] [CrossRef]
- Ateia, M.; Arifuzzaman, M.; Pellizzeri, S.; Attia, M.F.; Tharayil, N.; Anker, J.N.; Karanfil, T. Cationic polymer for selective removal of GenX and short-chain PFAS from surface waters and wastewaters at ng/L levels. Water Res. 2019, 163, 114874. [Google Scholar] [CrossRef] [PubMed]
- Arkas, M.; Allabashi, R.; Tsiourvas, D.; Mattausch, E.-M.; Perfler, R. Organic/Inorganic Hybrid Filters Based on Dendritic and Cyclodextrin “Nanosponges” for the Removal of Organic Pollutants from Water. Environ. Sci. Technol. 2006, 40, 2771–2777. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Su, F.; Hu, S. Surface modification of carbon nanotubes for enhancing BTEX adsorption from aqueous solutions. Appl. Surf. Sci. 2008, 254, 7035–7041. [Google Scholar] [CrossRef]
- Su, F.; Lu, C.; Hu, S. Adsorption of benzene, toluene, ethylbenzene and p-xylene by NaOCl-oxidized carbon nanotubes. Colloids Surf. A Physicochem. Eng. Asp. 2010, 353, 83–91. [Google Scholar] [CrossRef]
- Liu, G.; Wang, J.; Zhu, Y.; Zhang, X. Application of Multiwalled Carbon Nanotubes as a Solid-Phase Extraction Sorbent for Chlorobenzenes. Anal. Lett. 2004, 37, 3085–3104. [Google Scholar] [CrossRef]
- Peng, X.; Li, Y.; Luan, Z.; Di, Z.; Wang, H.; Tian, B.; Jia, Z. Adsorption of 1,2-dichlorobenzene from water to carbon nanotubes. Chem. Phys. Lett. 2003, 376, 154–158. [Google Scholar] [CrossRef]
- Li, X.; Yuan, J.; Du, J.; Sui, H.; He, L. Functionalized Ordered Mesoporous Silica by Vinyltriethoxysilane for the Removal of Volatile Organic Compounds through Adsorption/Desorption Process. Ind. Eng. Chem. Res. 2020, 59, 3511–3520. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Deng, Y.; Liu, G.; Chen, Y.; Yang, J. Emerging nanostructured materials for the catalytic removal of volatile organic compounds. Nanotechnol. Rev. 2016, 5, 147–181. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Wang, Y.; He, H. Sodium-Promoted Pd/TiO2 for Catalytic Oxidation of Formaldehyde at Ambient Temperature. Environ. Sci. Technol. 2014, 48, 5816–5822. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, F.; Zhang, L.; Pan, S.; Bian, C.; Zheng, X.; Meng, X.; Xiao, F.-S. Importance of platinum particle size for complete oxidation of toluene over Pt/ZSM-5 catalysts. Chem. Commun. 2015, 51, 5936–5938. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, K.; Li, J.; Tang, W.; Xu, J.; Liu, H.; Yue, R.; Chen, Y. Surface Diffusion of Pt Clusters in/on SiO2 Matrix at Elevated Temperatures and Their Improved Catalytic Activities in Benzene Oxidation. J. Phys. Chem. C 2014, 118, 22719–22729. [Google Scholar] [CrossRef]
- Huang, H.; Leung, D.Y.C. Complete Oxidation of Formaldehyde at Room Temperature Using TiO2 Supported Metallic Pd Nanoparticles. ACS Catal. 2011, 1, 348–354. [Google Scholar] [CrossRef]
