Abstract
Mercury (Hg) is an important heavy metal to consider in marine predators, while selenium (Se) has a natural antagonistic effect on this metal in fish. The Atlantic bluefin tuna (ABFT, Thunnus thynnus) is a pelagic top-level predator of the trophic web and their Hg muscular content is an object of concern in food safety. Nevertheless, little is known about levels of this metal in remaining tissues, which may be important as by-product source, and its relationship with Se. Thus, concentration of both elements in liver, kidney, brain, gill and bone, in addition to muscle, of ABFT were determined. The kidney was the tissue with the highest concentration of Hg (Total-Hg, THg) and Se, and the Se/THg concentration ratio was similar in all tissues, except bone and muscle. The Selenium Health Benefit Value (HBVSe) was positive in each specimen and tissue, indicating that the Se plays an important role against Hg not only in the muscle.
1. Introduction
The presence of heavy metals in marine ecosystems has increased in recent years, derived from natural and anthropogenic sources [1,2] continuously depositing in the seabed and interacting directly with the biota [3,4]. Mercury (Hg) is highly toxic, and its pollution is understood to be global, diffuse and a chronic problem [5]. This heavy metal is included in the Substance Priority List from the Agency for Toxic Substances and Disease Registry [6], as a priority substance in the field of water policy [7,8], as a priority pollutant under the clean water act [9] and subject to international agreements [10]. In addition, this non-essential metal produces important effects on animal and human health, so it has been a frequent object of research [11]. The global costs to society by neurocognitive deficits due to Hg toxicity are estimated at several USD billions [12,13].
Although Hg pollution is a worldwide problem in seas and oceans, it is especially important in the Mediterranean Sea by mining and sub-marine volcano activities [14], with at least three primary sources of atmospheric deposition in the surface sediments: urban, industrial and global precipitation-derived [15]. Over the last decades, fish from the Mediterranean Sea have been linked with higher Hg concentration than other oceans [16,17,18,19], becoming a problem of great concern as fish plays a fundamental role in the Mediterranean diet. This diet recommends consuming fish at least twice a week, in order to reduce risk of contracting many pathologies such as cardiovascular disease, lung disease, cancer or Alzheimer’s disease, thanks to the intake of omega-3 fatty acids content [20].
Accumulation and biomagnification of Hg in top-level aquatic predators is well known, being considered that environmental concentrations in predator’s tissues are amplified by a million times or more [21]. Atlantic bluefin tuna (ABFT, Thunnus thynnus L.) is a top pelagic predator of the marine food web. In the last years the potential risks associated with excessive consumption of tuna have been largely discussed [22,23,24]. The problem of bioaccumulation of methylmercury (MeHg) in marine predators is expected to be exacerbated by climate change [21], which could increase concerns about the potential health risks derived from the consumption of this species. Maximum levels for Hg in certain foods have been established by European authorities, 1.0 µg g−1 (wet weight) being the maximum level in muscle meat of Thunnus species [25]. In this regard, some recent reviews highlighted the need of more knowledge about this issue [26,27], with more attention to the toxicology in marine fish due to its bioaccumulation and toxic effects [28]. As a result, many studies have been focused on measuring the Hg content in muscle as well as in liver of marine predators that are frequently consumed by humans, recommended as an environmental indicator of water pollution, even though the muscle can show higher Hg concentration [29]. On the other hand, by-products of the tuna aquaculture industry (such as gill, guts and bones) go to animal feed (pet food, fish meal for aquaculture), industrial uses (essential oils and other derivatives), or for human consumption [30,31]. However, scientific literature reporting tissue distribution of Hg in other tissues of ABFT is very scarce [29,32,33,34].
Finally, Selenium (Se) is an antagonist of Hg toxicity, which protects tissues against oxidative damage and prevents pathological consequences of Hg bioaccumulation [35]. If the Se supply is altered, the fish does not respond adequately to growth and physiological and biochemical functions (such as antioxidant response), but an excess could also be harmful [36]. Teleost fishes transport through their body more Se than most other vertebrates [37], and have developed greater dependence on environmental Se. In addition, some predatory species contain higher concentrations of Se than those reported in other fish species [38]. However, Se is only studied in fish muscle for human food safety. In fact, it is reported that the Selenium Health Benefit Value (HBVSe [39]) is a good instrument to better understand the available Se that remains after its interaction with Hg.
Thus, in light of the above, the aim of this study was to better understand the Hg and Se interaction in several tissues of ABFT from Mediterranean stock, since that could contribute to environmental knowledge in relation to this pollutant, its toxicokinetic properties, and the tuna health status by Hg exposure. Due to the lack of existing information regarding Hg tissular distribution, Total-Hg (THg) and Se concentration in gills, brain, kidney and bone in ABFT, in addition to liver and muscle, were assessed.
2. Material and Methods
2.1. Sample Collection
Samples (liver, kidney, muscle, brain, gill and bone) were taken from 43 specimens of ABFT that had been purse-seined in Balearic Sea (Mediterranean Sea) in May 2016 and transferred to sea cage culture facilities in San Pedro del Pinatar (Murcia, Spain) (37° 49′ 55.46″ N–0° 39′ 42.33″ W) where they were kept until slaughter for human consumption (September to October 2016). The specimens (length 202–248 cm, weight 206–360 kg, 27 males and 16 females) were shot underwater by lupara (shocking-death) then hoisted by crane, and the age was determined from the size [40]. The samples were carefully taken (0.5–1 g) according to the protocol established by the ICCAT through the Atlantic-Wide Research Programme for Bluefin Tuna. The muscle samples were dissected from the caudal peduncle, and bone samples were collected from the bony denticles of the branchial arch. Bone samples were dried at 40 °C to constant weight, and the dry matter percentages for each sample (specimen) were calculated. Gill samples were washed with purified water (MilliQ), nitric acid (2%), and again MilliQ water, and then dried at room temperature. All samples were stored at -20 °C until analysis.
2.2. Metal Analysis
The THg content in each tissue (samples in triplicate) was determined by using the pyrolysis atomic absorption spectrometry with gold amalgamation (AMA254 Advanced Mercury Analyzer, LECO Instrumentos S.A., Madrid, Spain), according to the methodology describe by Costley et al. [41]. In order to ensure quality of the results obtained, analytical blanks and certificate material (NIST2976) were employed. The method’s precision was 9%, calculated by analytical blanks coefficient of variation. The recovery of the certificated material was 110%, and the analytical detection limit (DL) was 0.01 ng.
To determine Se content, samples were analysed using inductively coupled plasma optical emission spectrometry (ICP-OES, ICAP 6500 Duo, Thermo Scientific, with One Fast System, ThermoFisher Scientific, Waltham, USA). Previously, the samples were digested in special Teflon reaction tubes with 4 mL of trace mineral-grade nitric acid (69%) and 1 mL of hydrogen peroxide (33%) (Suprapure, Merck) and heated for 20 min at 220 °C in a microwave digestion system (UltraClave-Microwave Milestone®, Sorisole, Italy). Finally, the samples were diluted to 10 mL with double deionised water. Two readings were taken for every sample, so the concentration values used were the mean of the two readings. In order to check any possible contamination, one blank sample for every eleven samples was also analysed. Element calibration standards (SCP Science, in 4% nitric acid) with specific concentrations of this element and intermediate patterns were prepared. The calibration device was established per batch, with a minimum of three points for every lot. Each run started with the calibration standards, continued with samples and intermediate patterns, and finished with the series with intermediate patterns (10% variation coefficient). The wavelengths were 196.090-203.985, and the recovery rates for reference materials (Standard Reference Material L577b) was 103.93%. The DL was 0.001 μg g−1.
For a better interpretation of results and to compare between different tissues, the element concentrations of bone were transformed to wet weight (the moisture percentage of each sample were measured, and then the individual THg and Se concentration in wet weight was calculated). Thus, inorganic element concentrations were expressed in micrograms per gram in wet weight (μg g−1 ww).
2.3. Data Analysis
The data given for the THg and Se concentrations are mean, standard error, minimum and maximum. The Kolmogorov–Smirnov and Shapiro–Wilk test were used to check the normality of data, and Student’s t-test, ANOVA, Games-Howell (post hoc) and Pearson tests were used as statistical methods. The significance level for all tests was set as 0.05. All statistical analyses were performed with SPSS v.24.0 for Windows.
In terms of food safety, the HBVSe [39] in muscle tissue was calculated:
where Se and Hg are molar concentrations (µmol kg−1) of these elements. A positive value of HBVSe is considered healthy [39,42]. The scale of the value proportionately reflects the Se deficit or surplus associated with eating that seafood [39]. For better knowledge of Se and Hg relation, we applied this formula to the remaining tissues. The Se/THg concentration ratio and the HBVSe were calculated for each individual sample, then averaged. The Se:Hg molar ratio is not presented (this can be obtained by multiplying the Se/THg concentration ratio by 2.54).
HBVSe = ([Se – Hg] / Se) × (Se + Hg)
3. Results
The birth-year of bluefin tuna analysed ranged from 2001 to 2006.
