Abstract
Few studies have investigated the relationship between ambient air pollution and cardiorespiratory outcomes in Africa. A cross-sectional study comprising of 572 adults from four informal settlements in the Western Cape, South Africa was conducted. Participants completed a questionnaire adapted from the European Community Respiratory Health Survey, and the National Health and Nutrition Examination Survey questionnaire. Exposure estimates were previously modelled using Land-Use Regression for Particulate Matter (PM2.5) and Nitrogen Dioxide (NO2) at participants’ homes. The median age of the participants was 40.7 years, and 88.5% were female. The median annual NO2 level was 19.7 µg/m3 (interquartile range [IQR: 9.6–23.7]) and the median annual PM2.5 level was 9.7 µg/m3 (IQR: 7.3–12.4). Logistic regression analysis was used to assess associations between outcome variables and air pollutants. An interquartile range increase of 5.12 µg/m3 in PM2.5 was significantly associated with an increased prevalence of self-reported chest-pain, [Odds ratio: 1.38 (95% CI: 1.06–1.80)], adjusting for NO2, and other covariates. The study found preliminary circumstantial evidence of an association between annual ambient PM2.5 exposure and self-reported chest-pain (a crude proxy of angina-related pain), even at levels below the South African National Ambient Air Quality Standards.
1. Introduction
In 2016, the World Health Organisation (WHO) estimated that 4.2 million deaths were attributed to ambient air pollution [1]. Furthermore, four air pollutants were found to have adverse effects on health, namely, particulate matter (PM), sulphur dioxide (SO2), nitrogen dioxide (NO2) and ozone (O3) [1]. Approximately 58% of the air pollution related deaths was due to ischaemic heart disease and stroke, 18% was due to chronic obstructive pulmonary disease and acute lower respiratory tract infections, respectively, and 6% was due to lung cancer [1]. In South Africa, air pollutants contributed to approximately 740,199 disability adjusted life years and 22971 deaths, all due to cardiopulmonary cancers and non-cancer diseases [2,3].
Despite the majority of ambient air pollution-related deaths occurring in low-and middle-income countries, most of the studies investigating the relationship between ambient air pollution and cardiorespiratory outcomes have been conducted in America, Asia and Europe where air pollution levels and composition as well as population characteristics differ from those in Africa and more specifically in South Africa’s informal settlements [1,4,5,6,7,8,9,10,11]. Furthermore, epidemiological studies conducted in Africa investigating this relationship have notable limitations, including a lack of robust exposure measurements, a lack of objective outcome measurements or inadequate adjustment of possible confounders [12,13,14,15,16].
There is therefore a paucity of robust data on the continent describing the relationship between ambient air pollution and cardiorespiratory outcomes, especially amongst adults residing in informal settlements, who might be disproportionately affected by air pollution due to underlying susceptibility and lifestyle behaviour in informal communities. This study aimed to determine the relationship between modelled annual exposure estimates of PM2.5 and NO2 concentrations with self-reported cardiorespiratory outcomes amongst adults residing in four informal settlements of the Western Cape province of South Africa.
This study involved the analysis of a subset of data that was collected as part of a larger cohort study conducted in 2016 investigating the association between ambient air quality and respiratory morbidities including childhood asthma among 590 primary school pupils in the Western Cape [16]. The current study is a cross-sectional study investigating the association between ambient air quality and self-reported cardiorespiratory outcomes among the parents/guardians of these primary school pupils.
2. Materials and Methods
2.1. Study Design, Population and Sampling
A detailed description of the study area and methodology have been previously published [17]. In brief, the study areas included informal settlements in three areas identified in the Western Cape. These areas were selected to maximise contrasts in exposure levels to the different ambient air pollutants. The areas included an urban industrialised area (Marconi-Beam in Milnerton), a peri-urban area with a large informal sector (Khayelitsha) and a semi-arid rural area (Oudtshoorn). An additional area served as a background (with a low air pollution score ranking) and with comparable socio-economic status as the three identified areas (Masiphumelele in Noordhoek).
For this study, all the parents of the primary school pupils selected for the larger cohort study were included [17]. Briefly, the pupils were recruited from schools located near the ambient air quality monitoring stations in the selected areas. Approximately 150 pupils in each study area were selected. After meeting each school’s principal, obtaining permission from the school board and obtaining class lists and addresses, the houses of the primary school children were visited by trained field staff to obtain consent from the caregivers (parent or guardian). Consent was also obtained from the parents/guardians for their own participation in the sub-study. Only those who consented were included in the study. Only the parents/guardians of the pupils selected in the larger study were recruited for this current study.
