Diagnostic Test Accuracy of First-Void Urine Human Papillomaviruses for Presence Cervical HPV in Women: Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Criteria for Search and Eligibility
2.2. Study Extraction, Quality and Selection
2.3. Data Synthesis and Statistical Analysis
3. Results
3.1. Studies Description
3.2. Quality of Studies
3.3. Meta-Analysis
3.4. Meta-Regression Analyses
3.5. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bosch, F.X.; Lorincz, A.; Munoz, N.; Meijer, C.J.L.M.; Shah, K.V. The causal relation between human papillomavirus and cervical cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef] [Green Version]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2014, 136, E359–E386. [Google Scholar] [CrossRef]
- Vergara, N.; Balanda, M.; Hidalgo, W.; San Martín, H.; Aceituno, A.; Roldán, F.; Villalón, T.; Hott, M.; Espinoza, G.; Quiero, A.; et al. Detection and genotyping of HPV in urine samples from Chilean women attending primary health care centers. Med. Microbiol. Immunol. 2017, 207, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human papillomavirus and cervical cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef]
- Clifford, G.M.; Smith, J.S.; Aguado, T.; Franceschi, S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: A meta-analysis. Br. J. Cancer 2003, 89, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Sanjose, S. de Human papillomavirus genotype attribution in invasive cervical cancer: A retrospective cross-sectional worldwide study. Lancet Oncol. 2010, 11, 1048–1056. [Google Scholar] [CrossRef]
- Greig, J.M.; Ellis, C.J. Occupational Hygiene of Chemical and Biological Agents, 3rd ed.; Elsevier Science: Amsterdam, The Netherlands, 2008; Volume 100, pp. 344–359. [Google Scholar] [CrossRef]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Gómez, D.; Muñoz, J.; Bosch, F.; de Sanjosé, S. Human Papillomavirus and Related Diseases in the World-Summary Rep; ICO/IARC Inf Cent HPV Cancer (HPV Inf Centre): Geneva, Switzerland, 2019; p. 307. [Google Scholar]
- Peto, J.; Gilham, C.; Fletcher, O.; Matthews, F.E. The cervical cancer epidemic that screening has prevented in the UK. Lancet 2004, 364, 249–256. [Google Scholar] [CrossRef]
- Safaeian, M.; Solomon, D.; Castle, P.E. Cervical Cancer Prevention—Cervical Screening: Science in Evolution. Obstet. Gynecol. Clin. N. Am. 2007, 34, 739–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasieni, P.; Adams, J. Effect of screening on cervical cancer mortality in England and Wales: Analysis of trends with an age period cohort model. BMJ 1999, 318, 1244–1245. [Google Scholar] [CrossRef] [Green Version]
- Louie, K.S.; Silvia de Sanjose, P.M. Epidemiology and prevention of human papillomavirus and cervical cancer in sub-Saharan Africa: A comprehensive review. Trop. Med. Int. Heal. 2009, 14, 1287–1302. [Google Scholar] [CrossRef]