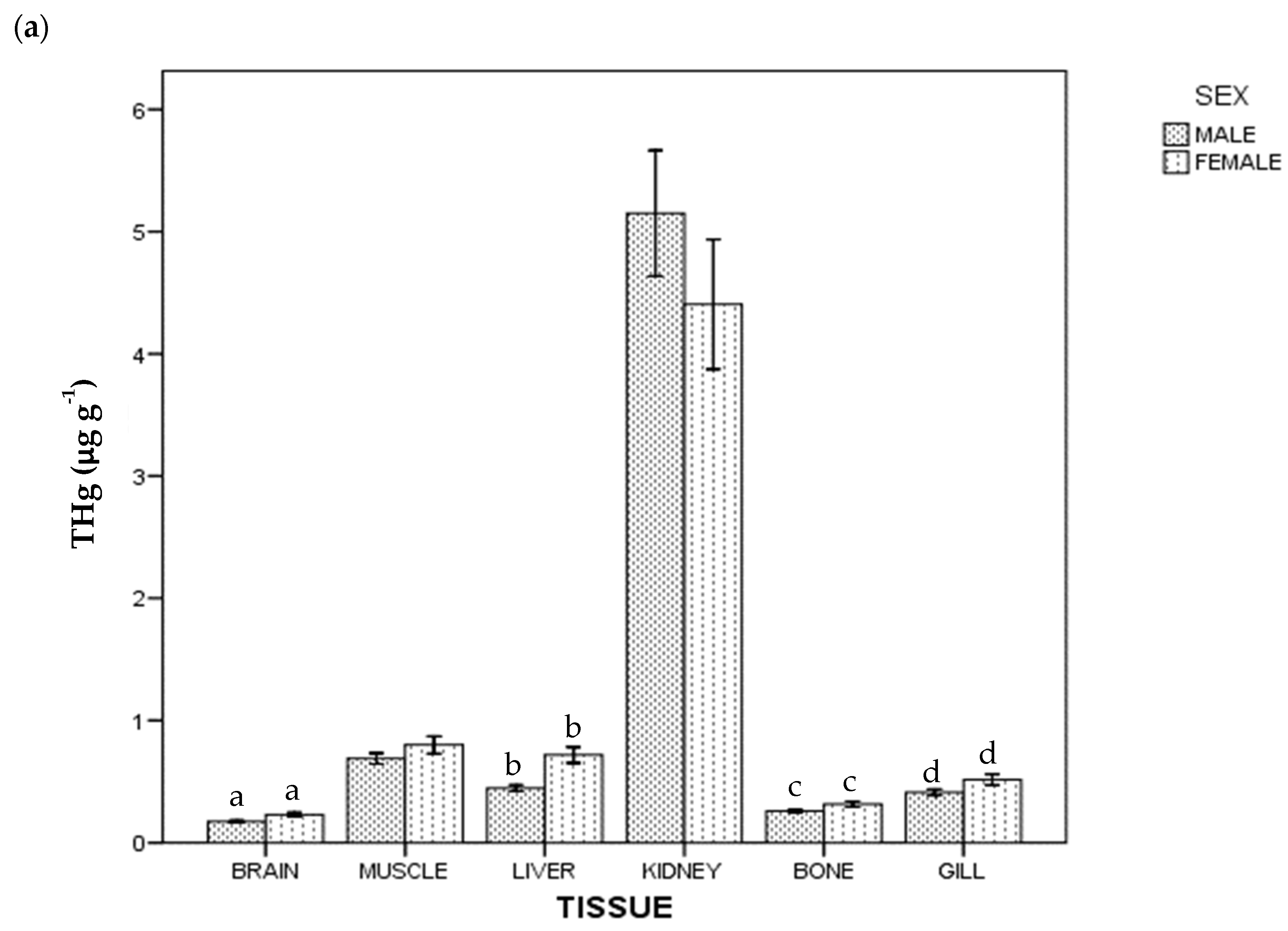
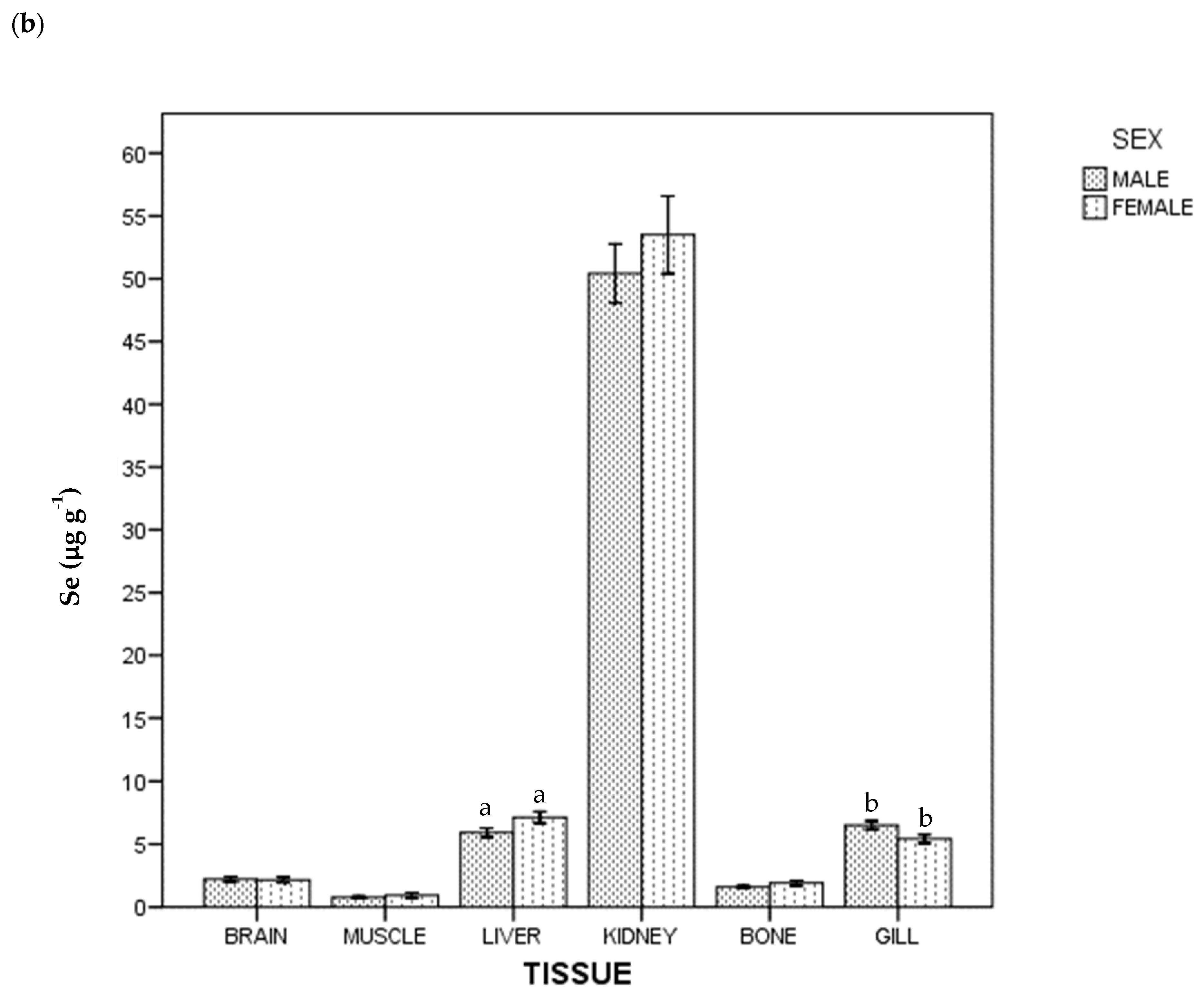
Regarding THg and Se concentration, all the samples were above the instrumental DL. The detected concentrations of THg and Se in tissues in whole population are given in Table 1. In general terms, the highest average concentration was of Se in kidney (51.776 µg g−1), and the lowest was of THg in brain (0.198 µg g−1). Figure 1 shows THg and Se concentrations by tissue and sex: females had a higher THg concentration than males (p < 0.05) in brain, liver, bone and gill; for Se, females had a higher concentration than males in liver, but lower in gill (p < 0.05).

Table 1.
Concentration of THg and Se (µg g−1, ww), Se/THg concentration ratio and HBVSe in tissues of ABFT. Data: mean±standard error, minimum-maximum.
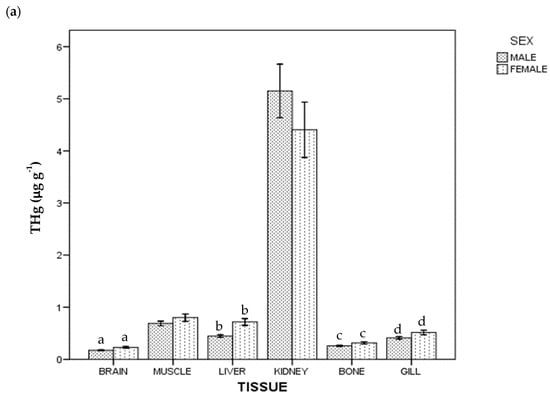
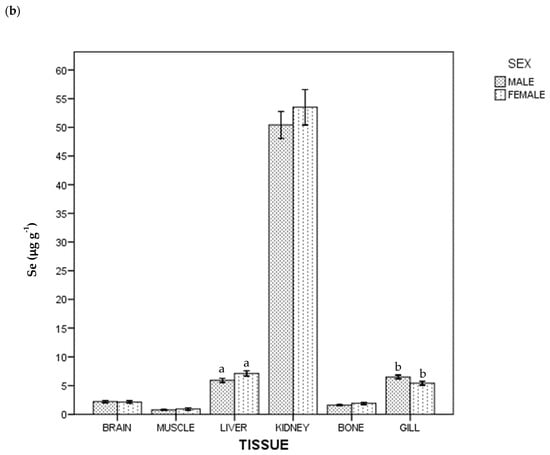
Figure 1.
