Reducing Biofilm Infections in Burn Patients’ Wounds and Biofilms on Surfaces in Hospitals, Medical Facilities and Medical Equipment to Improve Burn Care: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results
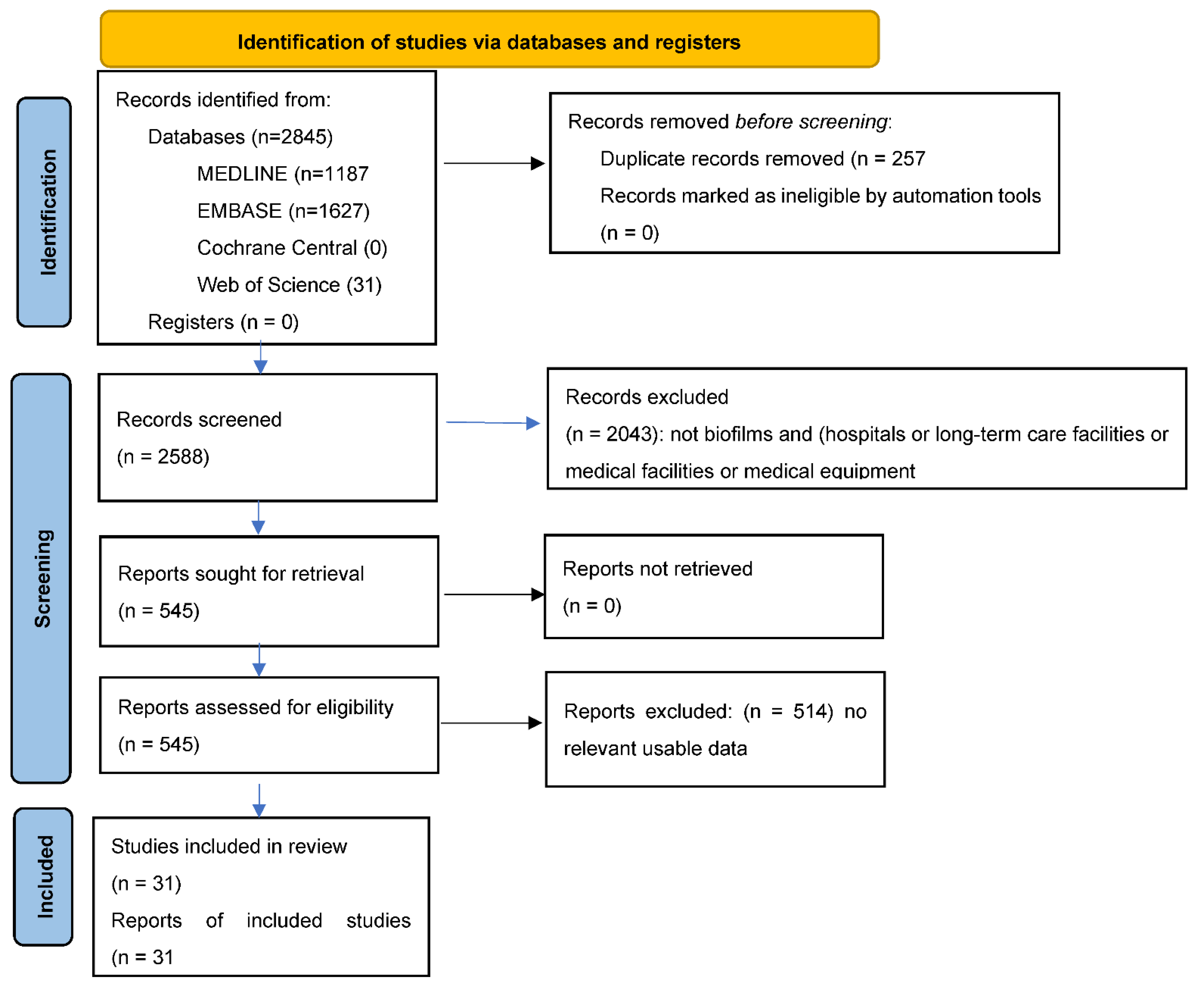
3.1. Literature Search
3.2. Interventions
3.2.1. Silver
3.2.2. Other Metals
3.2.3. Disinfectants
3.2.4. Hydrogels
3.2.5. Sound
3.2.6. Light
3.3. Small Molecules
3.4. Glycans
3.5. Lactobacilli
3.6. Bacteriophages
3.6.1. Risk of Bias Assessment: Numbers of Bacterial Strains Tested, Numbers of In Vivo Tests Using Animals, and Summary Measures Used in In Vitro and In Vivo Biofilm Outcomes
3.6.2. Identification of Candidate Interventions for Further Testing in Large Scale c-RCTs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodwine, J.; Gil, J.; Doiron, A.; Valdes, J.; Solis, M.; Higa, A.; Davis, S.; Sauer, K. Pyruvate-depleting conditions induce biofilm dispersion and enhance the efficacy of antibiotics in killing biofilms in vitro and in vivo. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Pourhajibagher, M.; Mahmoudi, H.; Rezaei-Soufi, L.; Alikhani, M.Y.; Bahador, A. Potentiation effects of antimicrobial photodynamic therapy on quorum sensing genes expression: A promising treatment for multi-species bacterial biofilms in burn wound infections. Photodiagn. Photodyn. Ther. 2020, 30, 101717. [Google Scholar] [CrossRef]
- Lu, M.; Wang, S.; Wang, T.; Hu, S.; Bhayana, B.; Ishii, M.; Kong, Y.; Cai, Y.; Dai, T.; Cui, W.; et al. Bacteria-specific phototoxic reactions triggered by blue light and phytochemical carvacrol. Sci. Transl. Med. 2021, 13, eaba3571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, X.; Chen, J.; Amin, R.; Lu, M.; Bhayana, B.; Zhao, J.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; et al. Antimicrobial Blue Light Inactivation of Gram-Negative Pathogens in Biofilms: In Vitro and In Vivo Studies. J. Infect. Dis. 2016, 213, 1380–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konai, M.M.; Haldar, J. Lysine-Based Small Molecule Sensitizes Rifampicin and Tetracycline against Multidrug-Resistant Acinetobacter baumannii and Pseudomonas aeruginosa. ACS Infect. Dis. 2019, 6, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gholamrezazadeh, M.; Shakibaie, M.R.; Monirzadeh, F.; Masoumi, S.; Hashemizadeh, Z. Effect of nano-silver, nano-copper, deconex and benzalkonium chloride on biofilm formation and expression of transcription regulatory quorum sensing gene (rh1 R) in drug-resistance Pseudomonas aeruginosa burn isolates. Burns 2018, 44, 700–708. [Google Scholar] [CrossRef]
- Yali, G.; Jing, C.; Chunjiang, L.; Cheng, Z.; Xiaoqiang, L.; Yizhi, P. Comparison of pathogens and antibiotic resistance of burn patients in the burn ICU or in the common burn ward. Burns 2014, 40, 402–407. [Google Scholar] [CrossRef]
- Karaky, N.; Kirby, A.; McBain, A.; Butler, J.; El Mohtadi, M.; Banks, C.E.; Whitehead, K.A. Metal ions and graphene-based compounds as alternative treatment options for burn wounds infected by antibiotic-resistant Pseudomonas aeruginosa. Arch. Microbiol. 2020, 202, 995–1004. [Google Scholar] [CrossRef] [Green Version]
- Andersson, M.; Madsen, L.; Schmidtchen, A.; Puthia, M. Development of an Experimental Ex Vivo Wound Model to Evaluate Antimicrobial Efficacy of Topical Formulations. Int. J. Mol. Sci. 2021, 22, 5045. [Google Scholar] [CrossRef]
- Thomas, R.E.; Thomas, B.C.; Conly, J.; Lorenzetti, D. Cleaning and disinfecting surfaces in hospitals and long-term care facilities for reducing hospital and facility-acquired bacterial and viral infections: A systematic review. J. Hosp. Infect. 2021, in press. [Google Scholar]
- Stewart, P.S.; Parker, A.E. Measuring Antimicrobial Efficacy against Biofilms: A Meta-analysis. Antimicrob. Agents Chemother. 2019, 63, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Schwarzer, S.; James, G.A.; Goeres, D.; Bjarnsholt, T.; Vickery, K.; Percival, S.L.; Stoodley, P.; Schultz, G.; Jensen, S.O.; Malone, M. The efficacy of topical agents used in wounds for managing chronic biofilm infections: A systematic review. J. Infect. 2020, 80, 261–270. [Google Scholar] [CrossRef]
