Relationship between Resting State Heart Rate Variability and Sleep Quality in Older Adults with Mild Cognitive Impairment
Abstract
:1. Introduction
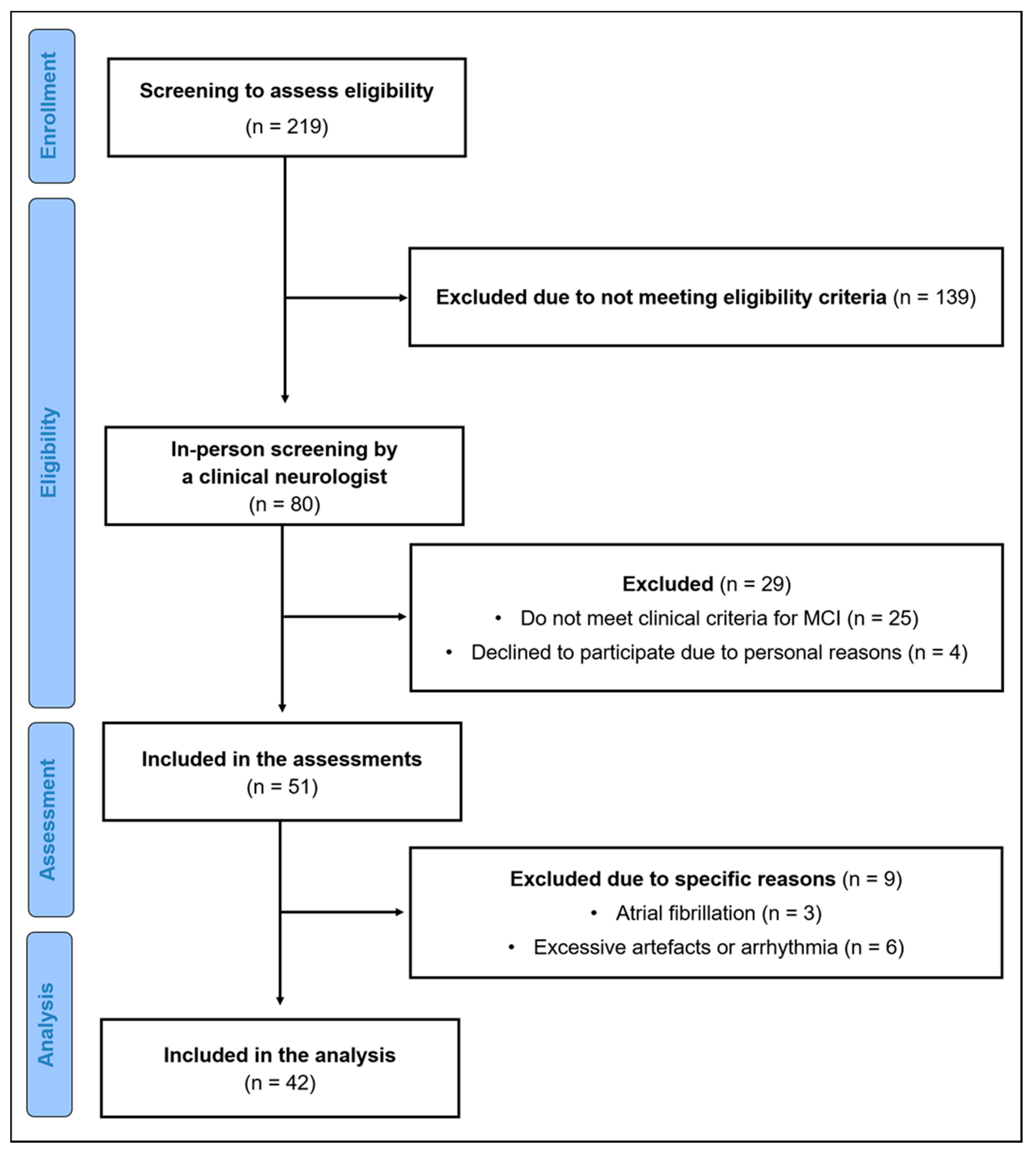
2. Materials and Methods
2.1. Participants
2.2. Ethical Approval
2.3. Experimental Design
2.4. Subjective Sleep Quality
2.5. Autonomic Data Collection and Processing
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Sample
3.2. Subjective Sleep Quality
3.3. Correlation between HRV and PSQI Components
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects. 2019. Available online: https://population.un.org/wpp/ (accessed on 27 August 2021).