- Cronjé, H.S. Cervical screening strategies in resourced and resource-constrained countries. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Monsonego, J.; Bosch, F.X.; Coursaget, P.; Cox, J.T.; Franco, E.; Frazer, I.; Sankaranarayanan, R.; Schiller, J.; Singer, A.; Wright, T.; et al. Cervical cancer control, priorities and new directions. Int. J. Cancer 2003, 108, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.W.; Sikon, A.; Yen-Lieberman, B. Cervical cancer screening: Less testing, smarter testing. Cleve. Clin. J. Med. 2011, 78, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Burroni, E.; Bonanni, P.; Sani, C.; Lastrucci, V.; Carozzi, F. Human papillomavirus prevalence in paired urine and cervical samples in women invited for cervical cancer screening. J. Med. Virol. 2014, 87, 508–515. [Google Scholar] [CrossRef]
- Cómbita, A.L.; Gheit, T.; González, P.; Puerto, D.; Murillo, R.H.; Montoya, L.; Vorsters, A.; Van Keer, S.; Van Damme, P.; Tommasino, M.; et al. Comparison between Urine and Cervical Samples for HPV DNA Detection and Typing in Young Women in Colombia. Cancer Prev. Res. 2016, 9, 766–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, S.; Umulisa, M.C.; Tshomo, U.; Gheit, T.; Baussano, I.; Tenet, V.; Tshokey, T.; Gatera, M.; Ngabo, F.; Van Damme, P.; et al. Urine testing to monitor the impact of HPV vaccination in Bhutan and Rwanda. Int. J. Cancer 2016, 139, 518–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorsters, A.; Micalessi, I.; Bilcke, J.; Ieven, M.; Bogers, J.; Damme, P. Detection of human papillomavirus DNA in urine. A review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 31, 627–640. [Google Scholar] [CrossRef]
- Vorsters, A.; Van Keer, S.; Biesmans, S.; Hens, A.; De Coster, I.; Goossens, H.; Ieven, M.; Damme, P. Van Long-Term Follow-up of {HPV} Infection Using Urine and Cervical Quantitative HPV DNA Testing. Int. J. Mol. Sci. 2016, 17, 750. [Google Scholar] [CrossRef] [Green Version]
- Pattyn, J.; Van Keer, S.; Téblick, L.; Van Damme, P.; Vorsters, A. HPV DNA detection in urine samples of women: `An efficacious and accurate alternative to cervical samples? Expert Rev. Anti. Infect. Ther. 2019, 17, 755–757. [Google Scholar] [CrossRef]
- Lefeuvre, C.; Pivert, A.; Guillou-Guillemette, H.L.; Lunel-Fabiani, F.; Veillon, P.; Le Duc-Banaszuk, A.-S.; Ducancelle, A. Urinary HPV DNA testing as a tool for cervical cancer screening in women who are reluctant to have a Pap smear in France. J. Infect. 2020, 81, 248–254. [Google Scholar] [CrossRef]
- Pathak, N.; Dodds, J.; Zamora, J.; Khan, K. Accuracy of urinary human papillomavirus testing for presence of cervical HPV: Systematic review and meta-analysis. BMJ 2014, 349, g5264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorsters, A.; Van Damme, P.; Clifford, G. Urine testing for HPV: Rationale for using first void. BMJ 2014, 349, g6252. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [Green Version]