- Halstead, F.D.; Rauf, M.; Bamford, A.; Wearn, C.M.; Bishop, J.R.; Burt, R.; Fraise, A.P.; Moiemen, N.S.; Oppenheim, B.A.; Webber, M.A. Antimicrobial dressings: Comparison of the ability of a panel of dressings to prevent biofilm formation by key burn wound pathogens. Burns 2015, 41, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Partoazar, A.; Alaeddini, M.; Etemad-Moghadam, S.; Bahador, A. Photodisinfection effects of silver sulfadiazine nanoliposomes doped-curcumin on Acinetobacter baumannii: A mouse model. Nanomedicine 2020, 15, 437–452. [Google Scholar] [CrossRef]
- Li, D.; Guo, Q.; Ding, L.; Zhang, W.; Cheng, L.; Wang, Y.; Xu, Z.; Wang, H.; Gao, L. Bimetallic CuCo 2 S 4 Nanozymes with Enhanced Peroxidase Activity at Neutral pH for Combating Burn Infections. ChemBioChem 2020, 21, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Nozari, M.; Gholizadeh, M.; Oghani, F.Z.; Tahvildari, K. Studies on novel chitosan/alginate and chitosan/bentonite flexible films incorporated with ZnO nano particles for accelerating dermal burn healing: In vivo and in vitro evaluation. Int. J. Biol. Macromol. 2021, 184, 235–249. [Google Scholar] [CrossRef]
- Halstead, F.D.; Rauf, M.; Moiemen, N.S.; Bamford, A.; Wearn, C.M.; Fraise, A.P.; Lund, P.A.; Oppenheim, B.A.; Webber, M.A. The Antibacterial Activity of Acetic Acid against Biofilm-Producing Pathogens of Relevance to Burns Patients. PLoS ONE 2015, 10, e0136190. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Sun, H.; Yang, Y.; Jing, H.; Yang, L.; Tong, Y.; Wei, C.; Wang, Z.; Zou, Q.; Zeng, H. Enhanced efficacy and anti-biofilm activity of novel nanoemulsions against skin burn wound multi-drug resistant MRSA infections. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1543–1555. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Rajak, S.; Mondal, D.P.; Biswas, D. Sodium hypochlorite is more effective than 70% ethanol against biofilms of clinical isolates of Staphylococcus aureus. Am. J. Infect. Control 2018, 46, e37–e42. [Google Scholar] [CrossRef]
- Chhibber, T.; Gondil, V.S.; Sinha, V.R. Development of Chitosan-Based Hydrogel Containing Antibiofilm Agents for the Treatment of Staphylococcus aureus–Infected Burn Wound in Mice. AAPS Pharm. Sci. Tech. 2020, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Pourhajibagher, M.; Rahimi-esboei, B.; Ahmadi, H.; Bahador, A. The anti-biofilm capability of nano-emodin mediated sonodynamic therapy on multi-species biofilms produced by burn wound bacterial strains. Photodiagn. Photodyn. Ther. 2021, 24, 102288. [Google Scholar] [CrossRef]
- Ishiwata, N.; Tsunoi, Y.; Sarker, R.R.; Haruyama, Y.; Kawauachi, S.; Sekine, Y.; Onuma, C.; Tsuda, H.; Saitoh, D.; Nichidate, I.; et al. Control of burn wound infection by methylene blue-mediated photodynamic treatment with light-emitting diode array illumination in rats. Lasers Surg. Med. 2021, 53, 1238–1246. [Google Scholar] [CrossRef]
- Banar, M.; Emaneini, M.; Satarzadeh, M.; Abdellahi, N.; Beigverdi, R.; Leeuwen, W.B.; Jabalameli, F. Evaluation of Mannosidase and Trypsin Enzymes Effects on Biofilm Production of Pseudomonas aeruginosa Isolated from Burn Wound Infections. PLoS ONE 2016, 11, e0164622. [Google Scholar]
- Ghosh, C.; Manjunath, G.B.; Konai, M.M.; Uppu, D.S.; Paramanandham, K.; Shome, B.R.; Ravikumar, R.; Haldar, J. Aryl-alkyl-lysines: Membrane-Active Small Molecules Active against Murine Model of Burn Infection. ACS Infect. Dis. 2016, 2, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Ma, Z.; Gao, P.; Lu, Z.; Liu, H.; Gao, L.; Lu, W.; Ju, X.; Lv, F.H.; Bie, X. The antibacterial activity of LI-F type peptide against methicillin-resistant Staphylococcus aureus (MRSA) in vitro and inhibition of infections in murine scalded epidermis. Appl. Microbiol. Biotechnol. 2018, 102, 2301–2311. [Google Scholar] [CrossRef]
- Memariani, H.; Shahbazzadeh, D.; Sabatier, J.M.; Memariani, M.; Karbalaeimahdi, A.; Bagheri, K.P. Mechanism of action and in vitro activity of short hybrid antimicrobial peptide PV3 against Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 2016, 479, 103–108. [Google Scholar] [CrossRef]