- WHO. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-155054-3. [Google Scholar]
- Etgen, T.; Sander, D.; Bickel, H.; Förstl, H. Mild cognitive impairment and dementia: The importance of modifiable risk factors. Dtsch. Arztebl. Int. 2011, 108, 743–750. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- Gillis, C.; Mirzaei, F.; Potashman, M.; Ikram, M.A.; Maserejian, N. The incidence of mild cognitive impairment: A systematic review and data synthesis. Alzheimer’s Dement. 2019, 11, 248–256. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Shiri-Feshki, M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr. Scand. 2009, 119, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Schmicker, M.; Müller, N.G. Präventionsstrategien gegen Demenz. Z. Gerontol. Geriatr. 2017, 50, 89–95. [Google Scholar] [CrossRef]
- Atri, A. The Alzheimer’s Disease Clinical Spectrum: Diagnosis and Management. Med. Clin. N. Am. 2019, 103, 263–293. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Caracciolo, B.; Brayne, C.; Gauthier, S.; Jelic, V.; Fratiglioni, L. Mild cognitive impairment: A concept in evolution. J. Intern. Med. 2014, 275, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Jongsiriyanyong, S.; Limpawattana, P. Mild Cognitive Impairment in Clinical Practice: A Review Article. Am. J. Alzheimer’s Dis. Other Demen. 2018, 33, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Mangialasche, F.; Ngandu, T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s Dement. 2016, 12, 292–323. [Google Scholar] [CrossRef] [PubMed]
- Sewell, K.R.; Erickson, K.I.; Rainey-Smith, S.R.; Peiffer, J.J.; Sohrabi, H.R.; Brown, B.M. Relationships between physical activity, sleep and cognitive function: A narrative review. Neurosci. Biobehav. Rev. 2021, 130, 369–378. [Google Scholar] [CrossRef]
- Brown, R.E.; Basheer, R.; McKenna, J.T.; Strecker, R.E.; McCarley, R.W. Control of sleep and wakefulness. Physiol. Rev. 2012, 92, 1087–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.-C.; Espeland, M.A.; Brunner, R.L.; Lovato, L.C.; Wallace, R.B.; Leng, X.; Phillips, L.S.; Robinson, J.G.; Kotchen, J.M.; Johnson, K.C.; et al. Sleep duration, cognitive decline, and dementia risk in older women. Alzheimer’s Dement. 2016, 12, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Spira, A.P.; Chen-Edinboro, L.P.; Wu, M.N.; Yaffe, K. Impact of sleep on the risk of cognitive decline and dementia. Curr. Opin. Psychiatry 2014, 27, 478–483. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Tan, C.-C.; Zou, J.-J.; Cao, X.-P.; Tan, L. Sleep problems and risk of all-cause cognitive decline or dementia: An updated systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2020, 91, 236–244. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Lee, H.-F.; Lin, M.-H. Exploring the Association between Sleep Quality and Heart Rate Variability among Female Nurses. Int. J. Environ. Res. Public Health 2021, 18, 5551. [Google Scholar] [CrossRef]
- Kong, S.D.X.; Hoyos, C.M.; Phillips, C.L.; McKinnon, A.C.; Lin, P.; Duffy, S.L.; Mowszowski, L.; LaMonica, H.M.; Grunstein, R.R.; Naismith, S.L.; et al. Altered heart rate variability during sleep in mild cognitive impairment. Sleep 2021, 44, zsaa232. [Google Scholar] [CrossRef]