Total Hg (a) and Se (b) concentrations of ABFT by tissue and sex. For each tissue, the same lowercase letter shows statistical differences between males and females. Bars show standard error. For each tissue, "the same lowercase letter (a, b, c and d in Figure 1a; a and b in Figure 1b) shows statistical differences between males and females”. For example, for liver, “a” is the same lowercase in male and female, so there is statistical differences between them.
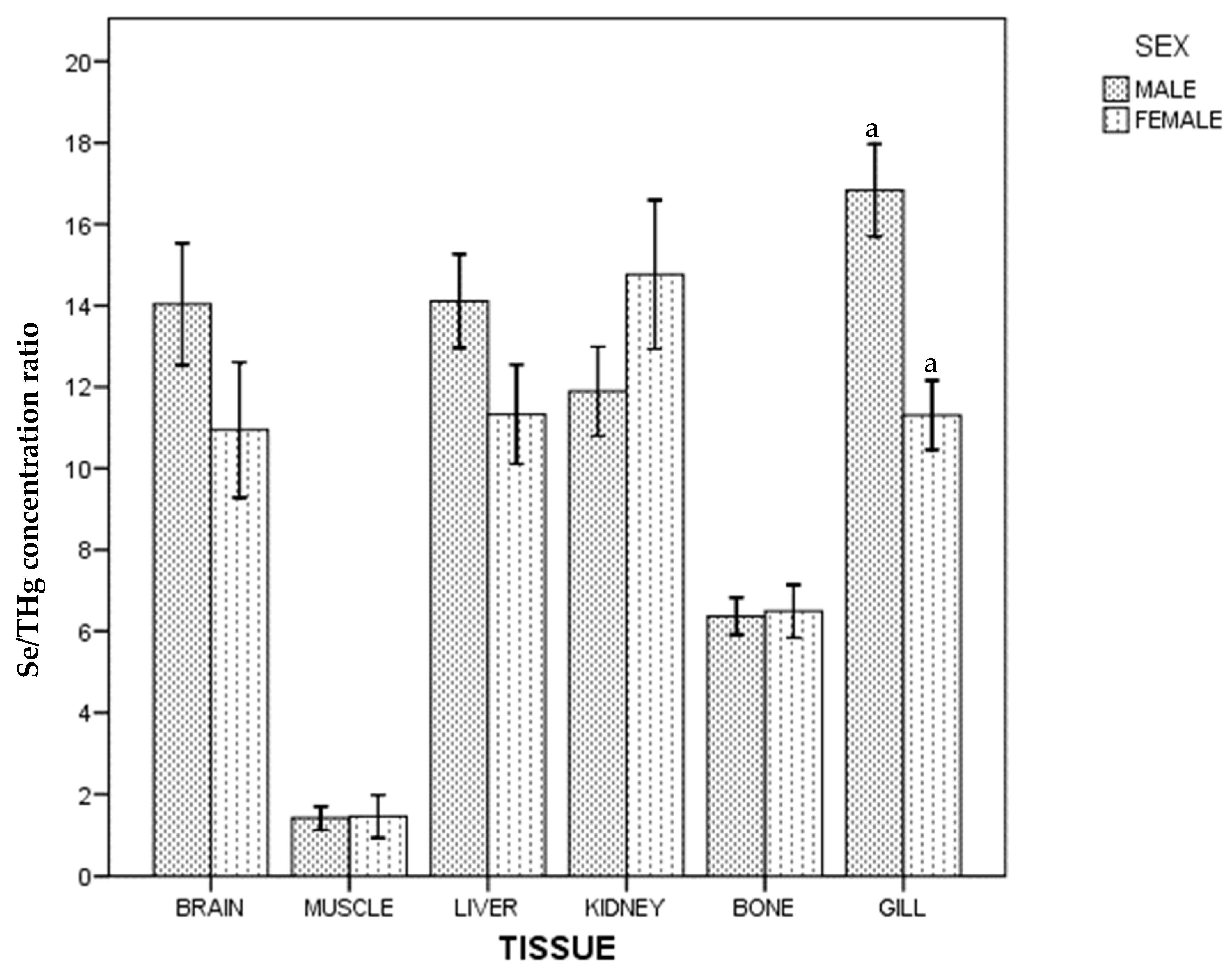
Regarding the whole population, no differences in Se/THg concentration ratio between gill, kidney, liver and brain were found, and these were higher than those found in bone and muscle ((gill~kidney~liver~brain) > bone > muscle). In males, differences between kidney and gill were also found, and no differences between brain and bone in female were found. Differences between sexes are shown in Figure 2.
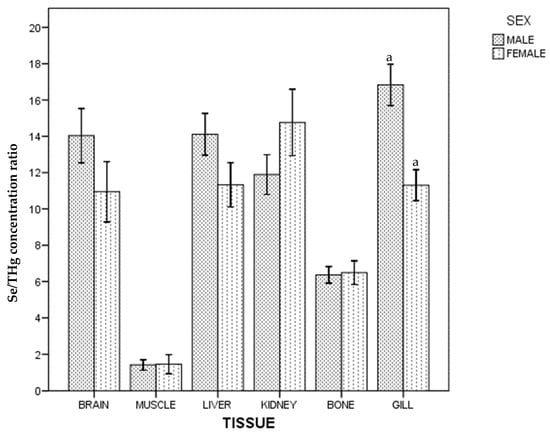
Figure 2.
Se/THg concentration ratio of ABFT by tissue and sex. For each tissue, the same lowercase letter shows statistical differences between males and females. Bars show standard error. For each tissue, "the same lowercase letter (a in Figure 2) shows statistical differences between males and females”. For gill, “a” is the same lowercase in male and female, so there is statistical differences between them.
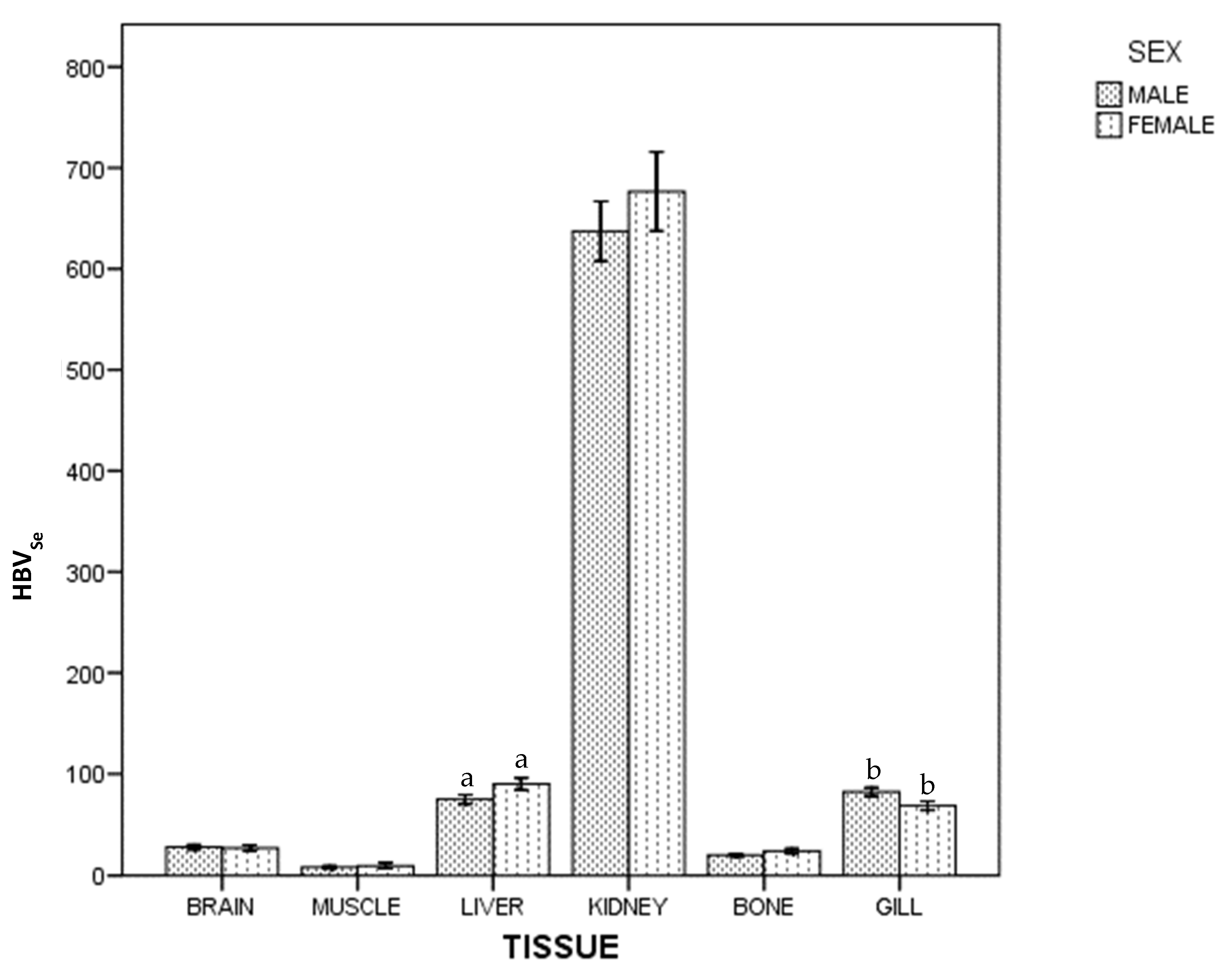
For HBVSe, in whole population and by sex, there were statistical differences between tissues: kidney > (liver~gill) > (brain~bone) > muscle. Figure 3 shows differences between females and males: females had a higher HBVSe than males in liver, but lower in gill (p < 0.05).