2.2. Questionnaire
Trained interviewers administered the questionnaires to participants in their spoken language (English, Xhosa or Afrikaans). The questionnaire was back-translated to ensure the consistency and reliability of the questionnaires. The use of mobile technology was implemented in the administration and capture of questionnaires.
The questionnaire used for this study included items on: demographic characteristics, residential history, respiratory health, cardiovascular disease, blood pressure and other chronic illnesses such as high cholesterol, occupational history, exposure to indoor pollutants, exposure to outdoor pollutants, physical activity, psychosocial stress and tobacco use.
Questions from the European Community Respiratory Health Survey [18], as well as the National Health and Nutrition Examination Survey questionnaire [19], were incorporated into this study’s questionnaire (Table 1).

Table 1.
Cardiorespiratory questions included in the questionnaire.
2.3. Outcome Characterisation
An asthma symptom score was created from the responses to eight (8) asthma-related questions (Table 1). One point was allocated for each positive response. A similar asthma symptom score, excluding the variable self-reported asthma, has been validated elsewhere [20]. Furthermore, additional cardiorespiratory self-reported outcomes include: doctor diagnosed asthma, chest-pain, hypertension, and high cholesterol (Table 1).
2.4. Exposure Characterisation
The annual average concentration of PM2.5 and NO2 was estimated at each participant’s address by land-use regression (LUR) models developed specifically for this study and have been previously published elsewhere. [21] In brief, the air pollution monitoring campaigns were performed during 2015–2016 in each study area. Weekly measurements of PM2.5 and NO2 were performed in both winter and summer at 140 sites (40 sites each in three study areas, and 20 sites in Masiphumulele) within a period of one year. These measurements were temporally adjusted using routinely monitored air quality measurements to obtain the seasonal (winter/summer) and annual averages. Predictors of exposure, obtained or collected on-site, such as household density, nearby traffic (e.g., major roads, bus stops, and train stations), waste burning sites, and land-use derived from geographic information system (GIS) were used to evaluate the spatial variation in the annual average concentrations. To maximize the adjusted explained variance, regression models were developed, using a supervised stepwise approach, and the models were validated using leave-one-out-cross-validation (LOOCV). The LUR models were used to estimate annual average concentration of PM2.5 and NO2 at each participant’s address. The annual NO2 LUR model explained 76% of the spatial variability in the NO2 adjusted concentrations, 62% for the warm dry summer season and 77% for the cold and wet winter season. The annual PM2.5 LUR model explained 29% of the spatial variability in the PM2.5 adjusted concentrations, 36% for the warm season and 29% for the cold season.
2.5. Statistical Analysis
Data was captured, cleaned and analysed in Stata: Release 11 (StataCorp. 2009. Statistical Software. StataCorp LP: College Station, TX, USA). Descriptive statistics were used to examine the characteristics of the study population, cardiorespiratory outcomes and various potential confounders. Bivariate regression was used to measure associations between various potential confounders and cardiorespiratory outcomes. Relevant confounders were identified a priori or based on bivariate associations, and they were incrementally included in multivariable logistic regression models (age, sex, use of paraffin, smoking, physical activity, and study area). The base model was a single-pollutant model comprising cardiorespiratory outcomes as the dependent variable with either annual NO2 or annual PM2.5 as the independent variable. The final model was a two-pollutant model, which included cardiorespiratory outcomes as the dependant variable and both, annual NO2 and annual PM2.5 as independent variables.
2.6. Ethical Considerations
The main study was approved by the University of Cape Town’s Research Ethics Committee (ethics number: 234/2009). The protocol for the sub-study was approved by the University of Cape Town’s Research Ethics Committee (ethics number: 639/2018).
3. Results
3.1. Demographic Characteristics of the Study Participants
Table 2 shows that the majority of participants were female (88.5%), that 75% of the participants completed high school, and that the majority of the participants spoke isiXhosa (69.6%). Masiphumelele (Noordhoek) had the highest proportion of employed participants (51.8%). The participants from Masiphumelele were also younger (median age, 38.2 years) than those from the other study areas. More than half (61.4%) of the participants made use of paraffin for cooking and heating. Khayelitsha had the highest proportion of participants who were physically active in the last month before the interview (31.4%), while Oudtshoorn had the highest proportion of participants who smoked cigarettes (32.1%).

Table 2.
Demographics and selected characteristics of the study participants across the four study areas.