- Pan, M.; Lu, C.; Zheng, M.; Zhou, W.; Song, F.; Chen, W.F.; Liu, D.; Cai, J. Unnatural Amino-Acid-Based Star-Shaped Poly(l-Ornithine)s as Emerging Long-Term and Biofilm-Disrupting Antimicrobial Peptides to Treat Pseudomonas aeruginosa-Infected Burn Wounds. Adv. Healthc. Mater. 2020, 9, e2000647. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Qiu, L.; Deng, Y.; Ruiz, C.H.; Rudolf, J.D.; Dong, L.B.; Feng, X.; Cameron, M.D.; Shen, B.; Duan, Y.; et al. Evaluation of Platensimycin and Platensimycin-Inspired Thioether Analogues against Methicillin-Resistant Staphylococcus aureus in Topical and Systemic Infection Mouse Models. Mol. Pharm. 2019, 16, 3065–3071. [Google Scholar] [CrossRef]
- Uusitalo, P.; Hagglund, U.; Rhoos, E.; Scherman, N.H.; Elofsson, M.; Sundin, C. The salicylidene acylhydrazide INP0341 attenuates Pseudomonas aeruginosa virulence in vitro and in vivo. J. Antibiot. 2017, 70, 937–943. [Google Scholar] [CrossRef]
- Wheeler, K.M.; Carcamo-Oyarce, G.; Turner, B.S.; Dellos-Nolan, S.; Co, J.Y.; Lehoux, S.; Cummings, R.D.; Wozniak, D.J.; Ribbeck, K. Mucin glycans attenuate the virulence of Pseudomonas aeruginosa in infection. Nat. Microbiol. 2019, 4, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- Lenzmeier, T.D.; Mudaliar, N.S.; Stanbro, J.A.; Watters, C.; Ahmad, A.; Simons, M.P.; Ventolini, G.; Zak, J.C.; Colmer-Hamood, J.A.; Hamood, A.N. Application of Lactobacillus gasseri 63 AM supernatant to Pseudomonas aeruginosa-infected wounds prevents sepsis in murine models of thermal injury and dorsal excision. J. Med. Microbiol. 2019, 68, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
- Ssekatawa, K.; Byarugaba, D.K.; Kato, C.D.; Wampande, E.M.; Ejobi, F.; Tweyongyere, R.; Nakavuma, J.L. A review of phage mediated antibacterial applications. Alex. J. Med. 2020, 57, 1–20. [Google Scholar] [CrossRef]
- Alves, D.R.; Booth, S.P.; Scavone, P.; Schellenberger, P.; Salvage, J.; Dedi, C.; Thet, N.T.; Jenkins, A.T.A.; Waters, R.; Ng, K.W.; et al. Development of a High-Throughput ex-Vivo Burn Wound Model Using Porcine Skin, and Its Application to Evaluate New Approaches to Control Wound Infection. Front. Cell Infect. Microbiol. 2018, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.H.; Tseng, C.C.; Wang, L.S.; Chen, Y.T.; Ho, G.J.; Lin, T.Y.; Wang, L.Y.; Chen, L.K. Application of Bacteriophage-containing Aerosol against Nosocomial Transmission of Carbapenem-Resistant Acinetobacter baumannii in an Intensive Care Unit. PLoS ONE. 2016, 11, e0168380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holguin, A.V.; Rangel, G.; Clavijo, V.; Prada, C.; Mantilla, M.; Gomez, M.C.; Kutter, E.; Taylor, C.; Fineran, P.C.; Barrios, A.F.; et al. Phage PHIPan70, a Putative Temperate Phage, Controls Pseudomonas aeruginosa in Planktonic, Biofilm and Burn Mouse Model Assays. Viruses 2015, 7, 4602–4623. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, S.; Ross, R.P.; Meaney, W.; Fitzgerald, G.F.; Elbreki, M.F.; Coffey, A. Potential of the polyvalent anti-Staphylococcus bacteriophage K for control of antibiotic-resistant staphylococci from hospitals. Appl. Environ. Microbiol. 2005, 71, 1836–1842. [Google Scholar] [CrossRef] [Green Version]
- Pallavali, R.R.; Degati, V.L.; Narala, V.R.; Velpula, K.K.; Yenugu, S.; Durbaka, V.R.P. Lytic bacteriophages against bacterial biofilms formed by multidrug-resistant Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and Staphylococcus aureus isolated form burn wounds. PHAGE Ther. Appl. Res. 2021, 2, 120–130. [Google Scholar]
- Jones, L.D.; Mana, T.S.C.; Cadnum, J.L.; Jencson, A.L.; Silva, S.Y.; Wilson, B.M.; Donskey, C.J. Effectiveness of foam disinfectants in reducing sink-drain gram-negative bacterial colonization. Infect Control Hosp. Epidemiol. 2020, 41, 280–285. [Google Scholar] [CrossRef]
- Mitchell, B.G.; Fasugba, O.; Russo, P.L. Where is the strength of evidence? A review of infection prevention and control guidelines. J. Hosp. Inf. 2020, 105, 242–252. [Google Scholar] [CrossRef]
- Akinbobola, A.B.; Sherry, L.; Mckay, W.G.; Ramage, G.; Williams, C. Tolerance of Pseudomonas aeruginosa in in-vitro biofilms to high-level peracetic acid disinfection. J. Hosp. Infect. 2017, 97, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Yang, Q.; Li, B.; Yao, Y. Establishment of a quality control circle to reduce biofilm formation in flexible endoscopes by improvement of qualified cleaning rate. J. Intern. Med. Res. 2020, 48, 300060520952983. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.M.; Graziano, K.U.; Olson, N.; Franca, R.; Alfa, M.J. The polytetrafluoroethylene (PTFE) channel model of cyclic-buildup biofilm and traditional biofilm: The impact of friction, and detergent on cleaning and subsequent high-level disinfection. Infect Control Hosp. Epidemiol. 2020, 41, 172–180. [Google Scholar] [CrossRef]