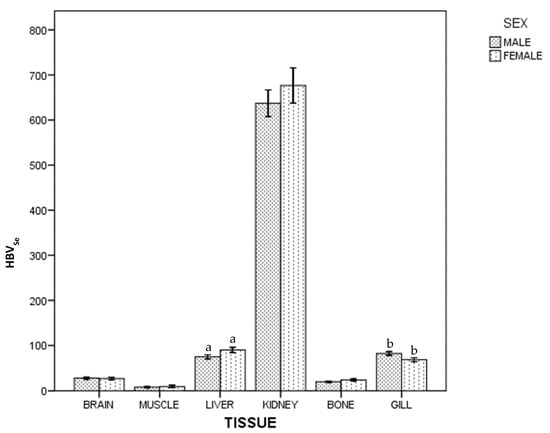
Figure 3.
Selenium Health Benefit Value of ABFT by tissue and sex. For each tissue, the same lowercase letter shows statistical differences between males and females. Bars show standard error. For each tissue, "the same lowercase letter (a and b in Figure 3) shows statistical differences between males and females”. For liver, “a” is the same lowercase in male and female, so there is statistical differences between them.
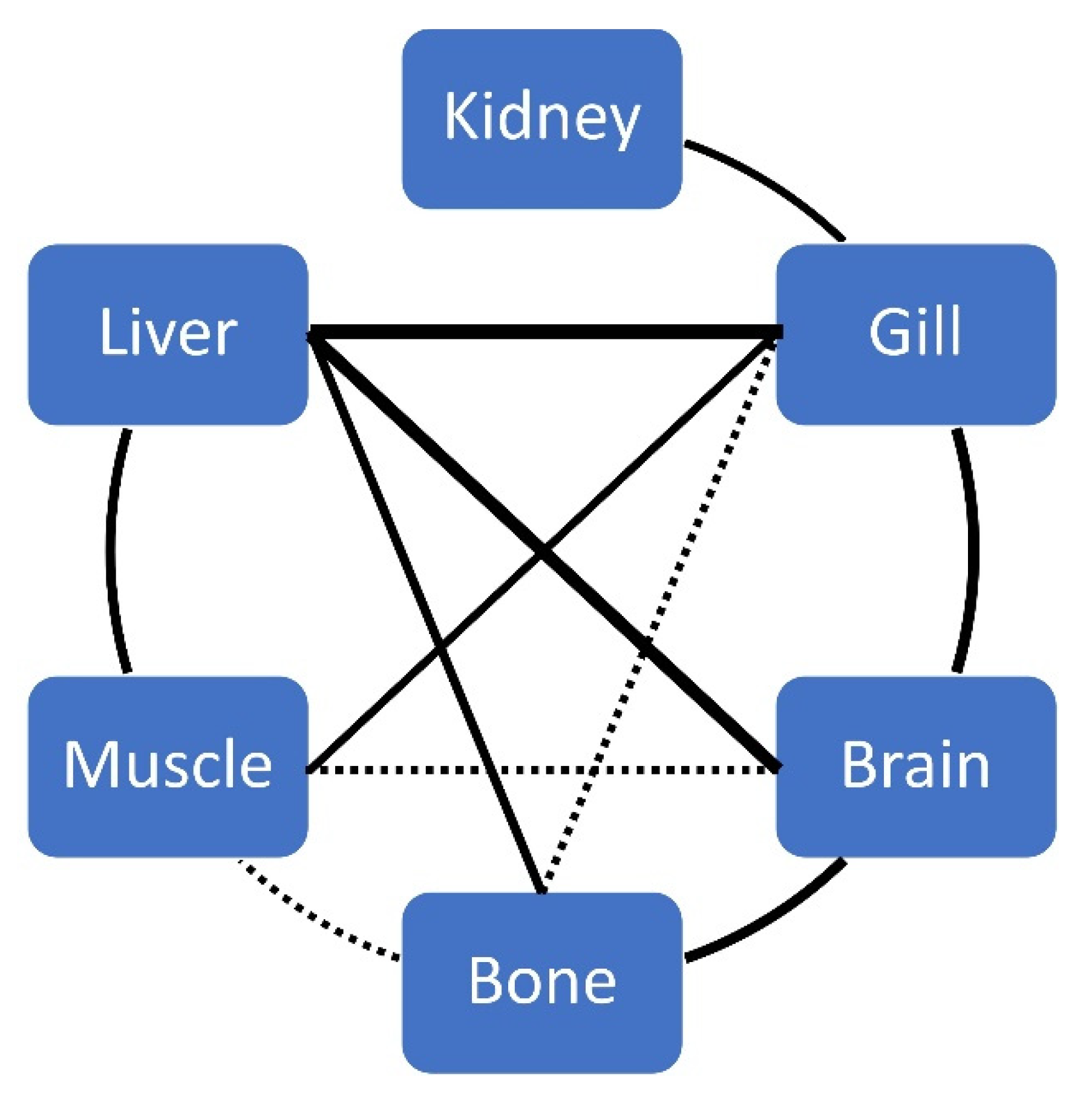
Significant correlations between biometric data, age and elements (by tissue) are given in Table 2. For each element, significant correlations between tissues are given in Table 3 and Table 4, and Figure 4 for Hg.

Table 2.
Statistically significant correlations between biometric data, age and elements concentration in tissues of ABFT; L = liver, K = kidney, Bo = bone, M = muscle, G = gills, Br = brain.

Table 3.
Statistically significant correlations for THg concentration between tissues of ABFT.

Table 4.
Statistically significant correlations for Se concentration between tissues of ABFT.
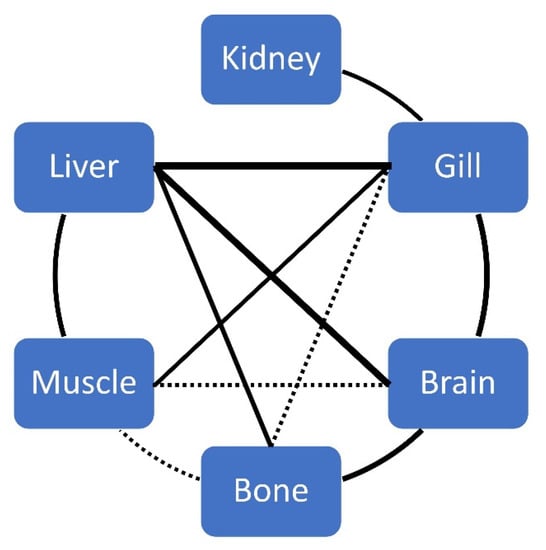
Figure 4.
Correlations between tissues for THg concentration in ABFT. The solid line indicates a p-value < 0.01, and a dashed line indicates a p-value < 0.05. The thickness of the line represents the strength of the relationship.
4. Discussion
Mercury is an important element related with the ABFT, and the scientific literature report concentrations of this heavy metal mainly in muscle (Table 5), by food safety. In fact, several studies report the importance of limiting the consumption of this fish, the need of continuous monitoring of Hg levels in this species and the control of tuna size by Hg accumulation [43,44,45]. However, the kinetic of this element in T. Thynnus specimens from the Mediterranean Sea is scarce (only Hg concentration in liver was reported). In addition, there are few studies regarding Se and Hg relation in tuna [39,42,46,47,48], and to our knowledge, none in T. thynnus. On the other hand, the differences between sex have been scarcely studied [29,48,49]. Thus, better knowledge regarding the distribution, tissue accumulation of THg, and Se concentrations in this species is necessary.

Table 5.
Total mercury and Se concentrations in ABFT from Mediterranean Sea. Data: mean ± standard deviation, µg g−1, ww.
4.1. Kidney
The kidney was the tissue with the highest concentration of Se and THg (Table 1), with a high variability for both elements between specimens. This is a well-perfused tissue, so the renal Hg concentration in fish is often high [53,54]. However, kidney is not usually analysed in tuna species, and only one work has reported this data in T. thynnus in the Arabian Sea [55], being much lower than those found in our study (0.187 vs. 4.821 µg g−1) and in agreement with fish from unpolluted marine water [56].