3.2. Cardiorespiratory Outcomes of the Study Participants
The prevalence of self-reported doctor-diagnosed asthma was 6.6%. Khayelitsha had the highest proportion of participants who reported having experienced wheezing (13.4%), shortness of breath (10.5%) and tight chest (12.2%) in the last 12 months (Table 3). Participants from Masiphumelele (Noordhoek) had the highest proportion of participants who reported having experienced shortness of breath after exercise (25.9%) and Oudtshoorn had the highest proportion of participants bringing up phlegm from the chest during winter (12.2%). Furthermore, all the study areas had participants who experienced at least one asthma symptom in the preceding year. Participants from Khayelitsha had the highest prevalence of self-reported chest-pain (14.5%) and self-reported cholesterol (8.7%). Khayelitsha and Oudtshoorn had the highest proportion of participants with self-reported hypertension (24.4%).

Table 3.
Cardiorespiratory outcomes of the study participants across the four study areas.
3.3. Air Pollution Characterization Based on LUR Modelling
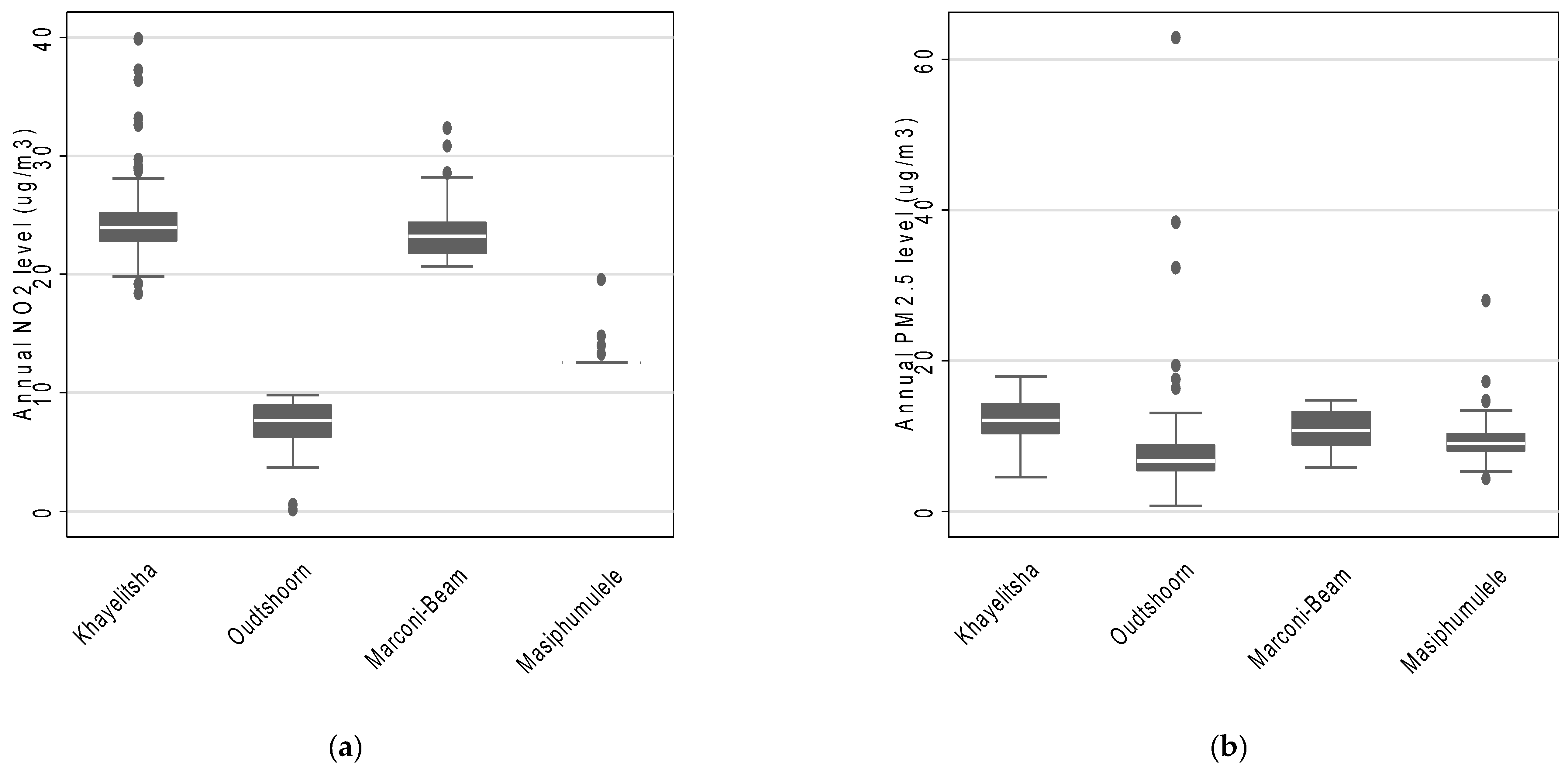
Across the four study areas, the estimated mean annual NO2 concentration was 16.9 µg/m3 (interquartile range: 9.6 µg/m3 to 23.7 µg/m3) and the estimated mean annual PM2.5 concentration was 10.1 µg/m3 (interquartile range: 7.3 µg/m3 to 12.4 µg/m3). Furthermore, the estimated mean annual concentrations suggest that participants residing in Khayelitsha were exposed to the highest levels of both NO2 and PM2.5 (Figure 1). Masiphumulele had the lowest variability in estimated annual average NO2 and PM2.5 concentrations (Figure 1). In addition, the annual estimated annual average NO2 and PM2.5 concentrations were slightly positively correlated (rho = 0.32).