- Bastien, C.H.; St-Jean, G.; Morin, C.M.; Turcotte, I.; Carrier, J. Chronic psychophysiological insomnia: Hyperarousal and/or inhibition deficits? An ERPs investigation. Sleep 2008, 31, 887–898. [Google Scholar] [CrossRef] [Green Version]
- Dlugaj, M.; Weinreich, G.; Weimar, C.; Stang, A.; Dragano, N.; Wessendorf, T.E.; Teschler, H.; Winkler, A.; Wege, N.; Moebus, S.; et al. Sleep-disordered breathing, sleep quality, and mild cognitive impairment in the general population. J. Alzheimer’s Dis. 2014, 41, 479–497. [Google Scholar] [CrossRef]
- van Egroo, M.; Narbutas, J.; Chylinski, D.; Villar González, P.; Maquet, P.; Salmon, E.; Bastin, C.; Collette, F.; Vandewalle, G. Sleep-wake regulation and the hallmarks of the pathogenesis of Alzheimer’s disease. Sleep 2019, 42, zsz017. [Google Scholar] [CrossRef] [Green Version]
- McKinnon, A.; Terpening, Z.; Hickie, I.B.; Batchelor, J.; Grunstein, R.; Lewis, S.J.G.; Naismith, S.L. Prevalence and predictors of poor sleep quality in mild cognitive impairment. J. Geriatr. Psychiatry Neurol. 2014, 27, 204–211. [Google Scholar] [CrossRef]
- Devore, E.E.; Grodstein, F.; Schernhammer, E.S. Sleep Duration in Relation to Cognitive Function among Older Adults: A Systematic Review of Observational Studies. Neuroepidemiology 2016, 46, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Bubu, O.M.; Brannick, M.; Mortimer, J.; Umasabor-Bubu, O.; Sebastião, Y.V.; Wen, Y.; Schwartz, S.; Borenstein, A.R.; Wu, Y.; Morgan, D.; et al. Sleep, Cognitive impairment, and Alzheimer’s disease: A Systematic Review and Meta-Analysis. Sleep 2017, 40, zsw032. [Google Scholar] [CrossRef]
- Virta, J.J.; Heikkilä, K.; Perola, M.; Koskenvuo, M.; Räihä, I.; Rinne, J.O.; Kaprio, J. Midlife sleep characteristics associated with late life cognitive function. Sleep 2013, 36, 1533–1541. [Google Scholar] [CrossRef] [Green Version]
- van Oostrom, S.H.; Nooyens, A.C.J.; van Boxtel, M.P.J.; Verschuren, W.M.M. Long sleep duration is associated with lower cognitive function among middle-age adults—The Doetinchem Cohort Study. Sleep Med. 2018, 41, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.R.; Gardener, H.; Rundek, T.; Elkind, M.S.V.; Boden-Albala, B.; Dong, C.; Cheung, Y.K.; Stern, Y.; Sacco, R.L.; Wright, C.B. Sleep disturbances and cognitive decline in the Northern Manhattan Study. Neurology 2016, 87, 1511–1516. [Google Scholar] [CrossRef] [Green Version]
- Johar, H.; Kawan, R.; Emeny, R.T.; Ladwig, K.-H. Impaired Sleep Predicts Cognitive Decline in Old People: Findings from the Prospective KORA Age Study. Sleep 2016, 39, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Kuperczkó, D.; Perlaki, G.; Faludi, B.; Orsi, G.; Altbacker, A.; Kovács, N.; Dóczi, T.; Komoly, S.; Schwarcz, A.; Clemens, Z.; et al. Late bedtime is associated with decreased hippocampal volume in young healthy subjects. Sleep Biol. Rhythm. 2015, 13, 68–75. [Google Scholar] [CrossRef]
- Sabeti, S.; Al-Darsani, Z.; Mander, B.A.; Corrada, M.M.; Kawas, C.H. Sleep, hippocampal volume, and cognition in adults over 90 years old. Aging Clin. Exp. Res. 2018, 30, 1307–1318. [Google Scholar] [CrossRef]