- Ousmane, M.; Liotta, L.F.; Pantaleo, G.; Venezia, A.M.; Di Carlo, G.; Aouine, M.; Retailleau, L.; Giroir-Fendler, A. Supported Au catalysts for propene total oxidation: Study of support morphology and gold particle size effects. Catal. Today 2011, 176, 7–13. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Zhang, Y.; Chen, M.; Wang, L.; Zhang, C.; He, H. Effect of Support on the Activity of Ag-based Catalysts for Formaldehyde Oxidation. Sci. Rep. 2015, 5, 12950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharlamova, T.; Mamontov, G.; Salaev, M.; Zaikovskii, V.; Popova, G.; Sobolev, V.; Knyazev, A.; Vodyankina, O. Silica-supported silver catalysts modified by cerium/manganese oxides for total oxidation of formaldehyde. Appl. Catal. A Gen. 2013, 467, 519–529. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, J.; Jaroniec, M. Efficient catalytic removal of formaldehyde at room temperature using AlOOH nanoflakes with deposited Pt. Appl. Catal. B Environ. 2015, 163, 306–312. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Chen, Y. Catalytic removal of gaseous benzene over Pt/SBA-15 catalyst: The effect of the preparation method. React. Kinet. Mech. Catal. 2014, 114, 711–723. [Google Scholar] [CrossRef]
- Huang, H.; Hu, P.; Huang, H.; Chen, J.; Ye, X.; Leung, D.Y.C. Highly dispersed and active supported Pt nanoparticles for gaseous formaldehyde oxidation: Influence of particle size. Chem. Eng. J. 2014, 252, 320–326. [Google Scholar] [CrossRef]
- Bendahou, K.; Cherif, L.; Siffert, S.; Tidahy, H.L.; Benaïssa, H.; Aboukaïs, A. The effect of the use of lanthanum-doped mesoporous SBA-15 on the performance of Pt/SBA-15 and Pd/SBA-15 catalysts for total oxidation of toluene. Appl. Catal. A Gen. 2008, 351, 82–87. [Google Scholar] [CrossRef]
- Jin, L.-Y.; Ma, R.-H.; Lin, J.-J.; Meng, L.; Wang, Y.-J.; Luo, M.-F. Bifunctional Pd/Cr2O3–ZrO2Catalyst for the Oxidation of Volatile Organic Compounds. Ind. Eng. Chem. Res. 2011, 50, 10878–10882. [Google Scholar] [CrossRef]
- Maira, A.J.; Yeung, K.L.; Lee, C.Y.; Yue, P.L.; Chan, C.K. Size Effects in Gas-Phase Photo-oxidation of Trichloroethylene Using Nanometer-Sized TiO2 Catalysts. J. Catal. 2000, 192, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Maira, A.J.; Coronado, J.M.; Augugliaro, V.; Yeung, K.L.; Conesa, J.C.; Soria, J. Fourier Transform Infrared Study of the Performance of Nanostructured TiO2 Particles for the Photocatalytic Oxidation of Gaseous Toluene. J. Catal. 2001, 202, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Weon, S.; Choi, W. TiO2 Nanotubes with Open Channels as Deactivation-Resistant Photocatalyst for the Degradation of Volatile Organic Compounds. Environ. Sci. Technol. 2016, 50, 2556–2563. [Google Scholar] [CrossRef]
- Lee, H.J.; Seo, H.O.; Kim, D.W.; Kim, K.-D.; Luo, Y.; Lim, D.C.; Ju, H.; Kim, J.W.; Lee, J.; Kim, Y.D. A high-performing nanostructured TiO2 filter for volatile organic compounds using atomic layer deposition. Chem. Commun. 2011, 47, 5605–5607. [Google Scholar] [CrossRef] [PubMed]
- Weon, S.; Choi, J.; Park, T.; Choi, W. Freestanding doubly open-ended TiO2 nanotubes for efficient photocatalytic degradation of volatile organic compounds. Appl. Catal. B Environ. 2017, 205, 386–392. [Google Scholar] [CrossRef]