- Whiting, P.F. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529. [Google Scholar] [CrossRef]
- Šimundič, A.M. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC 2009, 19, 203–211. [Google Scholar]
- Chu, H.; Cole, S.R. Bivariate meta-analysis of sensitivity and specificity with sparse data: A generalized linear mixed model approach. J. Clin. Epidemiol. 2006, 59, 1331–1332. [Google Scholar] [CrossRef]
- Harbord, R.M.; Deeks, J.J.; Egger, M.; Whiting, P.; Sterne, J.A.C. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2006, 8, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Lijmer, J.G.; Patrick, M.M.; Bossuyt, H.; Siem, H. Heisterkamp2, Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Statist. Med. 2002, 21, 1525–1537. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-W.; Hong, J.H.; Min, K.J.; Ouh, Y.-T.; Seong, S.J.; Moon, J.H.; Cho, S.H.; Lee, J.K. Performance and Diagnostic Accuracy of Human Papillomavirus Testing on Self-Collected Urine and Vaginal Samples in a Referral Population. Cancer Res. Treat. 2021, 53, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Tranberg, M.; Jensen, J.S.; Bech, B.H.; Andersen, B. Urine collection in cervical cancer screening–analytical comparison of two HPV DNA assays. BMC Infect. Dis. 2020, 20, 926. [Google Scholar] [CrossRef]
- Van Keer, S.; Tjalma, W.A.A.; Pattyn, J.; Biesmans, S.; Pieters, Z.; Van Ostade, X.; Ieven, M.; Van Damme, P.; Vorsters, A. Human papillomavirus genotype and viral load agreement between paired first-void urine and clinician-collected cervical samples. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 859–869. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, B.Y.; Tareg, A.C.; Reichhardt, M.; Agapito, A.; Zhu, X.; Sy, A.; Yuji, A.; Killeen, J.; Chan, O.; Buenconsejo-Lum, L.E. Randomized controlled trial evaluating the utility of urine HPV DNA for cervical cancer screening in a Pacific Island population. J. Glob. Heal. Rep. 2018, 2. [Google Scholar] [CrossRef]
- Leeman, A.; del Pino, M.; Molijn, A.; Rodriguez, A.; Torné, A.; de Koning, M.; Ordi, J.; van Kemenade, F.; Jenkins, D.; Quint, W. HPV testing in first-void urine provides sensitivity for CIN2+ detection comparable with a smear taken by a clinician or a brush-based self-sample: Cross-sectional data from a triage population. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1356–1363. [Google Scholar] [CrossRef]