- Coogan, A.N.; Schutová, B.; Husung, S.; Furczyk, K.; Baune, B.T.; Kropp, P.; Häßler, F.; Thome, J. The circadian system in Alzheimer’s disease: Disturbances, mechanisms, and opportunities. Biol. Psychiatry 2013, 74, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Hayes, T.L.; Riley, T.; Mattek, N.; Pavel, M.; Kaye, J.A. Sleep habits in mild cognitive impairment. Alzheimer Dis. Assoc. Disord. 2014, 28, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; Zhang, P.; Li, C.; Tan, Y.; Li, G.; Xu, D.; Chen, L. Sleep disturbance in mild cognitive impairment: A systematic review of objective measures. Neurol. Sci. 2017, 38, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Brachem, C.; Winkler, A.; Tebrügge, S.; Weimar, C.; Erbel, R.; Jöckel, K.-H.; Stang, A.; Dragano, N.; Moebus, S.; Kowall, B.; et al. Associations between self-reported sleep characteristics and incident mild cognitive impairment: The Heinz Nixdorf Recall Cohort Study. Sci. Rep. 2020, 10, 6542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potvin, O.; Lorrain, D.; Forget, H.; Dubé, M.; Grenier, S.; Préville, M.; Hudon, C. Sleep quality and 1-year incident cognitive impairment in community-dwelling older adults. Sleep 2012, 35, 491–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webers, A.; Heneka, M.T.; Gleeson, P.A. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol. Cell Biol. 2020, 98, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.R.; Tarraf, W.; Wu, B.; Redline, S.; Cai, J.; Daviglus, M.L.; Gallo, L.; Mossavar-Rahmani, Y.; Perreira, K.M.; Zee, P.; et al. Sleep and neurocognitive decline in the Hispanic Community Health Study/Study of Latinos. Alzheimer’s Dement. 2020, 16, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-B.; Myung, S.-K.; Lee, S.-M.; Park, Y.C. Longer Duration of Sleep and Risk of Cognitive Decline: A Meta-Analysis of Observational Studies. Neuroepidemiology 2016, 47, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Wennberg, A.M.V.; Wu, M.N.; Rosenberg, P.B.; Spira, A.P. Sleep Disturbance, Cognitive Decline, and Dementia: A Review. Semin. Neurol. 2017, 37, 395–406. [Google Scholar] [CrossRef]
- Lo, J.C.; Groeger, J.A.; Cheng, G.H.; Dijk, D.-J.; Chee, M.W.L. Self-reported sleep duration and cognitive performance in older adults: A systematic review and meta-analysis. Sleep Med. 2016, 17, 87–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozzini, L.; Conti, M.Z.; Riva, M.; Ceraso, A.; Caratozzolo, S.; Zanetti, M.; Padovani, A. Non-amnestic mild cognitive impairment and sleep complaints: A bidirectional relationship? Aging Clin. Exp. Res. 2018, 30, 661–668. [Google Scholar] [CrossRef]
- Gañán-Calvo, A.M.; Fajardo-López, J. Universal structures of normal and pathological heart rate variability. Sci. Rep. 2016, 6, 21749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, G. Heart-Rate Variability-More than Heart Beats? Front. Public Health 2017, 5, 240. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCraty, R.; Shaffer, F. Heart rate variability: New perspectives on physiological mechanisms, assessment of self-regulatory capacity, and health risk. Glob. Adv. Health Med. 2015, 4, 46–61. [Google Scholar] [CrossRef] [Green Version]
- La Rovere, M.T.; Pinna, G.D. Beneficial effects of physical activity on baroreflex control in the elderly. Ann. Noninvasive Electrocardiol. 2014, 19, 303–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thayer, J.F.; Lane, R.D. The role of vagal function in the risk for cardiovascular disease and mortality. Biol. Psychol. 2007, 74, 224–242. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef] [Green Version]