- Bhatt, S.; Mehta, P.; Chen, C.; Schneider, C.L.; White, L.N.; Chen, H.L.; Kong, M.G. Efficacy of low-temperature plasma-activated gas disinfection against biofilm on contaminated GI endoscope channels. Gastrointest Endosc. 2019, 89, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Rashid, T.; Poblete, K.; Amadio, J.; Hasan, I.; Begum, K.; Alam, M.J.; Garey, K.W. Evaluation of a shoe sole UVC device to reduce pathogen colonization on floors, surfaces and patients. J. Hosp. Infect. 2018, 98, 96–101. [Google Scholar] [CrossRef]
- Hooker, E.A.; Allen, S.; Gray, L.; Kaufman, C. A randomized trial to evaluate a launderable bed protection system for hospital beds. Antimicrob. Resist. Infect Control 2012, 1, 1–27. [Google Scholar] [CrossRef] [Green Version]
- Yuen, J.W.M.; Chung, T.W.K.; Loke, A.Y. Methicillin-resistant Staphylococcus aureus (MRSA) contamination in bedside surfaces of a hospital ward and the potential effectiveness of enhanced disinfection with an antimicrobial polymer surfactant. Int. J. Environ. Res. Public Health 2015, 12, 3026–3041. [Google Scholar] [CrossRef]
- Hooker, E.A.; Bochan, M.; Reiff, T.T.; Blackwell, C.; Webb, K.W.; Hart, K.W. Decreasing Clostridium difficile health care-associated infections through use of a launderable mattress cover. Am. J. Infect Control 2015, 43, 1326–1330. [Google Scholar] [CrossRef] [Green Version]
- Burns, S.; Tulpinski, J.; Som, A.; Taylor, T. Pushing our buttons: How many cycles does it take to remove an active disinfecting compound from a keypad? Am. J. Infect Control 2014, 42, S39–S40. [Google Scholar] [CrossRef]
- Gostine, A.; Gostine, D.; Donohue, C.; Carlstrom, L. Evaluating the effectiveness of ultraviolet-C lamps for reducing keyboard contamination in the intensive care unit: A longitudinal analysis. Am. J. Infect Control 2016, 44, 1089–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtin, A.M.; Buckley, H.L. Biofouling detection methods that are widely applicable and useful across disciplines: A mini-review. Biofouling 2021, 37, 494–505. [Google Scholar] [CrossRef]
- Astada, A.; Nakagami, G.; Minematsu, T.; Kitamura, A.; Mugita, Y.; Sanada, H. Concurrent validity of biofilm detection by wound blotting on hard-to-heal wounds. J. Wound Care 2021, 30, S4–S13. [Google Scholar] [CrossRef]
- Cieslinski, J.; Ribeiro, V.S.T.; Kraft, L.; Suss, P.H.; Rosa, E.; Morello, L.G.; Pillonetto, M.; Tuon, F.F. Direct detection of microorganisms in sonicated orthopedic devices after in vitro biofilm production and different processing conditions. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Locke, A.; Fitzgerald, S.; Mahadevan-Jansen, A. Advances in optical detection of human-associated pathogenic bacteria. Molecules 2020, 25, 5256. [Google Scholar] [CrossRef] [PubMed]
- Nutman, A.; Marchaim, D. How to: Molecular investigation of a hospital outbreak. Clin. Microbiol. Infect. 2019, 25, 688–695. [Google Scholar] [CrossRef] [PubMed]
| Author, Date | Bacteria and Interventions | Number of Strains Tested and If In Vivo Number of Animals |
|---|---|---|
| Silver compounds | ||
| Gholamrezazadeh 2018 [6] *** | In vitro biofilms: Growth inhibited by Benzalkonium chloride of all P. aeruginosa isolates at MBC 0.1 ± 0.2 mg/mL, Deconex MIC 1.0 ± 0.2 mg/L; and nano-silver MBC 28.3 ± 2 mg/mL. In vitro biofilm formation: Decreased with bacterial concentration of 106 cfu/mL with nano-Ag 12.5 mg/mL nanomolecule formulation of silver (nano-Ag) at 12.5 ng/mL, causing a reduction in the number of P. aeruginosa bacteria forming biofilms from 28.5% to 3.5%, and benzalkonium MIC at 0.03 mg/mL reduced the number of bacteria forming biofilms from 28.5% to 18.7%. | Pa 28 |
| Pourhajibagher 2020 [14] *** | In vitro biofilms: With MIC90 doses of AgSD-NLs@Cur with LED, A. baumanii numbers decreased by 76.4%, with AgSD-NLs by 44.8%, and with AgSD 38.1%. | Ab 100 * |