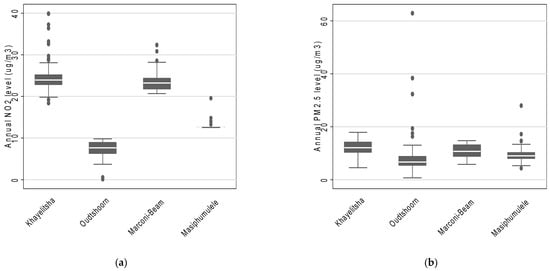
Figure 1.
The distribution of annual estimates of (a) NO2 and (b) PM2.5 concentrations across the four study areas based on LUR modelling.
3.4. Association between NO2 and PM2.5 Levels and Self-Reported Reported Cardiorespiratory Outcomes
An interquartile range increase of 5.12 µg/m3 in PM2.5 was statistically significantly associated with an increase in prevalence of self-reported chest-pain, both in the single—[odds ratio: 1.36 (95% CI: 1.05–1.78)] (Table 4 model F) and two-pollutant model [odds ratio: 1.38 (95% CI: 1.06–1.80)] (Table 5 model F), which included adjustment for NO2, and other covariates. There was also a marginal statistically significant positive association of PM2.5 with both doctor-diagnosed asthma and more than two asthma symptom score (ASS > 2), in both the single- and two-pollutant models (Table 4 and Table 5 model F). However, there was no significant association observed for NO2 with any of the outcomes assessed. A sensitivity analysis excluding males did not change the associations found (results not shown).

Table 4.
Association between interquartile increase in estimated annual NO2 and PM2.5 levels and self-reported cardiorespiratory outcomes in single-pollutant model (reported as odds ratios (95% confidence interval).

Table 5.
Association between interquartile increase in estimated annual NO2 and PM2.5 levels and self-reported cardiorespiratory outcomes in two-pollutant model, reported as odds ratios (95% confidence intervals).
4. Discussion
This cross-sectional study conducted amongst adults from four informal settlements in the Western Cape province of South Africa found an interquartile range increase of 5.12 µg/m3 in PM2.5 to be statistically significantly associated with an increase prevalence of self-reported chest-pain, both in the single—[1.36 (95% CI: 1.05–1.78)] and two-pollutant model [1.38 (95% CI: 1.06–1.80)], which controlled for NO2. However, none of the outcomes investigated were significantly associated with NO2.
All positive associations (both those statistically significant and those not significant) with PM2.5 in this study were at concentrations lower than National Ambient Air Quality Standards (NAAQS). For instance, the average annual estimated PM2.5 concentration of 10.1 µg/m3 was lower than the South African NAAQS of 25 µg/m3, while it was higher than the World Health Organisation (WHO) Air Quality Guideline of 5 µg/m3 [1,22,23]. The average annual estimated NO2 concentration of 16.9 µg/m3 was also lower than the South African NAAQS [22].
In comparison to previous studies within Africa, the estimated annual PM2.5 concentrations in this study were similar to those measured using stationary monitors in Zambia (10.2 µg/m3), while it was lower than measurements obtained in Zimbabwe (40.6 µg/m3), and in a previous study conducted in Bethlehem in South Africa (65 µg/m3) [24,25,26]. Moreover, the estimated annual NO2 levels in the current study were lower than levels measured in a previous study in Cape Town (25.1 µg/m3) [13].
Only two previous studies conducted in Africa that measured the prevalence of at least one self-reported cardiorespiratory outcome as reported in this current study, could be found. The prevalence of self-reported asthma among residents in Windhoek (11%) [16] in which the participants were all younger than 30 years old, was higher compared to this current study (6.6%), while self-reported hypertension (5.6%) among the elderly (55 years old and older) living near mine dumps in Gauteng and North West, was considerably lower than in this current study (20.1%) [27].