- Cuzick, J.; Cadman, L.; Ahmad, A.S.; Ho, L.; Terry, G.; Kleeman, M.; Lyons, D.; Austin, J.; Stoler, M.H.; Vibat, C.R.T.; et al. Performance and Diagnostic Accuracy of a Urine-Based Human Papillomavirus Assay in a Referral Population. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1053–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilyanimit, P.; Chansaenroj, J.; Karalak, A.; Laowahutanont, P.; Junyangdikul, P.; Poovorawan, Y. Comparison of human papillomavirus (HPV) detection in urine and cervical swab samples using the HPV GenoArray Diagnostic assay. PeerJ 2017, 5, e3910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahasrabuddhe, V.V.; Gravitt, P.E.; Dunn, S.T.; Brown, D.; Allen, R.A.; Eby, Y.J.; Smith, K.; Zuna, R.E.; Zhang, R.R.; Gold, M.A.; et al. Comparison of Human Papillomavirus Detections in Urine, Vulvar, and Cervical Samples from Women Attending a Colposcopy Clinic. J. Clin. Microbiol. 2013, 52, 187–192. [Google Scholar] [CrossRef] [Green Version]
- Mendez, K.; Romaguera, J.; Ortiz, A.P.; López, M.; Steinau, M.; Unger, E.R. Urine-based human papillomavirus DNA testing as a screening tool for cervical cancer in high-risk women. Int. J. Gynecol. Obstet. 2013, 124, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Ducancelle, A.; Legrand, M.C.; Pivert, A.; Veillon, P.; Le Guillou-Guillemette, H.; De Brux, M.A.; Beby-Defaux, A.; Agius, G.; Hantz, S.; Alain, S.; et al. Interest of Human Papillomavirus DNA quantification and genotyping in paired cervical and urine samples to detect cervical lesions. Arch. Gynecol. Obstet. 2014, 290, 299–308. [Google Scholar] [CrossRef] [Green Version]
- Bernal, S.; Palomares, J.C.; Artura, A.; Parra, M.; Cabezas, J.L.; Robles, A.; Mazuelos, E.M. Comparison of urine and cervical samples for detecting human papillomavirus (HPV) with the Cobas 4800 HPV test. J. Clin. Virol. 2014, 61, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, E.; Bianchi, S.; Fasolo, M.M.; Frati, E.R.; Mazza, F.; Martinelli, M.; Colzani, D.; Beretta, R.; Zappa, A.; Orlando, G. High performance of a new PCR-based urine assay for HPV-DNA detection and genotyping. J. Med. Virol. 2012, 85, 91–98. [Google Scholar] [CrossRef]
- Vorsters, A.; Van den Bergh, J.; Micalessi, I.; Biesmans, S.; Bogers, J.; Hens, A.; De Coster, I.; Ieven, M.; Van Damme, P. Optimization of HPV DNA detection in urine by improving collection, storage, and extraction. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 2005–2014. [Google Scholar] [CrossRef]
- Tshomo, U.; Franceschi, S.; Tshokey, T.; Tobgay, T.; Baussano, I.; Tenet, V.; Snijders, P.J.F.; Gheit, T.; Tommasino, M.; Vorsters, A.; et al. Evaluation of the performance of Human Papillomavirus testing in paired urine and clinician-collected cervical samples among women aged over 30~years in Bhutan. Virol. J. 2017, 14, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifford, G.M.; Vaccarella, S.; Franceschi, S.; Tenet, V.; Umulisa, M.C.; Tshomo, U.; Dondog, B.; Vorsters, A.; Tommasino, M.; Heideman, D.A.M.; et al. Comparison of Two Widely Used Human Papillomavirus Detection and Genotyping Methods, GP5+/6+-Based PCR Followed by Reverse Line Blot Hybridization and Multiplex Type-Specific E7-Based PCR. J. Clin. Microbiol. 2016, 54, 2031–2038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begg, C.B. Meta-analysis methods for diagnostic accuracy. J. Clin. Epidemiol. 2008, 61, 1081–1082. [Google Scholar] [CrossRef] [PubMed]