| Halstead 2015 [13] *** | In vitro biofilms: Reduction compared to control after 72 h incubation: (a) A. baumanii 1701 Acticoat 96%; Mepilex Ag 95.9%; acetic acid (concentrations 0.31% 5%) 90–93%: A. baumanii 721 Acticoat 100%; Mepilex Ag 100%; acetic acid 5% (concentrations 0.31% and 5%) 90–93%: (b) P. aeruginosa 15692 Acticoat 100%; Mepilex Ag 100%; acetic acid (concentrations 0.31% to 5%) 86–96%; P. aeruginosa 1586 Acticoat 94%; Mepilex Ag 99.9%; acetic acid (concentrations 0.31% to 5%) 88–97%. | |
| Other metals | ||
| Karaky 2020 [8] *** | In vitro biofilms: Graphene reduced the biofilm forms of the bacteria significantly more than the planktonic forms (p < 0.0001). Eight metal–graphene combinations reduced the amount of intact biofilm by 90% or more (platinum–graphene oxide, gallium–graphene oxide, molybdenum–graphene oxide, gold–graphene oxide, silver–graphene, gallium–graphene and molybdenum–graphene. The greatest reduction in P. aeruginosa biofilm metabolic activity occurred with gold–graphene oxide (94%), molybdenum–graphene oxide (93%), silver (91%), and silver–graphene (91%). | Pa 2 |
| Li 2020 [15] *** | In Vitro: After treatment with H2O2 (2 nM) and CuCo2S4 nanoparticles (100 μg/mL) after one hour, 3.6 log reduction was shown in viability of MRSA, 3.3 log in S. aureus, and 4.7 log reduction in E. coli. H2O2 and CuCo2S4 nanoparticles (100 μg/mL) separately showed no biocidal activity. In vivo mouse burns: After 2 days treatment with H2O2 (2 nM) and CuCo2S4 nanoparticles (100 μg/mL), no inflammatory response and burn wound contracted, and control group showed severe inflammatory response with suppuration. After 6 days group treated with H2O2 (2 nM) and CuCo2S4 showed enhanced healing and 83.7% wound closure; CuCo2S4 71%, H2O2 63.3%, and control 59%. At 2 weeks H2O2 (2 nM) and CuCo2S4 wounds completely closed and healed. | MRSA 1 (32 mice) |
| Nozari 2021 [16] | In vitro: After 18 h, S. aureus with chitosan–alginate–gelatin film for three samples ranged from 1 × 104 cfu/mL to 3.2 × 105 cfu/mL compared to control 1.5 × 109 cfu/mL; with chitosan-bentonite–gelatin film ranged from 7.8 × 105 cfu/mL to 3 × 106 cfu/mL compared to control 1.5 × 109 cfu/mL; (99.99% reduction); (2) P. aeruginosa with alginate film 1 × 104 cfu/mL to 8.2 × 105 cfu/mL compared to control 1.5 × 109 cfu/mL; with bentonite film 1 × 104 to 1.9 × 105 cfu/mL compared to control 1.5 × 109 cfu/mL; (99.99% reduction) In vivo mouse burns at 7 days histology: For the treated rats, there was re-epithelialisation, active fibroblasts, and hair follicles and sebaceous glands were detected. No re-epithelialisation was detected in untreated rats. | Sa 1; Pa 1; (3 rats) |
| Disinfectants | ||
| Halstead 2015 [17] *** | In vitro biofilms: For 23 isolates, acetic acid MBIC 0.31% and MBEC against formed biofilms ranged from ≤0.10% to 2.5% and eradication of mature biofilms was observed for all isolates after three hours of exposure. | Pa 9; Ab 8; Sa 3; Kp 2 |
| Song 2016 [18] *** | In vitro: Reduced bacterial viability 90% by CNE at 8 μg/mL within 5 min and bacteria completely killed with 8 μg/mL by 1440 min. CHX at 8 μg/mL reduced bacterial viability by 90% at 240 min but bacteria were not completely killed by 1440 min. In vivo: Mouse burn wound with CNE 5 mg/mL scab detached from wound on the 8th day and completely detached on the 29th day; CHX 13th and 33rd days. In vivo biofilms: As shown by scanning electron microscopy, MRSA biofilms treated with 2 μg/mL CNE biofilms were “dispersed and disrupted and obvious reduction in number of bacteria” and large vacuoles between cell wall and cytoplasm. Dead/live cell ratio with CNE 83.6%, with CHX 13%. | MRSA 1 (mice; n = ?) |
| Tiwari 2018 [19] | In vitro biofilm % reduction in biofilm optical density (OD): No significant differences between reductions in strong and weak biofilm formers for either sodium hypochlorite or ethanol. With 0.6% sodium hypochlorite for strong biofilms, 34.27% ± 15.30, and for weak biofilms, 35.07 ± 12.98 (p = 0.897); (2) with 70% ethanol for strong biofilms, 18.14% ± 11.56 and for weak biofilms, 20% (p = 0.488). On electron microscopy, strong biofilm producers showed significant depressions and irregular craters on their surface. | Sa 29 * |