Associations between air pollutants and adverse cardiovascular outcomes have been found in a number of international studies [4,5,6,7,8,28,29,30,31,32,33] and a previous study in Cape Town [13]. These studies differed from the current study in one or more respects such the health outcome (mortality or hospital admissions), PM exposure being short-term and >25.1 µg/m3 and air pollution measured from stationary monitors.
A study conducted in Canada involving more than 5 million participants found a 3.5 µg/m3 interquartile range increase in PM2.5 to be associated with 1.04 (95% CI: 1.03–1.05) risk of acute myocardial infarction [33]. The estimated mean PM2.5 concentration of 9.6 µg/m3 in the latter study was similar to the annual estimated PM2.5 levels in the current study. It is thus likely that adverse cardiovascular effects can occur at PM2.5 levels that comply with the thresholds of the NAAQS. However, it should also be noted that there are non-cardiac causes of chest pain and so the likely association observed in the current study could likely reflect adverse effects from other systems [34]. In the study conducted in Gauteng and North West among elderly poor communities, those living near mine dumps (1–2 km) had a significantly higher prevalence of cardiovascular disease than those living further away (≥5 km). A cohort of 21 countries, found an association between increased PM2.5 levels and cardiovascular disease mortality and the effect in low-and middle-income countries was similar to that of communities with PM2.5 levels > 35 μg/m3 [35].
This study, to the best of our knowledge, is the only to have been conducted on the African continent that used Land-Use Regression to estimate annual exposure to ambient air pollution to assess its relationship with cardiorespiratory outcomes among adults residing in informal settlements. Land-use regression models were used to estimate each participant’s annual concentration of exposure to NO2 and PM2.5 at their current address during the study period. We assumed that these annual exposures were not temporally variable in previous years prior to the study. The annual NO2 LUR model explained 76% of the spatial variability in the NO2 adjusted concentrations, 62% for the warm dry summer season and 77% for the cold and wet winter season. The annual PM2.5 LUR model explained 29% of the spatial variability in the PM2.5 adjusted concentrations, 36% for the warm season and 29% for the cold season [21]. However, the lower predictive power of the PM2.5 model could have been caused by non-differential exposure misclassification, thereby biasing the association with the other outcomes investigated towards the null (reduced the significance of the association).
However, there are several limitations in the current study. Outcomes were self-reported symptoms, and objective measurements such as lung function testing and forced-exhaled nitric oxide were not available in the study. Such self-reporting could have introduced both recall and reporting biases and may have introduced significant outcome misclassification. It is possible that this non-differential outcome misclassification may thus bias the association between air pollutants and respiratory outcomes towards the null (reduced the significance of the association). Additionally, the less-than-ideal exposure estimation in the study with low exposure variability for PM 2.5 (IQR ≈ 5 µg/m3) and a model with R2 ≈ 0.29 to generate exposure predictions could lead to bias to the null [36]. Although data collected from several local sources such as housing and population density, waste burning, outdoor grilling, bus routes, and construction sites were found to be predictors of PM 2.5 concentrations, a lack of comprehensive data on such sources, especially their transient nature, could explain a significant part of the low variability found for PM2.5 [21]. The low PM2.5 variability in the study is not necessarily unexpected considering the small scale of the four study neighbourhoods (<40 km2) and the relatively small distances between the four sites (<500 km). PM2.5 mostly reflects background pollution with low small-scale variability. The cross-sectional design of the study limits inference of the association to prevalence, and the temporality of such association cannot be ascertained. In addition, with more than four-fifths of the study participants being women, it is difficult to generalize the findings to men nor the general population. Lastly, the study was not sufficiently powered to detect most associations.
5. Conclusions
This study provided some preliminary evidence of the association between ambient PM2.5 levels and the prevalence of self-reported cardiovascular morbidity (chest-pain) at levels below the NAAQS. The presence of an association below the NAAQS supports the need to revisit it to determine its efficacy in terms of protecting vulnerable population, especially those from informal settlements who may have underlying vulnerability and/or are disproportionately affected by the burden of air pollution. It is important to conduct further research amongst this population using a more objective outcome with a longitudinal study design.