- Lijmer, J.G. Empirical Evidence of Design-Related Bias in Studies of Diagnostic Tests. JAMA 1999, 282, 1061. [Google Scholar] [CrossRef] [PubMed]
- Leeflang, M.M.G.; Bossuyt, P.M.M.; Irwig, L. Diagnostic test accuracy may vary with prevalence: Implications for evidence-based diagnosis. J. Clin. Epidemiol. 2009, 62, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, J.; Van Keer, S.; Biesmans, S.; Ieven, M.; Vanderborght, C.; Beyers, K.; Vankerckhoven, V.; Bruyndonckx, R.; Van Damme, P.; Vorsters, A. Human papillomavirus detection in urine: Effect of a first-void urine collection device and timing of collection. J. Virol. Methods 2019, 264, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Snijders, P.J.; Meijer, C.J.; Berkhof, J.; Cuschieri, K.; Kocjan, B.J.; Poljak, M. Which high-risk HPV assays fulfil criteria for use in primary cervical cancer screening? Clin. Microbiol. Infect. 2015, 21, 817–826. [Google Scholar] [CrossRef] [Green Version]
- Sehgal, A.; Gupta, S.; Parashari, A.; Sodhani, P.; Singh, V. Urine HPV-DNA detection for cervical cancer screening: Prospects and prejudices. J. Obstet. Gynaecol. 2009, 29, 583–589. [Google Scholar] [CrossRef]

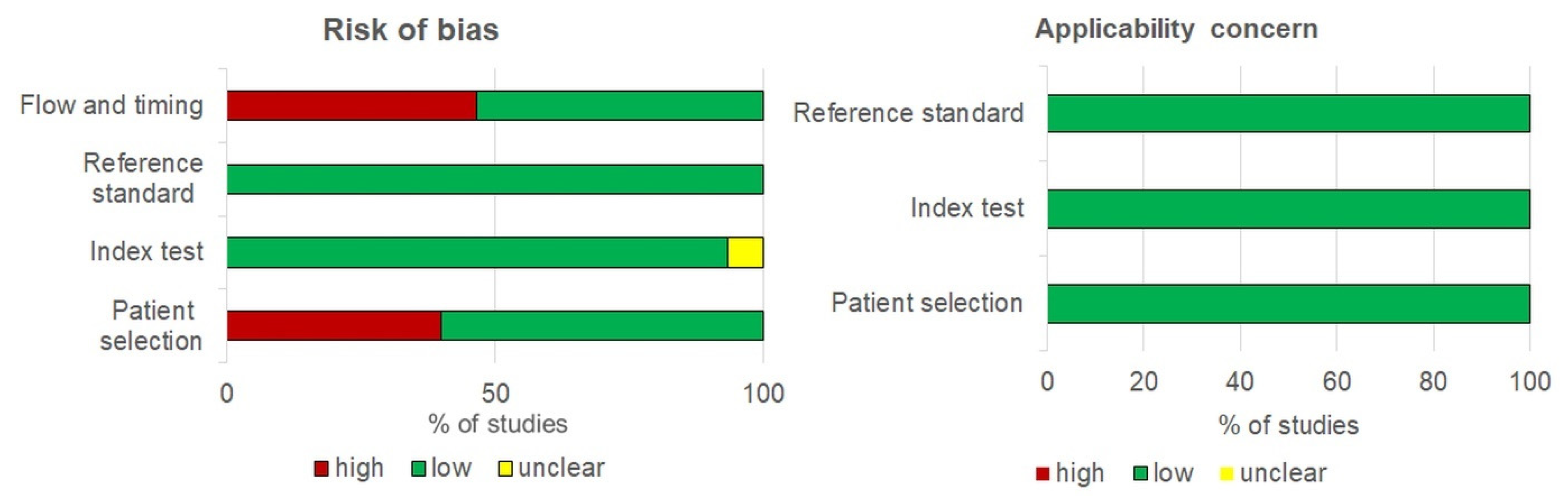

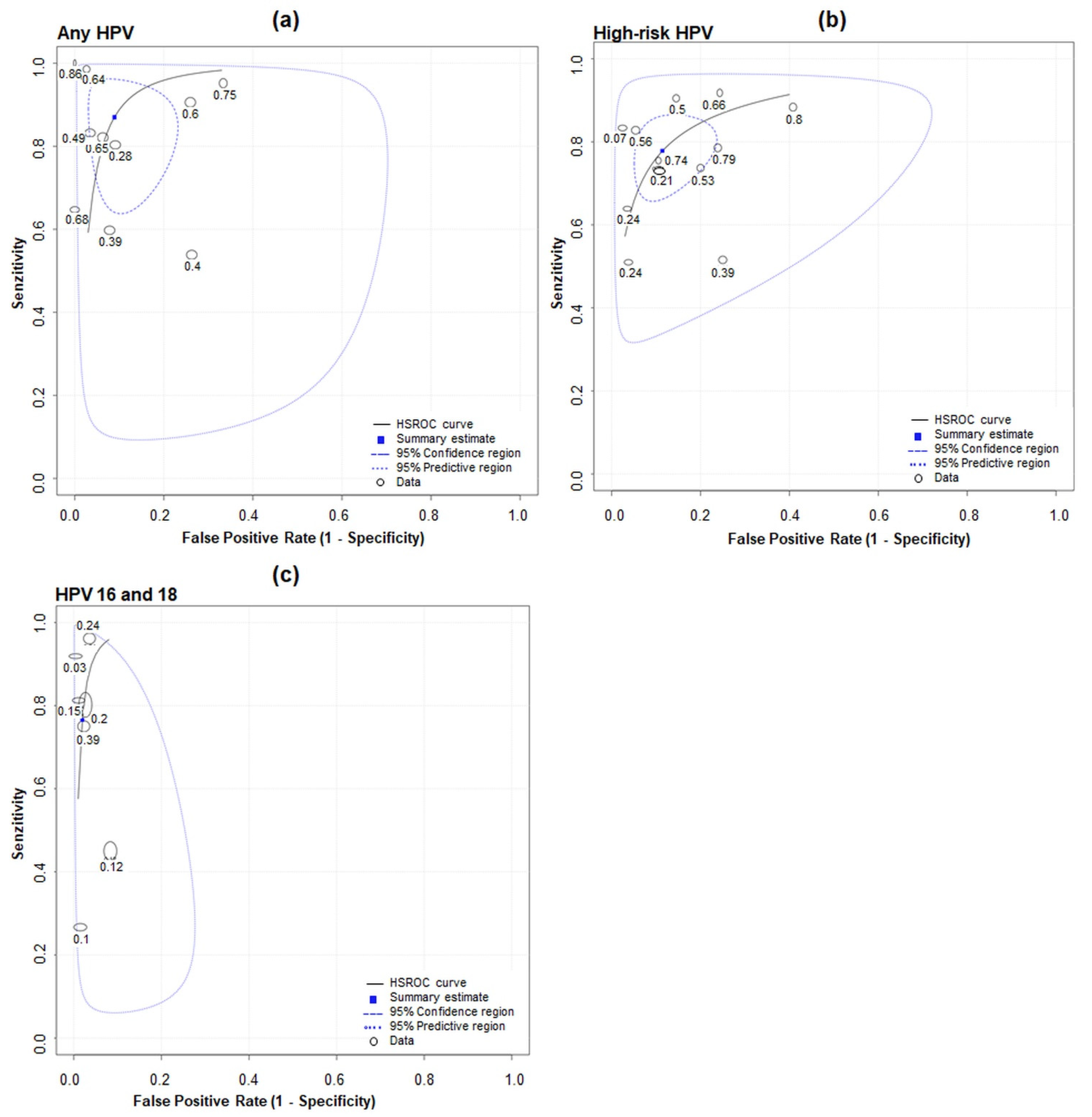

| Author, Year, [Ref] | Country | Study Context (Purpose) | Cytology (Histology) | Timing | HPV Detection Method | DNA Extraction Method | DNA Amplification Platform | Primers |
|---|---|---|---|---|---|---|---|---|
| Hyun-Woong Cho, 2020, [31] | South Korea | colposcopy (follow-up of CIN) | abnormal (CIN2, CIN3, cervical cancer) | urine after cervical | real-time PCR | QIAamp DNA blood minikit | Seegene | L1 |
| Mette Tranberg, 2020, [32] | Denmark | general practitioner (cancer screening) | ASC-US (normal, CIN1, CIN2+) | another day, urine after cervical | real-time PCR | MagNA Pure LC total nucleic acid isolation kit | Roche | L1 |
| Severien Van Keer, 2018, [33] | Belgium | colposcopy (HPV surveillance) | NILM, ASCUS/LSIL, ASC-H/HSIL (normal, CIN1, CIN2, CIN3) | same day, urine before cervical | qPCR | Non-commercial | — | — |
| Nicolás Vergara, 2018, [3] | Chile | primary health care centre (cancer screening) | normal, ASC-US, HSIL, LSIL (—) | same day, urine before cervical | conventional PCR | — | Agilent Technologies | L1/PGMY 09/11 |
| Brenda Y. Hernandez, 2018, [34] | Yap | community health centre (cancer screening) | normal, ASC-US, HSIL, LSIL (normal, CIN2, CIN3, cervical cancer) | urine before cervical and urine after cervical | real-time PCR | — | Roche (Linear Array) | L1/PGMY 09/11 |