| Andersson 2021 [9] | S. aureus (1) Wound surface reductionsS. aureus from cfu 108 to cfu log 106 for levofloxacin 2 μg/mL compared to control cfu increased to log 1010 (p < 0.0001); (2) wound tissue reductions to cfu log 106 for levofloxacin 2 μg/mL compared to control cfu Log 108.5 (p < 0.001); (3) wound surface reductions to cfu log 106 for Prontosan compared to control cfu log 1010 (p < 0.0001); (4) wound tissue reductions to cfu log 107 for Prontosan compared to control cfu log 108 (p < 0.05) P. aeruginosa (1) P. aeruginosa wound surface no change in cfu log 108 for levofloxacin 2 μg/mL, but control increased to cfu log 1011 (p < 0.001); (2) wound tissue no change cfu log 108 for levofloxacin 2 μg/mL and control cfu log 108 (n.s.); (3) wound surface reduction from cfu 108 to cfu log 106 for Prontosan compared to increase in control to cfu log 1011 (p < 0.001); (4) wound tissue reductions to cfu log 107 for Prontosan compared to control cfu log 108.5 (p < 0.05) | Sa 1; Pa 1 (pigs n = ?) |
| Chhibber 2020 [20] *** | In vivo biofilm: (1) Conventional hydrogel 2.8 log10 cfu/mL reduction on day 1; 4.2 log10 cfu/mL reduction day 2; and wound became sterile (day not stated). (2) Novel hydrogel 3.5 log10 cfu/mL reduction on day 1, 4.8 log10 cfu/mL reduction day 2, and wound sterile (day not stated). (3) Control 6.9 log10 cfu/mL count day 3. (4) At 4 h, complete eradication of MRSA from wounds with conventional and novel hydrogels but MRSA established in control mice. | MRSA b 1 (54 mice) |
| Sonotherapy | ||
| Pourhajibagher 2021 [21] *** | In vitro biofilms: Reduction in multi-species bacterial growth following SDT at ½ MIC of N-EMO was 81.5 %; at 1/16 MBIC 71.0%; and at 1/128 MBEC 57.8; (reductions in log10 cfu/mL 99.99%, 99.97%, 99.48%) | Pa 1; Sa 1. Ab 1 |
| Light therapy | ||
| Ishiwata 2021 [22] | In vivo: Baseline: 8.9 × 104 cfu/mL; Day 0 post infection: aPDT group, no bacteria, control 3.4 × 108 cfu/mL; Day 1: aPDT 3.5 × 105 cfu/mL, PS 4.7 × 105 cfu/mL indicating rapid regrowth; Days 2–7: rapid regrowth each day and aPDT group day 6 × 104 cfu/mL Rat survival at day 7: aPDT 11/14. PS 3/10, control 2/14 | Pa 1; (34 rats) |
| Lu 2021 [3] *** | In vitro biofilms: A.b, P.aAF0001 and MRSA IQ0064 biofilms at 107 cfu/mL completely eliminated after 22.5 min of blue light + carvacrol (p < 0.0001) and reduced Ab biofilm from 58.6 μm to 1.4 μm thickness and MRSA from 32.4 μm to 1.7 μm; six first-line antibiotics inactivated < 1.5 log CFU after 6 h. In vivo mouse burns: Carvacrol 50 μL at 1 mg/mL + blue light for 12 min (40 J/cm2) with luminescent bacteria eliminated log 8 luminescence, blue light alone 2.3 log, and carvacrol 0.8 log. | Ab 1 Pa 1 MRSA 1; (mice n = ?) |
| Pourhajibagher 2020 [2] | In vitro: Reduction in cell viability by ICG at 1000 μg/mL significant reduction in cell viability of A. baumannii 1.5 × 105 cfu/mL, P. aeruginosa 1 × 105 cfu/mL; S. aureus 1.0 × 105 cfu/mL compared to control 4.5 × 105 cfu/mL (all p < 0.05). | Ab 1, Pa 1, Sa 1 |
| Wang 2016 [4] *** | In vitro: (1) Exposure of 24 h old and 72 h old A. baumanii biofilms to aBL 432 J/cm2 for 72 min resulted in inactivation of 3.59 log10 and 3.18 log10 cfu/mL. (2) Exposure of P. aeruginosa biofilms to aBL 432 J/cm2 resulted in inactivation of 3.02 log10 cfu/mL and 3.12 log10 cfu/mL. Control biofilms showed <0.27 log10 cfu/mL loss of viability for A. baumanii and <0.42 log10 cfu/mL for P. aeruginosa. In vivo: Mouse burn wounds infected with 5 × 106 cfu/mL A. baumanii at 24 h required 360 J/cm2 at 48 h 540 J/cm2 to inactivate 3 log10 cfu/mL in biofilms. | Ab 1, Pa 1; (mice n = ?) |
| Small molecules | ||
| Banar 2016 [23] *** | Minimum biofilm eradicating concentration (MBEC): Strain 1: ceftazidime (CAZ) 1024 μg/mL, CAZ + α-mannosidase 128 μg/mL, CAZ + β-mannosidase 128 μg/mL, CAZ + trypsin 512 μg/mL; Strain 2: ceftazidime (CAZ) 1024 μg/mL, CAZ + α-mannosidase 4 μg/mL, CAZ + β-mannosidase 4 μg/mL, CAZ + trypsin 8 μg/mL; Strain 3: ceftazidime (CAZ) 1024 μg/mL, CAZ + α-mannosidase 4 μg/mL, CAZ + β-mannosidase 8 μg/mL, CAZ + trypsin 32 μg/mL. All tested concentrations killed biofilm bacterial cells. | Pa 57 * |