Author Contributions
Conceptualization, H.B., T.O., B.P., J.L. and M.A.D.; data curation, H.B. and T.O.; formal analysis, T.O. and M.A.D.; funding acquisition, M.R. and M.A.D.; investigation, H.B., T.O., K.d.H., A.S., B.P., J.L, M.R. and M.A.D.; methodology, H.B., T.O., K.d.H., A.S., B.P., J.L., M.R. and M.A.D.; project administration, H.B., T.O., M.R. and M.A.D.; resources, B.P., J.L, M.R. and M.A.D.; supervision, T.O. and M.A.D.; validation, H.B., T.O. and M.A.D.; visualization, H.B., T.O. and M.A.D.; writing—original draft, H.B., T.O. and M.A.D.; writing—review and editing, H.B., T.O., K.d.H., A.S., B.P., J.L., M.R. and M.A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Western Cape Government Department of Environmental Affairs and Development Planning (DEA&DP), South Africa (Study Tender: Conduct Comprehensive Human Health Risk Assessment (HRA) Studies within identified areas across the Western Cape, Ref: EADP7/2013), the South African National Research Foundation (SA-NRF) (Grant number 94883), and the South African Medical Research Council (SAMRC).
Institutional Review Board Statement
The study was conducted according to the guidelines of Declaration of Helsinki. The main study was approved by the University of Cape Town’s Research Ethics Committee (ethics number: 234/2009). The protocol for the sub-study was approved by the University of Cape Town’s Research Ethics Committee (ethics number: 639/2018). Permission was also obtained from the Western Cape Government, Department of Education (reference: 20140917-36653) to conduct the study.
Informed Consent Statement
Informed Consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request and with permission of DEA&DP, South Africa in a form which ensures privacy of study participants.
Acknowledgments
We thank the NOVA institute, the holder of the tender with DEA&DP under which the study falls for managing the tender and assisting in co-ordinating the fieldwork on this project. We acknowledge ACSA, especially Sean Bradshaw for providing air pollution data for the study and Ian Gildenhuys and Haithum Wingrove from the City of Cape Town for assisting with this. We also extend our appreciation to the Project Steering Committee (Ref: EADP/7/2013), particularly Gottlieb Arendse and Sally Benson from the DEA&DP, during project management and review of this article, and our research nurses, including the local fieldworkers for their commitment and diligence through the fieldwork. Lastly, we thank the participants—primary school pupils, parents, caregivers, teachers, principals and the school boards in the selected areas—for giving their time and support during the data collection across the study periods.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- WHO Global Ambient Air Quality Database (Update 2018). Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 1 November 2021).
- Altieri, K.; Keen, S. The Cost of Air Pollution in South Africa. International Growth Centre Blog. 22 November. Available online: http://eprints.lse.ac.uk/id/eprint/81698 (accessed on 1 November 2021).
- World Health Organisation. Ambient Air Pollution Attributable DALYs. 2018. Available online: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/mbd-aap-ambient-air-pollution-attributable-dalys (accessed on 30 November 2021).
- Dockery, D.W.; Pope, C.A.; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G.; Speizer, F.E. An Association between Air Pollution and Mortality in Six U.S. Cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, C.A.; Thun, M.J.; Namboodiri, M.M.; Dockery, D.W.; Evans, J.S.; Speizer, F.E.; Heath, C.W. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am. J. Respir. Crit. Care Med. 1995, 151, 669–674. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.; Kim, S.; Kang, S.H.; Kim, H.J.; Kim, H.; Heo, J.; Yi, S.-M.; Kim, K.; Youn, T.-J. Cardiovascular Effects of Long-Term Exposure to Air Pollution: A population Based Study With 900 845 Person-Years of Follow up. J. Am. Heart Assoc. 2017, 6, e007170. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Guo, C.; Lau, A.K.; Chan, T.-C.; Chuang, Y.C.; Lin, C.; Jiang, W.K.; Yeoh, E.-K.; Tam, T.; Woo, K.S.; et al. Long-Term Exposure to Fine Particulate Matter, Blood Pressure, and Incident Hypertension in Taiwanese Adults. Environ. Health Perspect. 2018, 126, 017008. [Google Scholar] [CrossRef]