- Azzouz, I.; Habba, Y.G.; Capochichi-Gnambodoe, M.; Marty, F.; Vial, J.; Leprince-Wang, Y.; Bourouina, T. Zinc oxide nano-enabled microfluidic reactor for water purification and its applicability to volatile organic compounds. Microsyst. Nanoeng. 2018, 4, 17093. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Z.; Zhao, Q.; Wang, L. Photocatalytic degradation of gaseous toluene over ZnAl2O4 prepared by different methods: A comparative study. J. Hazard. Mater. 2011, 186, 2089–2096. [Google Scholar] [CrossRef] [PubMed]
- Genuino, H.C.; Dharmarathna, S.; Njagi, E.C.; Mei, M.C.; Suib, S.L. Gas-Phase Total Oxidation of Benzene, Toluene, Ethylbenzene, and Xylenes Using Shape-Selective Manganese Oxide and Copper Manganese Oxide Catalysts. J. Phys. Chem. C 2012, 116, 12066–12078. [Google Scholar] [CrossRef]
- Miyawaki, J.; Lee, G.-H.; Yeh, J.; Shiratori, N.; Shimohara, T.; Mochida, I.; Yoon, S.-H. Development of carbon-supported hybrid catalyst for clean removal of formaldehyde indoors. Catal. Today 2012, 185, 278–283. [Google Scholar] [CrossRef]
- Schick, L.; Sanchis, R.; González-Alfaro, V.; Agouram, S.; López, J.M.; Torrente-Murciano, L.; García, T.; Solsona, B. Size-activity relationship of iridium particles supported on silica for the total oxidation of volatile organic compounds (VOCs). Chem. Eng. J. 2019, 366, 100–111. [Google Scholar] [CrossRef]
- Li, J.-J.; Cai, S.-C.; Yu, E.-Q.; Weng, B.; Chen, X.; Chen, J.; Jia, H.-P.; Xu, Y.-J. Efficient infrared light promoted degradation of volatile organic compounds over photo-thermal responsive Pt-rGO-TiO2 composites. Appl. Catal. B Environ. 2018, 233, 260–271. [Google Scholar] [CrossRef]
- Samaddar, P.; Son, Y.S.; Tsang, D.C.W.; Kim, K.H.; Kumar, S. Progress in Graphene-Based Materials as Superior Media for Sensing, Sorption, and Separation of Gaseous Pollutants. Coord. Chem. Rev. 2018, 368, 93–114. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Z.-R.; Fu, X.; Xu, Y.-J. TiO2−Graphene Nanocomposites for Gas-Phase Photocatalytic Degradation of Volatile Aromatic Pollutant: Is TiO2−Graphene Truly Different from Other TiO2−Carbon Composite Materials? ACS Nano 2010, 4, 7303–7314. [Google Scholar] [CrossRef] [PubMed]
- Roso, M.; Boaretti, C.; Pelizzo, M.G.; Lauria, A.; Modesti, M.; Lorenzetti, A. Nanostructured Photocatalysts Based on Different Oxidized Graphenes for VOCs Removal. Ind. Eng. Chem. Res. 2017, 56, 9980–9992. [Google Scholar] [CrossRef]
- Liu, G.-Q.; Wan, M.-X.; Huang, Z.-H.; Kang, F.-Y. Preparation of graphene/metal-organic composites and their adsorption performance for benzene and ethanol. New Carbon Mater. 2015, 30, 566–571. [Google Scholar] [CrossRef]
- Kumar, V.; Lee, Y.-S.; Shin, J.-W.; Kim, K.-H.; Kukkar, D.; Tsang, Y.F. Potential applications of graphene-based nanomaterials as adsorbent for removal of volatile organic compounds. Environ. Int. 2020, 135, 105356. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lee, C.Y.; Jerng, D.W.; Ahn, H.S. Toluene and acetaldehyde removal from air on to graphene-based adsorbents with microsized pores. J. Hazard. Mater. 2018, 344, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yu, Y.; Xiao, J.; Li, Y.; Li, Z. Improved Ethanol Adsorption Capacity and Coefficient of Performance for Adsorption Chillers of Cu-BTC@GO Composite Prepared by Rapid Room Temperature Synthesis. Ind. Eng. Chem. Res. 2016, 55, 11767–11774. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, L.; Meng, T.; Yu, F.; Chen, J.; Ma, J. Facile Synthesis of 3D Amino-Functional Graphene-Sponge Composites Decorated by Graphene Nanodots with Enhanced Removal of Indoor Formaldehyde. Aerosol Air Qual. Res. 2015, 15, 1028–1034. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Qin, Z.; Zhang, L.; Meng, T.; Yu, F.; Ma, J. CNT-enhanced amino-functionalized graphene aerogel adsorbent for highly efficient removal of formaldehyde. New J. Chem. 2017, 41, 2527–2533. [Google Scholar] [CrossRef]
- Zheng, Y.; Chu, F.; Zhang, B.; Yan, J.; Chen, Y. Ultrahigh adsorption capacities of carbon tetrachloride on MIL-101 and MIL-101/graphene oxide composites. Microporous Mesoporous Mater. 2017, 263, 71–76. [Google Scholar] [CrossRef]


| Class | Examples of Compounds | Boiling Point Range °C |
|---|---|---|
| Very volatile organic compounds (VVOCs) | propane, butane, methyl-chloride | 0 to 50–100 |
| Volatile organic compounds (VOCs) | formaldehyde, toluene, acetone, isopropyl alcohol | 50–100 to 240–260 |
| Semi volatile organic compounds (SVOCs) | pesticides (chlordane, DDT), plasticizers (phthalates) | 240–260 to 380–400 |
| Catalyst | Preparation Method | Loading (wt%) | Catalyst Mass (mg) | VOC Type | VOC Concentration (ppm) | Reference |
|---|---|---|---|---|---|---|
| Pt/TiO2 | impregnation | 0.01–1.00 | 250 | formaldehyde | 22 | [43] |
| Pt/ZSM-5 | reduction | 0.50–2.00 | 100 | toluene | 1000 | [44] |
| Pt/SiO2 | flame spray pyrolysis | 0.21 | 100 | benzene | 100 | [45] |
| Pd/TiO2 | impregnation + reduction | 1.00 | 500 | formaldehyde | 10 | [46] |
| Pd/TiO2 | deposition-precipitation + reduction | 1.00 | 500 | formaldehyde | 10 | [46] |
| Au/TiO2 | deposition-precipitation | 1.00 | 200 | propene | 1000 | [47] |
| Ag/TiO2 | impregnation | 8.00 | 60 | formaldehyde | 110 | [48] |
| Ag/CeO2/SiO2 | impregnation | 5.00 | 145 | formaldehyde | 18,000–22,000 | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
David, E.; Niculescu, V.-C. Volatile Organic Compounds (VOCs) as Environmental Pollutants: Occurrence and Mitigation Using Nanomaterials. Int. J. Environ. Res. Public Health 2021, 18, 13147. https://doi.org/10.3390/ijerph182413147
David E, Niculescu V-C. Volatile Organic Compounds (VOCs) as Environmental Pollutants: Occurrence and Mitigation Using Nanomaterials. International Journal of Environmental Research and Public Health. 2021; 18(24):13147. https://doi.org/10.3390/ijerph182413147
Chicago/Turabian StyleDavid, Elena, and Violeta-Carolina Niculescu. 2021. "Volatile Organic Compounds (VOCs) as Environmental Pollutants: Occurrence and Mitigation Using Nanomaterials" International Journal of Environmental Research and Public Health 18, no. 24: 13147. https://doi.org/10.3390/ijerph182413147
APA StyleDavid, E., & Niculescu, V.-C. (2021). Volatile Organic Compounds (VOCs) as Environmental Pollutants: Occurrence and Mitigation Using Nanomaterials. International Journal of Environmental Research and Public Health, 18(24), 13147. https://doi.org/10.3390/ijerph182413147