| Ghosh 2015 [24] *** | Persister cells: NCK-10 completely lysed persister cells of 5 log cfu/mL E. coli after 2 h, but colonies persisted in control group at 5 log cfu/mL. Disruption of biofilms: EC50 = 30 μM against biofilms of A. baumanii (MTCC 1425); 20 μM against E. coli MTCC 443); 26 μM against K. pneumoniae (ATCC 700603), and 19 μM against P. aeruginosa (MTCC 424). On confocal microscopy in the treated samples, the biofilms were completely disrupted, and the untreated samples had biofilms 12.6 μm thick. | Ab 3; Pa 3; Ec 3; Kp 2; (20 mice) |
| Goodwine 2019 [1] *** | In vitro (1) Samples from human wounds: 2.2-fold reduction after exposure to 5 mU pyruvate-dehydrogenase (PDH) and by 2.9-fold after 10–20 mU; In vitro biofilms (1) On confocal laser scanning microscopy 60% of microcolonies in PDH-treated biofilms showed signs of dispersion with central voids and 8% of untreated biofilms. (2) Four-day old human wound samples of S. aureus biofilms exposed to PDH 10 mU had 40% reduction in mass. In vivo (1) Pig burn wounds: P. aeruginosa biofilm population mass reduced 2-log with tobramycin 200 μg/mL compared to untreated control. (2) A 4-log reduction by tobramycin 200 μg/mL + PDH 200 mU compared to control. (3) Silver sulfadiazine 2-log reduction in biofilm and 4-log in planktonic populations. | P.a 1; (3 pigs) |
| Han 2018 [25] *** | Biofilms: reduction in cell viability of S. aureus CICC10790 to 10% with 8 × MIC vancomycin (8 μg/mL) and to 10% with AMP-jsa9 at 8 × MIC (128 μg/mL); reduction in biomass to 15% with 8 × MIC vancomycin (8 μg/mL) and to 15% with AMP-jsa9 at 8 × MIC (128 μg/mL). In vivo: In mouse scalded skin burns viable cell count treated with vancomycin or AMP-jsa9 were 101 to 102 on days 3 and 7 and in those treated with kanamycin or saline 2–3 × 104 at 3 days and 5–6 × 105 at 7 days with a large infiltrate of inflammatory cells. | MRSA 1; (mice n = ?) |
| Konai 2020 [5] *** | In vitro biofilms: With confocal scanning electron microscopy, D-LANA-14 8 μg/mL plus 8 μg/mL colistin resulted in >80% reduction in biofilm mass of A. baumanii-R674 and P. aeruginosa-R590; D-LANA-14 8 μg/mL showed no effect, and rifampicin 8μg/mL showed 25–30% disruption. In vivo: Burn wounds in mice: D-LANA-14 40 mg/kg plus rifampicin 40 mg/kg caused 4.9 log reduction in A. baumanii-R674 and 4.0 log in P. aeruginosa-R5902; D-LANA-14 2.3 log and 1.3 log; and rifampicin 3.0 log and 1.6 log. | Ab 3; Pa 5; (mice n = ?) |
| Memariani 2016 [26] *** | In vitro biofilms: Scanning electron microscopy with acridine-orange/ethidium bromide staining: PV3 treated cells were shorter, blisters on membranes, roughness, and blebbing. For PV3 at 8 × MIC at 24 h resulted in “almost” 100% killing of cells and 95% biomass removal. | Pa 7 |
| Pan 2020 [27] *** | In vitro biofilms: P03 reduced the biomass of P. aeruginosa biofilms by 76.9%, PL2 by 35.1%, and PH2 by 31.45%, Polymixin by 7.8%. In Vivo: In mice burn wounds: P03 caused 78.2% reduction in P. aeruginosa and PL2 caused 49.3% reduction compared to Polymixin B. | Pa 1; MRSA 1 (mice n = ?) |
| Su 2019 [28] | In vitro biofilms: Microtitre dish biofilm formation assays: after 2 μg/mL PTM or PTM-2t biofilm formation for S. aureus ATCC 291213 reduced 95%. In vivo: Mouse burns treated with 4 mg of PTM or PTM-2t on burn wound twice daily × 7 days. PTM reduced S. aureus to 2 × 106 cfu/g and PTM-2t to 8.6 × 106 cfu/g compared to 2.5 × 106 cfu/g for mupirocin and untreated mice 4.3 × 108 cfu/g. | MRSA 1, Sa 1; (20 mice) |
| Uusitalo 2017 [29] | In vitro biofilms: (1) INP0321 at 100 μM reduced biofilm to 40% of control (p < 0.05); (2) INP0341 inhibited P. aeruginosa swarming and prevented movement across semisolid surfaces which requires flagella and type IV pili. In vivo: Treated mice died at 36 h, controls as 42 h (p < 0.05) | Pa 1; (mice n = ?) |
| Glycans | ||
| Wheeler 2019 [30] | In vitro: (1) P. aeruginosa PA01 biofilms exposed to mucins 70% of cells dissociated from surface into planktonic phase (p < 0.0001). (2) MUC5AC and MUC5B 0.5% w/v suppressed virulence pathways 1, 2, 3, and 6 secretion systems; siderophore biosynthesis; pyoverdine and pyochelin; and quorum sensing. (3) MUC5AC suppressed P. aeruginosa PA01 association with plastic and glass surfaces and attachment to live HT human epithelial cells in a concentration dependent manner. In vivo: Pig burn wounds with MUC5AC 1 week post infection, two-log reductions in P. aeruginosa CFUs, no reduction without mucins. | Pa 1; (4 pigs) |