In other tuna species, renal Hg concentrations were also lower than those found in T. thynnus: 0.216 µg g−1 (Katsuwonus pelamis), 0.260 µg g−1 (Thunnus albacares) and 0.201 µg g−1 (Thunnus obesus) [57]; even in T. albacares and K. pelamis the kidney had a mean concentration of 0.39 to 0.45 and 0.20 µg g−1, respectively [58]. In some works, the renal Hg concentration has been reported greater than muscle, liver and brain, in the same order of magnitude (e.g., Burger et al. [59], regarding Pomatomus saltratix), but kidney of T. thynnus (present study) had up to 25 times more Hg than the concentration reported by the cited authors. In this sense, some factors could explain these results: feeding, binding of Hg–protein and sea pollution. First, farm tuna is fed intensively with fish such as sardine (Sardina pilchardus), round sardinella (Sardinella aurita), scad (Trachurus trachurus), Atlantic herring (Clupea arengus) or mackerel (Scomber spp.), inter alia, and it is known that MeHg is bioaccumulated in aquatic organisms. According to Raihan et al. [60], the kidney had the highest MeHg concentration, followed by liver and gill, in Paralichthys olivaceus fed with a dietary organic Hg; the liver was the tissue in specimens fed with dietary inorganic Hg. On the other hand, an increase in MeHg exposure could accelerate the demethylation in liver [61], binding and immobilizing to metallothionine and other proteins containing sulfhydryl groups [62,63]. The liver’s ability to metabolise or eliminate Hg may determine concentrations in other tissues of the fish [64], and Hg may clear via kidneys as inorganic Hg [65,66]. Metallothionein in fish kidney induced by Hg has been discussed [67], and several studies showed that Hg shifts from low weight thiol proteins to the higher affinity Se containing selenoproteins when there is an increase of dose and time [68], so the target of Hg is Se, not sulphur.
In our study, there was a high Se concentration in kidney (51.776 ± 1.884 µg g−1). In other predator species (Merluccius productus and M. merluccius), some authors have reported the kidney as the tissue with the highest Se levels, although the concentrations of this element were 11-16 times lower than those found in ABFT [69,70]. Furthermore, a moderate to high relationship between both elements in this tissue (r = 0.531, p < 0.01) was found, while this relation was lower in liver (r = 0.395, p < 0.01), and a moderate to low relationship between renal THg and Se concentration and fork length were found (Table 2). In addition, the kidney had the highest Se-Hg molar relation (in form of HBVSe), followed by the liver (Table 1). Thus, a possible speculation of this high renal concentration of Hg may result from intensive diets of farmed tuna, insufficient ability of liver to store high concentrations of this metal, and an effective Se-based renal protection.
4.2. Gills
The concentration of Hg in the tuna gills could be related to the presence of this metal in the Mediterranean Sea, since the studied fish population was caught in this area, and it was kept and fattened in these waters for 4-5 months. Regarding Storelli et al. [32], abiotic factors may affect the metal accumulation in fish, and it is generally believed that the Mediterranean Sea contains high Hg concentrations scattered throughout its basin, with high Hg levels in pelagic and benthic organisms [34,52]. In fact, some authors reported high Hg concentration in gills after water exposure [71], but other authors [72] reported low Hg concentrations in this tissue, indicating that Hg uptake in fish organs is not mainly from water.
In our study, ABFT had a similar THg concentration in gill than liver, and a significant correlation between gill and kidney was found (Table 3, Figure 4), which could add the transport of Hg through the body to our hypothesis, at least in this area (kidney was correlated only with gill). Furthermore, THg concentration in gill of T. thynnus was similar than those reported by Koffi et al. [57] in T. albacares, T. obesus and K. pelamis (0.440, 0.475 and 0.474 µg g−1, respectively) from the Atlantic Ocean, but in the present study gill was the tissue with more Hg concentration; there were also significative relationships between gill and remaining tissues (Table 3, Figure 4).
Finally, Se concentration in gill was high (seven times greater than those reported in the predator species M. productus [69]), with a similar Se/THg concentration ratio to that we found in kidney (14:1 vs. 13:1), but lower HBVSe (Table 1), so we could consider this tissue as a temporal store of Hg.
4.3. Muscle
The muscle is a tissue usually analysed in Mediterranean ABFT (Table 5), and it is considered as one of the main tissues of Hg accumulation. In this sense, some authors report that this tissue has a negligible elimination of Hg and an efficient uptake from other tissues [72]. In fact, there was a positive correlation between muscle and the rest of tissues, except kidney (Table 3, Figure 4). In T. thynnus, muscle had similar Hg concentration to those reported in farmed tuna in several Mediterranean areas, lower than those detected in wild tuna (Table 5), and no significant correlation with biometric measures (Table 2). Several authors have reported positive correlation between Hg muscle concentration and weight and/or length [43,44,48,50,52], but curiously, in these studies fish were wild and farmed tuna with low weight (except than those reported by Milatou et al. [50]). No correlation was reported in tuna of greater weight (Annibaldi et al. [48] in farmed tuna), and a negative relationship was reported by Licata et al. [29] in wild tuna. Some authors have reported an important relation of weight/Hg muscular concentration in tuna above 40 kg [43,44]. According to our results, we agree with Nakao et al. [73], who indicated that THg concentration in full-cycle reared bluefin tuna (T. orientalis) does not correspond to its body weight, being quite different to wild tuna, and associated to the rapidly growing fish (tissue mass increase faster than the pollutant input [74]).
In our study, five specimens had a THg concentration above 1.0 µg g−1 (1.032, 1.055, 1.083, 1.277 and 1.603 µg g−1). In this sense, Se concentration in ocean fish can alleviate Hg-exposure risk [35,75], so it is considered an element of great importance in terms of food safety. Muscular Se concentration was lower than that reported in farm tuna from the Mediterranean Sea with similar weight [48], and higher than that reported by Di Bella et al. [49] in wild T. thynnus (Table 5). In addition, those results were similar or lower than those reported in other tuna species [46,47,76,77,78,79]. In fact, the muscle was the tissue with the lowest concentration of Se, so the distribution observed in our study agreed with that previously reported by Acosta-Lizárraga et al. [69] in other predator species. However, an important concept to consider is the relation of Se/Hg, by the ability of Se to decrease or eliminate the toxic effects of Hg [68,80,81,82], which is relevant in fish muscle. Thus, some works have reported a good proportion between both elements in tuna [39,46,83]. In this sense, the muscle was the tissue with the lowest Se/THg concentration ratio (1.4/1), but the HBVSe was, in each specimen, positive (8.777 ± 1.500, 0.351-44.459), similar to that reported in the same and other tuna species [39,42,46,47,48], or even greater than those reported in small pelagic species [84], and thus safe to consumers.
4.4. Liver
Mercury concentration in liver presented intermediate values among the ones reported in previous studies from the Mediterranean Sea (Table 5). Attending the weight of tuna analysed, our results should be similar to the ones reported by Licata et al. [29] and Srebocan et al. [34], but it is necessary to take into account that there was a negative correlation between tuna weight and THg concentration in the liver (r = -0.326, p < 0.05), so we could assume that the hepatic mass also increases faster than the Hg input. Nevertheless, hepatic THg concentrations were higher than those reported in T. thynnus by Jaffar and Ashraf [55] in the Arabian Sea and in other tuna species (T. albacares, T. obesus and K. pelamis) in other marine areas (Atlantic, Pacific and Indian Ocean) [47,57,58,85]. Total mercury concentration in liver and gill was strongly correlated (r = 0.719, p < 0.01), and non-statistical differences between both tissues were found, so the Hg levels in the Mediterranean basin and a direct transport between gills–liver could explain these data. In addition, Se concentration in liver was also negatively correlated with the tuna weight (r = -0.399, p < 0.01); a positive relationship (p < 0.01) between Se and THg in liver, and no statistical differences in Se concentration between liver and gill were found. Thus, it seems that there was a similar performance pattern between both elements and tissues. In this sense, Tallandini et al. [86] reported similar kinetic characteristics between both tissues for Se. However, no relationship in Se concentration between liver and gill was found, so a transport of Se to the liver from other fish tissues could have happened. On the other hand, liver Se concentrations were lower than those reported in T. albacares and K. pelamis [47,58], agreeing in order with those reported by other authors in other predator species (second tissue with the highest concentration [69]).
The European Union, the world’s second largest trader of fishery and aquaculture products after China [87], promotes the use of fishery and aquaculture side streams. In the present study, the THg accumulation in liver was higher than 0.1 µg g−1 in all specimens, so theoretically, and according to the Commission Regulation (EU) 2019/1869 [88], these data should be taken in consideration if liver is used as a by-product source. Nevertheless, and as was the case with other tissues, the liver Se/THg concentration ratio was higher than 10, and the HBVSe was also high (Table 1), with a minimum of 17.543, so these data should be taken into account in regulations regarding substances in animal feed.
4.5. Brain
Brain had the lowest THg concentration in ABFT (Table 1). Mercury is neurotoxic, its toxicity is widely reported [89], and the risk in humans has been studied [24]. Thus, many studies in human populations are published, including the relationship of Hg with cardiovascular disease, effects in gastrointestinal tract, kidneys, liver, skin, genotoxicity and cancer [90], autism spectrum disorders, and Alzheimer’s Disease [91,92]; however, information regarding its threat to fish nervous system and their mechanisms is still scarce [93]. In fact, there are few studies regarding Hg concentration in the tuna brain, and to our knowledge, none in T. thynnus. Regarding several authors, Hg crosses the blood–brain barrier, reaches the fish brain and produces toxicity [94,95,96]. In other tuna species (T. obesus, T. albacares and K. pelamis), Koffi et al. [57] reported very similar Hg concentrations to those we have found in ABFT (0.162 to 0.186 vs. 0.198 µg g−1), while the Hg concentration found in other species varies between 0.02 (Trichiurus lepturus [97]) and 1.33 µg g−1 (Carcharhinus limbatus [98]). Regarding Burger et al. [59], the Se/Hg relation maybe important in tissue such as kidney, liver and brain. In T. thynnus, no statistical differences between these tissues (plus gill) in Se/THg concentration were found, and these ratio data were higher (p < 0.05) than those found in bone and muscle (Table 1), so we agree with these authors about the possible protection role of Se against Hg levels in important tissues such as the brain.