- Le Tertre, A.; Medina, S.; Samoli, E.; Forsberg, B.; Michelozzi, P.; Boumghar, A.; Vonk, J.M.; Bellini, A.; Atkinson, R.; Ayres, J.G.; et al. Short-term effects of particulate air pollution on cardiovascular diseases in eight European cities. J. Epidemiol. Community Health 2002, 56, 773–779. [Google Scholar] [CrossRef]
- Adam, M.; Schikowski, T.; Carsin, A.E.; Cai, Y.; Jacquemin, B.; Sanchez, M.; Vierkötter, A.; Marcon, A.; Keidel, D.; Sugiri, D.; et al. Adult lung function and long-term air pollution exposure. ESCAPE: A multicentre cohort study and meta-analysis. Eur. Respir. J. 2015, 45, 38–50. [Google Scholar] [CrossRef] [Green Version]
- Ackermann-Liebrich, U.; Leuenberger, P.; Schwartz, J.; Schindler, C.; Monn, C.; Bolognini, G.; Bongard, J.P.; Brändli, O.; Domenighetti, G.; Elsasser, S.; et al. Lung function and long term exposure to air pollutants in Switzerland. Study on Air Pollution and Lung Diseases in Adults (SAPALDIA) Team. Am. J. Respir. Crit. Care Med. 1997, 155, 122–129. [Google Scholar] [CrossRef] [PubMed]
- McCreanor, J.; Cullinan, P.; Nieuwenhuijsen, M.J.; Stewart-Evans, J.; Malliarou, E.; Jarup, L.; Harrington, R.; Svartengren, M.; Han, I.-K.; Ohman-Strickland, P.; et al. Respiratory Effects of Exposure to Diesel Traffic in Persons with Asthma. N. Engl. J. Med. 2007, 357, 2348–2358. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Guo, Y.; Di, Q.; Zheng, Y.; Kowal, P.; Xiao, J.; Tao, L.; Xing, L.; Wenlin, Z.; Steven, W.H.; et al. Ambient PM2.5 and Stroke: Effect Modifiers and Population Attributable Risk in Six Low- and Middle- Income Countries. Stroke 2017, 48, 1191–1197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wichmann, J.; Voyi, K. Ambient air pollution exposure and respiratory, cardiovascular and cerebrovascular mortality in Cape Town, South Africa: 2001–2006. Int. J. Environ. Res. Public Health 2012, 9, 3978–4016. [Google Scholar] [CrossRef]
- Benaissa, F.; Alkama, R.; Annesi-Maesano, I. Assessment of air pollution impacts on population health in Bejaia city, Northern Algeria. Iran. J. Public Health 2014, 43, 1221–1228. [Google Scholar] [PubMed]
- Ana, G.; Odeshi, T.; Sridhar, M.; Ige, M. Outdoor respirable particulate matter and the lung function status of residents of selected communities in Ibadan, Nigeria. Perspect. Public Health 2014, 134, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Hamatui, N.; Beynon, C. Particulate Matter and Respiratory Symptoms among Adults Living in Windhoek, Namibia: A Cross Sectional Descriptive Study. Int. J. Environ. Res. Public Health 2017, 14, 110. [Google Scholar] [CrossRef] [Green Version]
- Olaniyan, T.; Jeebhay, M.; Röösli, M.; Naidoo, R.; Baatjies, R.; Künzil, N.; Tsai, M.; Davey, M.; De Hoogh, K.; Berman, D.; et al. A prospective cohort study on ambient air pollution and respiratory morbidities including childhood asthma in adolescents from the western Cape Province: Study protocol. BMC Public Health 2017, 17, 712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarvis, D.; Knox, J.; Burney, P.; Chinn, S.; Luczynska, C.; Anto, J.M.; Cerveri, I.; de Marco, R.; Gislasson, T.; Heinrich, J.; et al. The European Community Respiratory Health Survey II. Steer. Comm. Eur. Respir. J. 2002, 20, 1071–1079. [Google Scholar]
- Curtin, L.R.; Mohadjer, L.K.; Dohrmann, S.M.; Kruszon-Moran, D.; Mirel, L.B.; Carroll, M.D.; Hirsch, R.; Burt, V.L.; Johnson, C.L. National Health and Nutrition Examination Survey: Sample design, 2007–2010. Vital Health Stat. 2013, 2, 1–23. [Google Scholar]
- Calciano, L.; Corsico, A.G.; Pirina, P.; Trucco, G.; Jarvis, D.; Janson, C.; Accordini, S. Assessment of asthma severity in adults with ever asthma: A continuous score. PLoS ONE 2017, 12, e0177538. [Google Scholar] [CrossRef]
- Saucy, A.; Röösli, M.; Künzli, N.; Tsai, M.-Y.; Sieber, C.; Olaniyan, T.; Baatjies, R.; Jeebhay, M.; Davey, M.; Flückiger, B.; et al. Land Use Regression Modelling of Outdoor NO2 and PM2.5 Concentrations in Three Low Income Areas in the Western Cape Province, South Africa. Int. J. Environ. Res. Public Health 2018, 15, 1452. [Google Scholar] [CrossRef] [Green Version]
- Suid-Afrika RVAN. National Ambient Air Quality Standards [Internet]. Government Gazette; 2009; p. 4. Available online: https://www.gov.za/sites/default/files/gcis_document/201409/35463gon486.pdf (accessed on 1 February 2019).
- DEA. Ambient Air Quality Standards. Republic of South Africa, GN 35072, 2 March 2012. Available online: https://www.gov.za/sites/default/files/gcis_document/201409/35072144.pdf (accessed on 1 November 2021).