| Lactobacilli | ||
| Lenzmeier 2019 [31] | In vivo: LgCS inhibited the growth of P. aeruginosa strain PAO1, reduced biofilm development 40-fold at 8 h (control significantly increased), and eliminated biofilms at 28 h. In vitro: Mouse burns: local treatment of wound by LgCS did not inhibit P. aeruginosa growth in wound at 24 h but prevented transfer to blood stream with 100% survival of mice at 7 days treated with LgCS (no P. aeruginosa in livers or spleens), 100% death due to sepsis in untreated mice (~107 cfu/mL P. aeruginosa g−1 in livers and spleens). Second dose of LgCS 24 h after first dose completely eliminated P. aeruginosa in wound. | Pa 1; (20 mice) |
| Phage Therapy | ||
| Alves 2018 [33] | Ex vivo biofilms: 24 h after phage treatment, phage treated 106.5 cfu/mL compared to control 107.5 cfu/mL, (p ≤ 0.0001); 48 h after phage treatment 107 cfu/mL compared to control 107 cfu/mL (n.s.); | MRSA 27; (pig skins, not live pigs, n = ?) |
| Ho 2016 [34] *** | In vivo: Carbapenem-resistant Acinetobacter baumanii (CRAB) 8.57/1000 patient days pre-intervention, 5.11 during aerosol phage intervention period (p =.0029), resistant isolates decreased 87.6% to 46.07% (p = 0.001) Decreased drug use: colistin 7876 DDD/1000 patient days decreased to 3158 (p =0.0177); tigecycline 2737 to 753 (p = 0.0005); meropenem 5084 to 2469 (p = 0.0385); imipenem 1384 to 1101 (ns). | |
| Holguín 2015 [35] | In vitro: at 18 h after phage therapy, P1 107.5 decreased to 104 pfu/mL, P2 108 to 104.5, P4 107.5 to 102.5 (by visual inspection of Figure 2 in Holguín’s article), P2 not reported) In vitro biofilms: P1 17% reduction at 0 h (p = 0.003), 34% at 24 h (p = 0.134), 55% at 48 h (p = 0.005), P3 59% reduction at 0 h (p = 0.00001), 56% at 24 h (p = 0.034), 75% at 48 h (p = 0.0004), P4 68% reduction at 0 h (p = 0.015), 15% at 24 h (p = 0.036; 21% at 48 h (p = 0.286) In vivo: ΦPan70 immediately after P. aeruginosa infection 4/5 mice survived; ΦPan70 45 min after infection 5/5 survived; 24 and 48 h after infection 4/5 mice survived; controls all mice died days 3 or 4. | |
| O’Flaherty 2005 [36] *** | In vitro: 14/28 S. aureus strains sensitive to phage K 107 cfu/mL and no bacteria remained after 2 h; no bacteriophage-insensitive mutants (BIMs) after 25 h In vivo: (1) MRSA strain DPC5645 decreased within 2 h from 5.7 × 106 cfu/mL to undetectable levels; (2) MRSA strain DPC5246 on skin reduced 100-fold with phage K 1.4 × 108 pfu/mL (10 replications, no statement of numbers of participants or hands) | |
| Pallavali 2021 [37] | In vitro biofilms: At 96 hours after 4 h phage therapy optical density (OD, which corresponds to biomass): (1) P. aeruginosa 0.47 ± 0.035 decreased to 0.17 ± 0.024; (2) E. coli 0.47 ± 0.035 decreased to 0.15 ± 0.026; (3) K. pneumoniae 0.47 ± 0.035 decreased to 0.17 ± 0.022; (4) S. aureus 0.47 ± 0.036 decreased to 0.16 ± 0.032. In vitro confocal microscopy: Predominant numbers of dead cells after 4 h phage therapy | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, R.E.; Thomas, B.C. Reducing Biofilm Infections in Burn Patients’ Wounds and Biofilms on Surfaces in Hospitals, Medical Facilities and Medical Equipment to Improve Burn Care: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 13195. https://doi.org/10.3390/ijerph182413195
Thomas RE, Thomas BC. Reducing Biofilm Infections in Burn Patients’ Wounds and Biofilms on Surfaces in Hospitals, Medical Facilities and Medical Equipment to Improve Burn Care: A Systematic Review. International Journal of Environmental Research and Public Health. 2021; 18(24):13195. https://doi.org/10.3390/ijerph182413195
Chicago/Turabian StyleThomas, Roger E., and Bennett Charles Thomas. 2021. "Reducing Biofilm Infections in Burn Patients’ Wounds and Biofilms on Surfaces in Hospitals, Medical Facilities and Medical Equipment to Improve Burn Care: A Systematic Review" International Journal of Environmental Research and Public Health 18, no. 24: 13195. https://doi.org/10.3390/ijerph182413195