On the other hand, a positive and significant correlation in THg concentration between brain and the rest of tissue (except kidney) was found (Table 3, Figure 4). Regarding some authors, the MeHg can be demethylated in the brain, and liver and gill also participate in this process, while muscles store MeHg [99,100,101], which could explain these high correlations (Table 3).
4.6. Bone
Bone is reported as the main tissue of the bioaccumulation of metals such as Cu, Zn or Fe [102], mainly by the hydroxyapatite. To the best of our knowledge, no data about Hg concentration in T. thynnus bone have been reported to date, so the results were compared first with dorsal spine in the same species [103]; the THg concentration in bone was higher (0.283 vs. 0.11 µg g−1). In other fish species, Hg concentrations were very low (mean lower than 0.012 µg g−1 [102]), or similar (mean of 0.18 to 0.33 µg g−1 [104]) to those found in ABFT. Mercury and Se were not correlated in bone, but THg concentration in this tissue was correlated with remaining tissues, except kidney, being high with brain (r = 0.509, p < 0.01). Therefore, a priori, one could think that this is a useful tissue for bioaccumulate, but the low THg concentration seems to indicate that this is a temporal place for accumulation prior to elimination by gills or kidney (the gills have been described as the most important route in Hg elimination in marine fish [105], and the kidney was the tissue with highest THg concentration). Regarding Se concentration, no differences between bone and brain were found (neither in HBVSe), but the Se/THg concentration ratio was in the middle of those found in gill, kidney, liver and brain, so further studies could explain the relation between both tissues.
4.7. Mercury and Selenium by Sex
In ABFT, differences between sexes have been reported in few studies. Thus, some authors have found no differences in Hg and Se muscle and liver concentrations between females and males [29,48], even if other authors found differences in Se concentration [49]. In this sense, it is interesting to note that a recent review with several species showed that males had higher Hg concentration in muscle than females in eight studies, while the opposite situation was reported in seven studies, concluding that sex is not the main driver of Hg bioaccumulation [106]. On the other hand, in T. thynnus, a specific pattern in Se and THg (concentration and distribution) between females and males was not found, even if THg concentration in liver was correlated with brain and gill for both groups (Table 3), and Se and THg concentrations were only correlated in kidney, both in males and females (Table 2). In addition, statistical differences in THg concentration (between sexes) in liver, brain and gill, plus bone, were found (Figure 1a), and only in liver and gill for Se concentration (Figure 1b). This is in agreement with that reported by Madenjian et al. [107], regarding that in teleost fishes, males eliminate Hg faster than females. Finally, regarding tissues, it seems that muscle is the tissue that contributes the least to establishing differences between sexes.
5. Conclusions
The results reported in the present study show an important role of Se against Hg in all tissues analysed, similar in brain, liver, kidney and gill. Total mercury concentration in muscle, remaining soft tissues and hard tissues of ABFT contributes to increasing the knowledge regarding pollution, fish health and food risks for human, but the Se/THg concentration ratio and the HBVSe should be taken into account in each tissue, since high Hg concentrations are important in both meat and by-products of the tuna aquaculture industry. A specific pattern in Se and THg (concentration and distribution) between females and males was not found, and it seems that muscle is the tissue that contributes the least to establishing differences between sexes.
Author Contributions
Conceptualization, A.B. and D.R.; methodology, A.B., J.S.-E. and D.R.; validation, P.M. and D.R.; formal analysis, A.B. and D.R.; investigation, A.B., J.S.-E., P.M. and D.R.; resources, A.B. and P.M.; data curation, A.B., J.S.-E.; writing—original draft preparation, A.B.; writing—review and editing, P.M., J.S.-E. and D.R.; visualization, D.R.; supervision, D.R.; project administration, D.R.; funding acquisition, D.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The authors are grateful to Desi, Carlos, David, Ezequiel and Alberto for sampling and technical assistance, and to Francisco San Nicolas for assistance in the analysis of the samples.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A global map of human impact on marine ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef] [PubMed]
- Serrano, O.; Mateo, M.A.; Dueñas-Bohórquez, A.; Renom, P.; López-Sáez, J.A.; Martínez Cortizas, A. The posidonia oceanica marine sedimentary record: A holocene archive of heavy metal pollution. Sci. Total Environ. 2011, 409, 4831–4840. [Google Scholar] [CrossRef]
- Li, H.; Gao, X.; Gu, Y.; Wang, R.; Xie, P.; Liang, M.; Ming, H.; Su, J. Comprehensive large-scale investigation and assessment of trace metal in the coastal sediments of Bohai Sea. Mar. Pollut. Bull. 2018, 129, 126–134. [Google Scholar] [CrossRef]
- Tarnawski, M.; Baran, A. Use of chemical indicators and bioassays in bottom sediment ecological risk assessment. Arch. Environ. Contam. Toxicol. 2018, 74, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Communication from the Commission to the Council and the European Parliament. Community Strategy Concerning Mercury SEC (2005) 10OM/2005/0020 final. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52005DC0020&from=EN (accessed on 28 October 2021).
- ATSDR. ATSDR’s Substance Priority List. Available online: https://www.atsdr.cdc.gov/spl/index.html#2019spl (accessed on 28 October 2021).
- Decision Nº 2455/2001/EC of the European Parliament and of the Council of 20 November 2001 establishing the list of priority substances in the field of water policy and amending Directive 2000/60/EC. Off. J. Eur. Union 2001, L331, 1–5.
- Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off. J. Eur. Union 2001, L226, 1–17.
- U.S.E.P.A. United States Environmental Protection Agency, List of Priority Pollutants. Available online: http://water.epa.gov/scitech/methods/cwa/pollutants.cfm (accessed on 28 October 2021).
- UNEP. Minamata Convention on Mercury. Available online: www.mercuryconvention.org (accessed on 28 October 2021).
- de Almeida Rodrigues, P.; Ferrari, R.G.; Dos Santos, L.N.; Conte Junior, C.A. Mercury in aquatic fauna contamination: A systematic review on its dynamics and potential health risks. J. Environ. Sci. 2019, 84, 205–218. [Google Scholar] [CrossRef]
- Bellanger, M.; Pichery, C.; Aerts, D.; Berglund, M.; Castaño, A.; Cejchanová, M.; Crettaz, P.; Davidson, F.; Esteban, M.; Fischer, M.E.; et al. Economic benefits of methylmercury exposure control in Europe: Monetary value of neurotoxicity prevention. Environ. Health 2013, 12, 3. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Bellanger, M. Calculation of the disease burden associated with environmental chemical exposures: Application of toxicological information in health economic estimation. Environ. Health 2017, 16, 123. [Google Scholar] [CrossRef] [PubMed]
- Cinnirella, S.; Bruno, D.E.; Pirrone, N.; Horvat, M.; Živković, I.; Evers, D.C.; Johnson, S.; Sunderland, E.M. Mercury concentrations in biota in the Mediterranean Sea, a compilation of 40 years of surveys. Sci. Data 2019, 6, 205. [Google Scholar] [CrossRef]
- Ogrinc, N.; Hintelmann, H.; Kotnik, J.; Horvat, M.; Pirrone, N. Sources of mercury in deep-sea sediments of the Mediterranean Sea as revealed by mercury stable isotopes. Sci. Rep. 2019, 9, 11626. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, M.; Renzoni, A. Mercury concentration in Mediterranean marine organisms and their environment: Natural and anthropogenic origin. Thalassia Jugosl. 1977, 13, 265. [Google Scholar]
- Bacci, E. Mercury in the Mediterranean. Mar. Pollut. Bull. 1989, 20, 59–63. [Google Scholar] [CrossRef]
- Lahaye, V.; Bustamante, P.; Dabin, W.; Van Canneyt, O.; Dhermain, F.; Cesarini, C.; Pierce, G.J.; Caurant, F. New insights from age determination on toxic element accumulation in striped and bottlenose dolphins from Atlantic and Mediterranean waters. Mar. Pollut. Bull. 2006, 52, 1219–1230. [Google Scholar] [CrossRef]
- Cossa, D.; Harmelin-Vivien, M.; Mellon-Duval, C.; Loizeau, V.; Averty, B.; Crochet, S.; Chou, L.; Cadiou, J.F. Influences of Bioavailability, Trophic Position, and Growth on Methylmercury in Hakes (Merluccius merluccius) from North-western Mediterranean and North-eastern Atlantic. Environ. Sci. Technol. 2012, 46, 4885–4893. [Google Scholar] [CrossRef]
- Prato, E.; Biandolino, F. The contribution of fish to the Mediterranean diet. In The Mediterranean Diet; Preedy, V.R., Watson, R.S., Eds.; Academic Press: Tucson, AZ, USA, 2015; pp. 165–174. [Google Scholar]
- Schartup, A.T.; Thackray, C.P.; Qureshi, A.; Dassuncao, C.; Gillespie, K.; Hanke, A. Sunderland EM. Climate change and overfishing increase neurotoxicant in marine predators. Nature 2019, 572, 648–650. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA J. 2012, 10, 2985. [Google Scholar]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on health benefits of seafood (fish and shellfish) consumption in relation to health risks associated with exposure to methylmercury. EFSA J. 2014, 12, 3761. [Google Scholar] [CrossRef]
- EFSA Scientific Committee. Statement on the benefits of fish/seafood consumption compared to the risks of methylmercury in fish/seafood. EFSA J. 2015, 13, 3982. [Google Scholar] [CrossRef]
- Commission Regulation (EC) N 629/2008 of 2 July 2008 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2008, L173, 6–9.