- Malik, M. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Sammito, S.; Thielmann, B.; Seibt, R.; Klussmann, A.; Weippert, M.; Böckelmann, I. Guideline for the application of heart rate and heart rate variability in occupational medicine and occupational science. ASUI 2015, 2015, 1–29. [Google Scholar] [CrossRef]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes de Godoy, M. Nonlinear Analysis of Heart Rate Variability: A Comprehensive Review. J. Cardiol. Ther. 2016, 3, 528–533. [Google Scholar] [CrossRef]
- Forte, G.; Favieri, F.; Casagrande, M. Heart Rate Variability and Cognitive Function: A Systematic Review. Front. Neurosci. 2019, 13, 710. [Google Scholar] [CrossRef]
- Grässler, B.; Hökelmann, A.; Cabral, R.H. Resting heart rate variability as a possible marker of cognitive decline. Kinesiology 2020, 52, 72–84. [Google Scholar] [CrossRef]
- Thayer, J.F.; Hansen, A.L.; Saus-Rose, E.; Johnsen, B.H. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation, and health. Ann. Behav. Med. 2009, 37, 141–153. [Google Scholar] [CrossRef]
- Castro-Diehl, C.; Diez Roux, A.V.; Redline, S.; Seeman, T.; McKinley, P.; Sloan, R.; Shea, S. Sleep Duration and Quality in Relation to Autonomic Nervous System Measures: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2016, 39, 1927–1940. [Google Scholar] [CrossRef]
- Kageyama, T.; Nishikido, N.; Kobayashi, T.; Kurokawa, Y.; Kaneko, T.; Kabuto, M. Self-reported sleep quality, job stress, and daytime autonomic activities assessed in terms of short-term heart rate variability among male white-collar workers. Ind. Health 1998, 36, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Sajjadieh, A.; Shahsavari, A.; Safaei, A.; Penzel, T.; Schoebel, C.; Fietze, I.; Mozafarian, N.; Amra, B.; Kelishadi, R. The Association of Sleep Duration and Quality with Heart Rate Variability and Blood Pressure. Tanaffos 2020, 19, 135–143. [Google Scholar] [PubMed]
- Jackowska, M.; Dockray, S.; Endrighi, R.; Hendrickx, H.; Steptoe, A. Sleep problems and heart rate variability over the working day. J. Sleep Res. 2012, 21, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Hovland, A.; Pallesen, S.; Hammar, A.; Hansen, A.L.; Thayer, J.F.; Sivertsen, B.; Tarvainen, M.P.; Nordhus, I.H. Subjective sleep quality in relation to inhibition and heart rate variability in patients with panic disorder. J. Affect. Disord. 2013, 150, 152–155. [Google Scholar] [CrossRef]
- Dupuy, O.; Bosquet, L.; Fraser, S.A.; Labelle, V.; Bherer, L. Higher cardiovascular fitness level is associated to better cognitive dual-task performance in Master Athletes: Mediation by cardiac autonomic control. Brain Cogn. 2018, 125, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.-C.; Huang, C.-J.; Yang, T.-T.; Tsai, P.-S. Heart rate variability and daytime functioning in insomniacs and normal sleepers: Preliminary results. J. Psychosom. Res. 2008, 65, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Werner, G.G.; Ford, B.Q.; Mauss, I.B.; Schabus, M.; Blechert, J.; Wilhelm, F.H. High cardiac vagal control is related to better subjective and objective sleep quality. Biol. Psychol. 2015, 106, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Schierholz, R.S. Herzratenvariabilität als Möglicher Objektiver Beanspruchungsparameter für die Einschätzung Subjektiv Angegebener Schlafstörungen im Kontext Psychischer Gesundheit. Ph.D. Dissertation, Medizinische Fakultät der Otto-von-Guericke-Universität Magdeburg, Magdeburg, Germany, 2019. [Google Scholar]