| A Leeman, 2017, [35] | Spain | colposcopy (follow-up of CIN) | normal, ASCUS/LSIL, ASC-H/HSIL (normal, CIN1, CIN2, CIN3) | same day, urine before cervical | conventional PCR | — | Innogeneticstechnology | L1/SPF10 |
| Jack Cuzick, 2017, [36] | United Kingdom | colposcopy (follow-up of CIN) | ASCUS, LSIL, HSIL (normal, CIN1, CIN2, CIN3, cervical cancer) | same day, urine before cervical | conventional PCR | QIAamp DNA Mini Kit | — | E1 |
| Pornjarim Nilyanimit, 2017, [37] | Thailand | (cancer screening) | normal, LSIL, HSIL (—) | urine after cervical | PCR based DNA microarray | HPV GenoArray Diagnostic Kit | HybriBio | L1 |
| Alba Lucía Combita, 2016, [17] | Colombia | health center (cancer screening) | normal, ASCUS/LSIL, ASC-H/HSIL (—) | same day, urine before cervical | multiplex PCR | NucliSENS easyMAG Extraction Kit | Luminex technology | E7 |
| Elena Burroni, 2014, [16] | Italy | (cancer screening) | normal, ASCUS/LSIL, ASC-H/HSIL (—) | 8 days (median), urine after cervical | conventional PCR | QIAamp DNA Mini Kit | Innogenetics | L1 |
| Vikrant V. Sahasrabuddhe, 2014, [38] | USA | colposcopy (cancer screening) | NILM, ASCUS/LSIL, HSIL (normal, CIN1, CIN2, CIN3) | same day, urine before cervical | conventional PCR | QIAamp DNA Blood Kit | Roche (Linear Array) | — |
| Keimari Mendez, 2014, [39] | USA | gynaecology clinic (cancer screening) | ASCUS/LSIL, ASC-H/HSIL (CIN1, CIN2) | same day, urine before cervical | conventional PCR | MagNA PureDNA Isolation Kit 1 | Roche (Linear Array) | — |
| A. Ducancelle, 2014, [40] | France | colposcopy (cancer screening) | normal, ASCUS/LSIL, HSIL (-) | — | real-time PCR | QIAamp viral RNA mini kit | Innogenetics | L1 |
| Samuel Bernal, 2014, [41] | Spain | gynaecology clinic (HPV surveillance) | normal, ASCUS/LSIL, HSIL (normal, CIN1, CIN2, CIN3) | same day, urine before cervical | real-time PCR | Cobas X 480 | Roche | — |
| Elisabetta Tanzi, 2013, [42] | Italy | genitourinary clinic (cancer screening) | normal, ASCUS/LSIL, HSIL (-) | same day | conventional PCR | BioMérieux NucliSENS1 MiniMAG1 | Innogenetics | L1(MY09/MY11) |
| Author, Year [Ref] | Sample Recruited (Sample Detection) | Median Age (Range) | Normal | ASCUS/LSIL | ASC-H/HSIL | Normal | CIN1 | CIN2 (CIN2/3) | CIN3 (Cancer) | First-Void Urine (Volume Analysed in mL) | Storage Temperature in °C |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hyun-Woong Cho, 2020, [31] | 314 (314) | 40 (20–60) | ─ | 244/─ | ─/70 | ─ | ─ | 21 | 104 (4) | (30) | 4 |
| Mette Tranberg, 2020, [32] | 150 (150) | 45 (30–59) | ─ | 150/─ | ─ | 11 | 10 | 11 | (10–12) | 4 | |
| Severien Van Keer, 2018, [33] | 110 (110) | 36 (25–64) | 58 | 36/─ | ─/15 | 7 | 11 | 6 | 9 | (median; 19) | −80 |