- Ha, E.; Basu, N.; Bose-O’Reilly, S.; Dórea, J.G.; McSorley, E.; Sakamoto, M.; Chan, H.M. Current progress on understanding the impact of mercury on human health. Environ. Res. 2017, 152, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Rodríguez, N.; Castro, A.J.; Tweedy, B.N.; Quintas-Soriano, C.; Vaughn, C.C. Mercury consumption and human health: Linking pollution and social risk perception in the southeastern United States. J. Environ. Manage. 2021, 282, 111528. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Wang, S.; Dong, W.; Hua, X.; Li, Y.; Song, X.; Chu, Q.; Hou, S.; Li, Y. The Toxicological Effects of Mercury Exposure in Marine Fish. Bull. Environ. Contam. Toxicol. 2019, 102, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Licata, P.; Trombetta, D.; Cristani, M.; Naccari, C.; Martino, D.; Calò, M.; Naccari, F. Heavy metals in liver and muscle of bluefin tuna (Thunnus thynnus) caught in the Straits of Messina (Sicily, Italy). Environ. Monit. Assess. 2005, 107, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Garrido Gamarro, E.; Orawattanamateekul, W.; Sentina, J.; Srinivasa Gopal, T.K. By-Products of Tuna Processing. In Globefish Research Programme; FAO: Rome, Italy, 2013; Volume 112, pp. 1–48. [Google Scholar]
- Hosch, G. Design options for the development of tuna catch documentation schemes. In FAO Fisheries and Aquaculture Technical Paper; FAO: Rome, Italy, 2016; Volume 596, pp. 1–144. [Google Scholar]
- Storelli, M.M.; Giacominelli-Stuffler, R.; Storelli, A.; Marcotrigiano, G.O. Accumulation of mercury, cadmium, lead and arsenic in swordfish and bluefin tuna from the Mediterranean Sea: A comparative study. Mar. Pollut. Bull. 2005, 50, 1004–1007. [Google Scholar] [CrossRef]
- Hernández-Hernández, F.; Medina, J.; Nsuategui, J.; Conesa, M. Heavy metal concentrations in some marine organisms from the Mediterranean Sea (Castellón, Spain): Metal accumulation in different tissues. Sci. Mar. 1990, 54, 113–129. [Google Scholar]
- Srebocan, E.; Pompe-Gotal, J.; Prevendar-Crnic, A.; Ofner, E. Mercury concentrations in captive Atlantic bluefin tuna (Thunnus thynnus) farmed in the Adriatic Sea. Vet. Med. 2007, 52, 175–177. [Google Scholar] [CrossRef]
- Ralston, N.V.C.; Kaneko, J.J.; Raymond, L.J. Selenium health benefit values provide a reliable index of seafood benefits vs. risks. J. Trace Elem. Med. Biol. 2019, 55, 50–57. [Google Scholar] [CrossRef]
- Khan, K.U.; Zuberi, A.; Fernandes, J.B.K.; Ullah, I.; Sarwar, H. An overview of the ongoing insights in selenium research and its role in fish nutrition and fish health. Fish Physiol. Biochem. 2017, 43, 1689–1705. [Google Scholar] [CrossRef]
- Sarangi, G.K.; Romagné, F.; Castellano, S. Distinct patterns of selection in selenium-dependent genes between land and aquatic vertebrates. Mol. Biol. Evol. 2018, 35, 1744–1756. [Google Scholar] [CrossRef]
- Olmedo, P.; Hernández, A.F.; Pla, A.; Femia, P.; Navas-Acien, A.; Gil, F. Determination of essential elements (copper, manganese, selenium and zinc) in fish and shellfish samples. Risk and nutritional assessment and mercury–selenium balance. Food Chem. Toxicol. 2013, 62, 299–307. [Google Scholar]
- Ralston, N.V.C.; Ralston, C.R.; Raymond, L.J. Selenium health benefit values: Updated criteria for mercury risk assessments. Biol. Trace Elem. Res. 2016, 171, 262–269. [Google Scholar] [CrossRef]
- Cort, J.L. Age and growth of the bluefin tuna, Thunnus thynnus (L.) of the Northwest Atlantic. ICCAT Coll. Vol. Sci. Pap. 1991, 35, 213–230. [Google Scholar]
- Costley, C.T.; Mossop, K.F.; Dean, J.R.; Garden, L.M.; Marshall, J.; Carrol, J. Determination of mercury in environmental and biological samples using pyrolysis atomic absorption spectrometry with gold amalgamation. Anal. Chim. Acta 2000, 405, 179–183. [Google Scholar] [CrossRef]
- Melgar, M.J.; Núñez, R.; García, M.A. Selenium intake from tuna in Galicia (Spain): Health risk assessment and protective role against exposure to mercury and inorganic arsenic. Sci. Total Environ. 2019, 694, 133716. [Google Scholar] [CrossRef]
- Storelli, M.M.; Marcotrigiano, G.O. Total mercury levels in muscle tissue of swordfish (Xiphias gladius) and bluefin tuna (Thunnus thynnus) from the Mediterranean Sea (Italy). J. Food Prot. 2001, 64, 1058–1061. [Google Scholar] [CrossRef]
- Storelli, M.M.; Giacominelli Stuffler, R.; Marcotrigiano, G.O. Total and methylmercury residues in tuna-fish from the Mediterranean Sea. Food Addit. Contam. 2002, 19, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Özden, Ö.; Erkan, N.; Kaplan, M.; Karakulak, F.S. Toxic metals and omega 3 fatty acids of bluefin tuna from aquaculture: Health risk and benefits. Expos. Health 2018, 12, 9–18. [Google Scholar] [CrossRef]
- Kaneko, J.J.; Ralston, N.V.C. Selenium and mercury in pelagic fish in the central north Pacific near Hawaii. Biol. Trace Elem. Res. 2007, 119, 242–254. [Google Scholar] [CrossRef]
- Ruelas-Inzunza, J.; Šlejkovec, Z.; Mazej, D.; Fajon, V.; Horvat, M.; Ramos-Osuna, M. Bioaccumulation of As, Hg, and Se in tunas Thunnus albacares and Katsuwonus pelamis from the Eastern Pacific: Tissue distribution and As speciation. Environ. Sci. Pollut. Res. 2018, 25, 19499–19509. [Google Scholar] [CrossRef]
- Annibaldi, A.; Truzzi, C.; Carnevali, O.; Pignalosa, P.; Api, M.; Scarponi, G.; Illuminati, S. Determination of Hg in farmed and wild Atlantic bluefin tuna (Thunnus thynnus L.) muscle. Molecules 2019, 24, 1273. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, G.; Potortì, A.G.; Lo Turco, V.; Bua, D.; Licata, P.; Cicero, N.; Dugo, G. Trace Elements in Thunnus thynnus from Mediterranean Sea and benefit–risk assessment for consumers. Food Addit. Contam. Part B Surveill. 2015, 8, 175–181. [Google Scholar] [CrossRef]
- Milatou, N.; Dassenakis, M.; Megalofonou, P. Do fattening process and biological parameters affect the accumulation of metals in Atlantic Bluefin Tuna? Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Storelli, M.M.; Barone, G.; Cuttone, G.; Giungato, D.; Garofalo, R. Occurrence of toxic metals (Hg, Cd and Pb) in fresh and canned tuna: Public health implications. Food Chem. Toxicol. 2010, 48, 3167–3170. [Google Scholar] [CrossRef]
- Renzi, M.; Cau, A.; Bianchi, N.; Focardi, S.E. Levels of mercury and polychlorobiphenyls in bluefin tuna from the Western Mediterranean Sea: A food safety issue? J. Environ. Prot. 2014, 5, 106–113. [Google Scholar] [CrossRef]
- Berntssen, M.; Hylland, K.; Julshamn, K.; Lundebye, A.K.; Waagbø, R. Maximum limits of organic and inorganic mercury in fish feed. Aquac. Nutr. 2004, 10, 83–97. [Google Scholar] [CrossRef]
- Sandheinrich, M.; Wiener, J. Methylmercury in freshwater fish: Recent advances in assessing toxicity of environmentally relevant exposures. Environ. Contam. Biota Interpret. Tissue Conc. 2011, 2, 169–190. [Google Scholar]
- Jaffar, M.; Ashraf, M. Selected trace metal concentrations in different tissues of fish from coastal waters of Pakistan (Arabian Sea). Indian, J. Mar. Sci. 1988, 17, 231–234. [Google Scholar]
- Martin, J.H.; Knauer, G.A. The elemental composition of plankton. Geochim. Cosmochim. Acta 1973, 37, 1639–1653. [Google Scholar] [CrossRef]
- Koffi, K.M.; Saki, S.J.; Kouadio, A.I.; Atse, B.C.; Biego, G.H.M. Accumulation of cadmium, lead, and mercury in different organs of three tuna fish species from coastal zone of Cote d’Ivoire. Int. J. Agric. Innov. Res. 2014, 3, 1473–2319. [Google Scholar]
- Kojadinovic, J.; Potier, M.; Le Corre, M.; Cosson, R.P.; Bustamante, P. Bioaccumulation of trace elements in pelagic fish from the Western Indian Ocean. Environ. Pollut. 2007, 146, 548–566. [Google Scholar] [CrossRef]