- Burton, A.R.; Rahman, K.; Kadota, Y.; Lloyd, A.; Vollmer-Conna, U. Reduced heart rate variability predicts poor sleep quality in a case-control study of chronic fatigue syndrome. Exp. Brain Res. 2010, 204, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Rongve, A.; Boeve, B.F.; Aarsland, D. Frequency and correlates of caregiver-reported sleep disturbances in a sample of persons with early dementia. J. Am. Geriatr. Soc. 2010, 58, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Thielmann, B.; Schierholz, R.S.; Böckelmann, I. Subjective and Objective Consequences of Stress in Subjects with Subjectively Different Sleep Quality—A Cross-Sectional Study. IJERPH 2021, 18, 9990. [Google Scholar] [CrossRef]
- Yang, A.C.; Tsai, S.-J.; Yang, C.-H.; Kuo, C.-H.; Chen, T.-J.; Hong, C.-J. Reduced physiologic complexity is associated with poor sleep in patients with major depression and primary insomnia. J. Affect. Disord. 2011, 131, 179–185. [Google Scholar] [CrossRef]
- Sammito, S.; Böckelmann, I. Factors influencing heart rate variability. ICFJ 2016, 6, 18–22. [Google Scholar] [CrossRef]
- Yesavage, J.A.; Sheikh, J.I. 9/Geriatric Depression Scale (GDS). Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Morris, J.C.; Heyman, A.; Mohs, R.C.; Hughes, J.P.; van Belle, G.; Fillenbaum, G.; Mellits, E.D.; Clark, C. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 1989, 39, 1159–1165. [Google Scholar] [CrossRef]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Grässler, B.; Herold, F.; Dordevic, M.; Gujar, T.A.; Darius, S.; Böckelmann, I.; Müller, N.G.; Hökelmann, A. Multimodal measurement approach to identify individuals with mild cognitive impairment: Study protocol for a cross-sectional trial. BMJ Open 2021, 11, e046879. [Google Scholar] [CrossRef] [PubMed]
- Rieman, D.; Backhaus, J. Behandlung von Schlafstörungen: Ein Psychologisches Gruppenprogramm, Beltz, PsychologiesUnion: Weinheim, Germany, 1996.
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Taylor and Francis: Hoboken, NJ, USA, 2013; ISBN 9780805802832. [Google Scholar]
- Conover, W.J. Practical Nonparametric Statistics, 3rd ed.; Wiley: New York, NY, USA; Weinheim, Germany, 1999; ISBN 978-0-471-16068-7. [Google Scholar]
- Jack, C.R.; Knopman, D.S.; Jagust, W.J.; Petersen, R.C.; Weiner, M.W.; Aisen, P.S.; Shaw, L.M.; Vemuri, P.; Wiste, H.J.; Weigand, S.D.; et al. Tracking pathophysiological processes in Alzheimer’s disease: An updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013, 12, 207–216. [Google Scholar] [CrossRef] [Green Version]
- André, C.; Tomadesso, C.; de Flores, R.; Branger, P.; Rehel, S.; Mézenge, F.; Landeau, B.; de la Sayette, V.; Eustache, F.; Chételat, G.; et al. Brain and cognitive correlates of sleep fragmentation in elderly subjects with and without cognitive deficits. Alzheimer’s Dement. 2019, 11, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Espinosa, M.P.; Atienza, M.; Cantero, J.L. Sleep deficits in mild cognitive impairment are related to increased levels of plasma amyloid-β and cortical thinning. Neuroimage 2014, 98, 395–404. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, A.C.; Lagopoulos, J.; Terpening, Z.; Grunstein, R.; Hickie, I.B.; Batchelor, J.; Lewis, S.J.G.; Duffy, S.; Shine, J.M.; Naismith, S.L. Sleep disturbance in mild cognitive impairment is associated with alterations in the brain’s default mode network. Behav. Neurosci. 2016, 130, 305–315. [Google Scholar] [CrossRef]