- Nkhama, E.; Ndhlovu, M.; Dvonch, J.T.; Lynam, M.; Mentz, G.; Siziya, S.; Voyi, K. Effects of airborne particulate matter on respiratory health in a community near a cement factory in Chilanga, Zambia results from a panel study. Int. J. Environ. Res. Public Health 2017, 14, 1351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuvarega, A.T.; Taru, P. Ambiental dust speciation and metal content variation in TSP, PM10 and PM2.5 in urban atmospheric air of Harare (Zimbabwe). Environ. Monit. Assess. 2008, 144, 1–14. [Google Scholar] [CrossRef]
- Worobiec, A.; Potgieter-Vermaak, S.S.; Berghmans, P.; Winkler, H.; Burger, R.; Van Grieken, R. Air Particulate Emissions in Developing Countries: A Case Study in South Africa. Anal. Lett. 2011, 44, 1907–1924. [Google Scholar] [CrossRef]
- Nkosi, V.; Wichmann, J.; Voyi, K. Comorbidity of respiratory and cardiovascular diseases among the elderly residing close to mine dumps in South Africa: A cross-sectional study. S. Afr. Med. J. 2016, 106, 290–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesaroni, G.; Forastiere, F.; Stafoggia, M.; Andersen, Z.J.; Badaloni, C.; Beelen, R.; Caracciolo, B.; De Faire, U.; Erbel, R.; Eriksen, K.T.; et al. Long term exposure to ambient air pollution and incidence of acute coronary events: Prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ 2013, 348, f7412. [Google Scholar] [CrossRef] [Green Version]
- Young, M.T.; Sandler, D.P.; DeRoo, L.A.; Vedal, S.; Kaufman, J.; London, S.J. Ambient Air Pollution Exposure and Incident Adult Asthma in a Nationwide Cohort of U.S. Women. Am. J. Respir. Crit. Care Med. 2014, 190, 914–921. [Google Scholar] [CrossRef] [Green Version]
- Jacquemin, B.; Siroux, V.; Sanchez, M.; Carsin, A.-E.; Schikowski, T.; Adam, M.; Bellisario, V.; Buschka, A.; Bono, R.; Brunekreef, B.; et al. Ambient Air Pollution and Adult Asthma Incidence in Six European Cohorts (ESCAPE). Environ. Health Perspect. 2015, 123, 613–621. [Google Scholar] [CrossRef]
- Zheng, X.-Y.; Ding, H.; Jiang, L.-N.; Chen, S.-W.; Zheng, J.-P.; Qiu, M.; Zhou, Y.-X.; Chen, Q.; Guan, W.-J. Association between Air Pollutants and Asthma Emergency Room Visits and Hospital Admissions in Time Series Studies: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0138146. [Google Scholar] [CrossRef]
- Xu, A.; Mu, Z.; Jiang, B.; Wang, W.; Yu, H.; Zhang, L.; Li, J. Acute Effects of Particulate Air Pollution on Ischemic Heart Disease Hospitalizations in Shanghai, China. Int. J. Environ. Res. Public Health 2017, 14, 168. [Google Scholar] [CrossRef]
- Bai, L.; Shin, S.; Burnett, R.T.; Kwong, J.C.; Hystad, P.; Van Donkelaar, A.; Goldberg, M.S.; Lavigne, E.; Copes, R.; Martin, R.V.; et al. Exposure to ambient air pollution and the incidence of congestive heart failure and acute myocardial infarction: A population-based study of 5.1 million Canadian adults living in Ontario. Environ. Int. 2019, 132, 105004. [Google Scholar] [CrossRef] [PubMed]
- William, E.; Cayley, J.R. Diagnosing the Cause of Chest Pain. Am. Fam. Physician 2005, 72, 1. [Google Scholar]
- Hystad, P.; Larkin, A.; Rangarajan, S.; AlHabib, K.F.; Avezum, Á.; Calik, K.B.T.; Chifamba, J.; Dans, A.; Diaz, R.; du Plessis, J.L.; et al. Associations of outdoor fine particulate air pollution and cardiovascular disease in 157 436 individuals from 21 high-income, middle-income, and low-income countries (PURE): A prospective cohort study. Lancet Planet. Health 2020, 4, e235–e245. [Google Scholar] [CrossRef]
- Sheppard, L.; Burnett, R.T.; Szpiro, A.A.; Kim, S.-Y.; Jerrett, M.; Pope, C.A.; Brunekreef, B. Confounding and exposure measurement error in air pollution epidemiology. Air Qual. Atmos. Health 2012, 5, 203–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).