| Nicolás Vergara, 2018, [3] | 543 (543) | (18–64) | 483 | 24/22 | ─/12 | ─ | ─ | ─ | ─ | (10–15) | −20 |
| Brenda Y. Hernandez, 2018, [34] | 217 (210) | (21–65) | 179 | 31/3 | ─/4 | 2 | ─ | 2 | 5 (2) | (30) | 4 |
| A Leeman, 2017, [35] | 113 (91) | (18–60) | 28 | 11/28 | 9/15 | 50 | 22 | 13 | 6 | (16) | −80 |
| Jack Cuzick, 2017, [36] | 652 (501) | 30 (18–69) | ─ | 160/292 | ─/49 | 185 | 99 | 64 | 79 | (0.5) | ─ |
| Pornjarim Nilyanimit, 2017, [37] | 164 (164) | (19–69) | 95 | ─/50 | ─/19 | ─ | ─ | ─ | ─ | (15) | 4 |
| Alba Lucía Combita, 2016, [17] | 540 (530) | (18–25) | 462 | 45/17 | 2/1 | ─ | ─ | ─ | ─ | (9) | −20 |
| Elena Burroni, 2014, [16] | 271 (215) | 25 | 205 | 3/4 | 1//1 | ─ | ─ | ─ | ─ | (60) | −20 |
| Vikrant V. Sahasrabuddhe, 2014, [38] | 72 (72) | 28 (20–61) | 18 | 23/11 | ─/16 | 17 | 28 | 16 | 10 | (0.53) | 20 |
| Keimari Mendez, 2014, [39] | 52 (50) | (21–60) | ─ | 27/13 | 2/5 | ─ | 42 | 7 | ─ | (6) | −20 |
| A. Ducancelle, 2014, [40] | 245 (230) | (18–55) | 34 | 70/59 | ─/25 | ─ | ─ | ─ | ─ | (1) | −80 |
| Samuel Bernal, 2014, [41] | 125 (125) | 36 (21–65) | 65 | 21/22 | ─/14 | 43 | 17 | 4 | 16 | (20) | ─ |
| Elisabetta Tanzi, 2013, [42] | 107 (107) | 42 (22–70) | 79 | 3/21 | ─/4 | ─ | ─ | ─ | ─ | (15) | −20 |
| Meta-Regression (Inverse Variance Weights 1) | |||||
|---|---|---|---|---|---|
| Var. | Coeff. | Std. Err. | p-Value | RDOR 2 | (95% CI) |
| Cte.3 | 4.202 | 0.900 | 0.006 | ||
| S 4 | −0.628 | 0.307 | 0.096 | ||
| Bias in patient selection | 0.170 | 0.866 | 0.852 | 1.19 | (0.13; 10.98) |
| Purposes | −2.708 | 1.759 | 0.184 | 0.07 | (0.00; 6.13) |
| Sample timing | −0.318 | 1.056 | 0.776 | 0.73 | (0.05; 11.00) |
| Storage temperature | 0.269 | 1.068 | 0.811 | 1.31 | (0.08; 20.39) |
| HPV detection method | 1.556 | 1.451 | 0.333 | 4.74 | (0.11; 197.51) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bober, P.; Firment, P.; Sabo, J. Diagnostic Test Accuracy of First-Void Urine Human Papillomaviruses for Presence Cervical HPV in Women: Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 13314. https://doi.org/10.3390/ijerph182413314
Bober P, Firment P, Sabo J. Diagnostic Test Accuracy of First-Void Urine Human Papillomaviruses for Presence Cervical HPV in Women: Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(24):13314. https://doi.org/10.3390/ijerph182413314
Chicago/Turabian StyleBober, Peter, Peter Firment, and Ján Sabo. 2021. "Diagnostic Test Accuracy of First-Void Urine Human Papillomaviruses for Presence Cervical HPV in Women: Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 24: 13314. https://doi.org/10.3390/ijerph182413314
APA StyleBober, P., Firment, P., & Sabo, J. (2021). Diagnostic Test Accuracy of First-Void Urine Human Papillomaviruses for Presence Cervical HPV in Women: Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 18(24), 13314. https://doi.org/10.3390/ijerph182413314