- Kreutzmann, J.C.; Havekes, R.; Abel, T.; Meerlo, P. Sleep deprivation and hippocampal vulnerability: Changes in neuronal plasticity, neurogenesis and cognitive function. Neuroscience 2015, 309, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Tononi, G.; Cirelli, C. Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron 2014, 81, 12–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backhaus, J.; Junghanns, K.; Broocks, A.; Riemann, D.; Hohagen, F. Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J. Psychosom. Res. 2002, 53, 737–740. [Google Scholar] [CrossRef]
- Manzar, M.D.; Moiz, J.A.; Zannat, W.; Spence, D.W.; Pandi-Perumal, S.R.; Hussain, M.E. Validity of the Pittsburgh Sleep Quality Index in Indian University Students. Oman Med. J. 2015, 30, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Nuccetelli, M.; Izzi, F.; Sancesario, G.; Romigi, A.; Martorana, A.; Amoroso, C.; Bernardini, S.; Marciani, M.G.; Mercuri, N.B.; et al. Rapid eye movement sleep disruption and sleep fragmentation are associated with increased orexin-A cerebrospinal-fluid levels in mild cognitive impairment due to Alzheimer’s disease. Neurobiol. Aging 2016, 40, 120–126. [Google Scholar] [CrossRef]
- Falck, R.S.; Davis, J.C.; Best, J.R.; Chan, P.C.Y.; Li, L.C.; Wyrough, A.B.; Bennett, K.J.; Backhouse, D.; Liu-Ambrose, T. Effect of a Multimodal Lifestyle Intervention on Sleep and Cognitive Function in Older Adults with Probable Mild Cognitive Impairment and Poor Sleep: A Randomized Clinical Trial. J. Alzheimer’s Dis. 2020, 76, 179–193. [Google Scholar] [CrossRef]
- Bademli, K.; Lok, N.; Canbaz, M.; Lok, S. Effects of Physical Activity Program on cognitive function and sleep quality in elderly with mild cognitive impairment: A randomized controlled trial. Perspect. Psychiatr. Care 2019, 55, 401–408. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284–290. [Google Scholar] [CrossRef]
- Gentili, A.; Weiner, D.K.; Kuchibhatla, M.; Edinger, J.D. Test-retest reliability of the Pittsburgh sleep quality index in nursing home residents. J. Am. Geriatr. Soc. 1995, 43, 1317–1318. [Google Scholar] [CrossRef] [PubMed]
| Variables | Total (n = 42) | Bad Sleepers (n = 19) | Good Sleepers (n = 23) | p-Value | Effect Size |
|---|---|---|---|---|---|
| Age (years) | 69.0 ± 5.5 | 69.3 ± 4.8 | 68.8 ± 6.1 | 0.711 a | 0.090 |
| Male/female (n) | 19/23 | 8/11 | 11/12 | 0.606 b | 0.114 |
| Height (cm) | 170.69 ± 8.81 | 170.42 ± 9.22 | 170.91 ± 8.67 | 0.860 a | 0. 055 |
| Body weight (kg) | 74.04 ± 11.58 | 72.32 ± 10.01 | 75.47 ± 12.77 | 0.595 c | 0.134 |
| BMI (kg/m2) | 25.32 ± 2.53 | 24.83 ± 2.10 | 25.72 ± 2.81 | 0.259 a | 0.354 |
| Years of education (years) | 15.33 ± 2.52 | 14.95 ± 2.00 | 15.65 ± 2.89 | 0.373 a | 0.277 |
| MMSE (score) | 27.24 ± 1.23 | 26.84 ± 0.83 | 27.57 ± 1.41 | 0.092 c | 0.294 |
| mHR (bpm) | 67.09 ± 9.89 | 63.61 ± 8.84 | 69.96 ± 9.96 | 0.037 a,* | 0.670 |
| RMSSD (ms) | 25.97 ± 15.76 | 30.16 ± 15.72 | 22.52 ± 15.27 | 0.063 c | 0.240 |
| HF nu | 47.82 ± 21.25 | 54.80 ± 22.51 | 42.05 ± 18.71 | 0.093 c | 0.297 |
| D2 | 0.75 ± 1.32 | 0.96 ± 1.49 | 0.58 ± 1.16 | 0.519 c | 0.143 |
| Variables | Total (n = 42) | Bad Sleeper (n = 19) | Good Sleepers (n = 23) | p-Value | Effect Size |
|---|---|---|---|---|---|
| Subjective sleep quality | 1.50 ± 0.74 | 1.89 ± 0.66 | 1.17 ± 0.65 | 0.002 ** | 0.482 |
| Sleep latency | 0.86 ± 0.98 | 1.53 ± 0.96 | 0.30 ± 0.56 | <0.001 *** | 0.626 |
| Sleep duration | 0.45 ± 0.80 | 0.89 ± 0.94 | 0.09 ± 0.42 | 0.001 ** | 0.494 |
| Sleep efficiency | 0.50 ± 0.86 | 1.00 ± 1.05 | 0.09 ± 0.29 | <0.001 *** | 0.526 |
| Sleep disturbance | 1.33 ± 0.53 | 1.63 ± 0.50 | 1.09 ± 0.42 | 0.001 ** | 0.508 |
| Sleep medication | 0.12 ± 0.33 | 0.26 ± 0.45 | 0.00 ± 0.00 | 0.010 * | 0.395 |
| Sleep dysfunction | 0.81 ± 0.77 | 1.11 ± 0.88 | 0.57 ± 0.59 | 0.036 * | 0.345 |
| Global score | 5.55 ± 3.15 | 8.32 ± 2.43 | 3.26 ± 1.25 | <0.001 *** | 0.803 |
| Variables | mHR | RMSSD | HF nu | D2 | ||||
|---|---|---|---|---|---|---|---|---|
| p-Value | rho | p-Value | rho | p-Value | rho | p-Value | rho | |
| Sleep quality | 0.174 | −0.219 | 0.390 | 0.140 | 0.344 | 0.154 | 0.766 | −0.049 |
| Latency | 0.512 | −0.107 | 0.987 | 0.003 | 0.054 | 0.307 | 0.279 | −0.176 |
| Duration | 0.106 | −0.259 | 0.135 | 0.240 | 0.548 | 0.098 | 0.725 | 0.057 |
| Efficiency | 0.156 | −0.229 | 0.099 | 0.264 | 0.048 * | 0.314 | 0.948 | −0.011 |
| Disturbance | 0.590 | −0.088 | 0.927 | −0.015 | 0.600 | −0.086 | 0.490 | 0.112 |
| Medication | 0.522 | 0.104 | 0.781 | −0.045 | 0.560 | 0.095 | 0.920 | −0.016 |
| Dysfunction | 0.666 | −0.070 | 0.653 | 0.073 | 0.579 | −0.090 | 0.448 | 0.123 |
| Global score | 0.132 | −0.239 | 0.251 | 0.184 | 0.177 | 0.215 | 0.989 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grässler, B.; Dordevic, M.; Herold, F.; Darius, S.; Langhans, C.; Halfpaap, N.; Labott, B.K.; Müller, P.; Ammar, A.; Thielmann, B.; et al. Relationship between Resting State Heart Rate Variability and Sleep Quality in Older Adults with Mild Cognitive Impairment. Int. J. Environ. Res. Public Health 2021, 18, 13321. https://doi.org/10.3390/ijerph182413321
Grässler B, Dordevic M, Herold F, Darius S, Langhans C, Halfpaap N, Labott BK, Müller P, Ammar A, Thielmann B, et al. Relationship between Resting State Heart Rate Variability and Sleep Quality in Older Adults with Mild Cognitive Impairment. International Journal of Environmental Research and Public Health. 2021; 18(24):13321. https://doi.org/10.3390/ijerph182413321
Chicago/Turabian StyleGrässler, Bernhard, Milos Dordevic, Fabian Herold, Sabine Darius, Corinna Langhans, Nicole Halfpaap, Berit K. Labott, Patrick Müller, Achraf Ammar, Beatrice Thielmann, and et al. 2021. "Relationship between Resting State Heart Rate Variability and Sleep Quality in Older Adults with Mild Cognitive Impairment" International Journal of Environmental Research and Public Health 18, no. 24: 13321. https://doi.org/10.3390/ijerph182413321
APA StyleGrässler, B., Dordevic, M., Herold, F., Darius, S., Langhans, C., Halfpaap, N., Labott, B. K., Müller, P., Ammar, A., Thielmann, B., Böckelmann, I., Müller, N. G., & Hökelmann, A. (2021). Relationship between Resting State Heart Rate Variability and Sleep Quality in Older Adults with Mild Cognitive Impairment. International Journal of Environmental Research and Public Health, 18(24), 13321. https://doi.org/10.3390/ijerph182413321