- Burger, J.; Jeitner, C.; Donio, M.; Pittfield, T.; Gochfeld, M. Mercury and selenium levels, and selenium:mercury molar ratios of brain, muscle and other tissues in bluefish (Pomatomus saltatrix) from New Jersey, USA. Sci. Total Environ. 2013, 15, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Raihan, S.M.; Moniruzzaman, M.; Park, Y.; Lee, S.; Bai, S.C. Evaluation of dietary organic and inorganic mercury threshold levels on induced mercury toxicity in a marine fish model. Animals 2020, 10, 405. [Google Scholar] [CrossRef]
- Cizdziel, J.; Hinners, T.; Cross, C.; Pollard, J. Distribution of mercury in the tissues of five species of freshwater fish from Lake Mead, U.S.A. J. Environ. Monit. 2003, 5, 802–807. [Google Scholar] [CrossRef]
- Hogstrand, C.; Haux, C. A radioimmunoassay for perch (Perca fluviatilis) metalothionein. Toxicol. Appl. Pharmacol. 1990, 102, 56. [Google Scholar] [CrossRef]
- Ajsuvakova, O.P.; Alexey, A.; Tinkov, M.; Aschner, J.; Rocha, B.T.; Michalke, B.; Skalnaya, M.G.; Skalny, A.N.; Butnariu, M.; Dadar, M.; et al. Sulfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef] [PubMed]
- Bradley, M.A.; Barst, B.D.; Basu, N. A review of mercury bioavailability in humans and fish. Int. J. Environ. Res. Public Health 2017, 14, 169. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.C.; Allen, P.V.; Turner, M.D.; Most, B.; Fisher, H.L.; Hall, L.L. The Kinetics of Intravenously Administered Methyl Mercury in Man. Toxicol. Appl. Pharmacol. 1994, 128, 251–256. [Google Scholar] [CrossRef]
- Smith, J.C.; Farris, F.F. Methyl mercury pharmacokinetics in man: A reevaluation. Toxicol. Appl. Pharmacol. 1996, 137, 245–252. [Google Scholar] [CrossRef]
- Mieiro, C.L.; Bervoets, L.; Joosen, S.; Blust, R.; Duarte, A.C.; Pereira, M.E.; Pacheco, M. Metallothioneins failed to reflect mercury external levels of exposure and bioaccumulation in marine fish–Considerations on tissue and species specific responses. Chemosphere 2011, 85, 114–121. [Google Scholar] [CrossRef]
- Spiller, H.A. Rethinking mercury: The role of selenium in the pathophysiology of mercury toxicity. Clin. Toxicol. 2017, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Lizárraga, L.G.; Bergés-Tiznado, M.E.; Bojórquez-Sánchez, C.; Osuna-Martínez, C.C.; Páez-Osuna, F. Bioaccumulation of mercury and selenium in tissues of the mesopelagic fish Pacific hake (Merluccius productus) from the northern Gulf of California and the risk assessment on human health. Chemosphere 2020, 255, 126941. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Eira, C.; Miquel, J.; Ferrer-Maza, D.; Delgado, E.; Casadevall, M. Effect of intestinal tapeworm Clestovothrium crassiceps on concentrations of toxic elements and selenium in European hake Merluccius merluccius from the Gulf of lion (northwestern Mediterranean Sea). J. Agric. Food Chem. 2015, 63, 9349–9356. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, D.A.; Rantin, F.T.; Kalinin, A.L. Inorganic mercury exposure: Toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxã, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology 2010, 19, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhao, L.; Wang, Y.; Xie, Q.; Yin, D.; Feng, X.; Wang, D. Bioaccumulation characteristics of mercury in fish in the Three Gorges Reservoir, China. Environ. Pollut. 2018, 243, 115–126. [Google Scholar] [CrossRef]
- Nakao, M.; Seoka, M.; Tsukamasa, Y.; Kawasaki, K.I.; Ando, M. Possibility for decreasing of mercury content in bluefin tuna Thunnus orientalis by fish culture. Fish Sci. 2007, 73, 724–731. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M.; Shukla, T.; Jeitner, C.; Burke, S.; Donio, M.; Shukla, S.; Snigaroff, R.; Snigaroff, D.; Stamm, T.; et al. Heavy metals in Pacific cod (Gadus macrocephalus) from the Aleutians: Location, age, size, and risk. J. Toxicol. Environ. Health 2007, 70, 1897–1911. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.X. Selenium induces the demethylation of mercury in marine fish. Environ. Pollut. 2017, 231, 1543–1551. [Google Scholar] [PubMed]
- Burger, J.; Gochfeld, M. Heavy metals in commercial fish in New Jersey. Environ. Res. 2005, 99, 403–412. [Google Scholar] [CrossRef]
- Yamashita, Y.; Amlund, H.; Suzuki, T.; Hara, T.; Hossain, A.M.; Yabu, T.; Touhata, K.; Yamashita, M. Selenoneine, total selenium, and total mercury content in the muscle of fishes. Fish. Sci. 2011, 77, 679–686. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yamashita, M.; Lida, H. Selenium content in seafood in Japan. Nutrients 2013, 5, 388–395. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M. Selenium and mercury molar ratios in commercial fish from New Jersey and Illinois: Variation within species and relevance to risk communication. Food Chem. Toxicol. 2013, 57, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, B.; Aaseth, J.; Ajsuvakovad, O.P.; Nikonorove, A.A.; Skalny, A.V.; Skalnay, M.G.; Tinkov, A.A. Molecular interaction between mercury and selenium in neurotoxicity. Coord. Chem. Rev. 2017, 332, 30–37. [Google Scholar] [CrossRef]
- Ralston, N.V.C. Effects of soft electrophiles on selenium physiology. Free Radic. Biol. Med. 2018, 127, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ralston, N.V.C.; Raymond, L.J. Mercury’s neurotoxicity is characterized by its disruption of selenium biochemistry. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2405–2416. [Google Scholar] [CrossRef]
- Ordiano-Flores, A.; Rosíles-Martínez, R.; Galván-Magaña, F. Biomagnification of mercury and its antagonistic interaction with selenium in yellowfin tuna Thunnus albacares in the trophic web of Baja California Sur, Mexico. Ecotoxicol. Environ. Saf. 2012, 86, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Grgec, A.S.; Kljaković-Gašpić, Z.; Orct, T.; Tičina, V.; Sekovanić, A.; Jurasović, J.; Piasek, M. Mercury and selenium in fish from the eastern part of the Adriatic Sea: A risk-benefit assessment in vulnerable population groups. Chemosphere 2020, 261, 127742. [Google Scholar] [CrossRef]
- Torres, P.; Rodrigues, A.; Soares, L.; Garcia, P. Metal concentrations in two commercial tuna species from an active volcanic region in the Mid-Atlantic Ocean. Arch. Environ. Contam. Toxicol. 2016, 70, 341–347. [Google Scholar] [CrossRef]
- Tallandini, L.; Cecchi, R.; De Boni, S.; Galassini, S.; Ghermandi, G.; Gialanella, G.; Liu, N.; Moro, R.; Turchetto, M.; Zhang, Y. Toxic levels of selenium in enzymes and selenium uptake in tissues of a marine fish. Biol. Trace Elem. Res. 1996, 51, 97–106. [Google Scholar] [CrossRef]
- Anonymous. The EU Trade Deficit for Fisheries and Aquaculture Products Continues to Rise. 2020. Available online: https://www.eumofa.eu/es/the-eu-fish-market-2020-edition-is-now-online (accessed on 28 October 2021).
- Commission Regulation (EU) 2019/1869 of 7 November 2019 amending and correcting Annex I to Directive 2002/32/EC of the European Parliament and of the Council as regards maximum levels for certain undesirable substances in animal feed. Off. J. Eur. Union 2019, L289, 32–36.
- Yang, L.; Zhang, Y.; Wang, F.; Luo, Z.; Guo, S.; Strähle, U. Toxicity of mercury: Molecular evidence. Chemosphere 2020, 245, 125586. [Google Scholar] [CrossRef] [PubMed]
- Risher, J.F. Elemental Mercury and Inorganic Mercury Compounds: Human Health Aspects. Concise International Chemical Assessment Document 50; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Kern, J.K.; Geier, D.A.; Sykes, L.K.; Haley, B.E.; Geier, M.R. The relationship between mercury and autism: A comprehensive review and discussion. J. Trace Elem. Med. Biol. 2016, 37, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Siblerud, R.; Mutter, J.; Moore, E.; Naumann, J.; Walach, H. A hypothesis and evidence that mercury may be an etiological factor in Alzheimer’s disease. Int. J. Environ. Res. Public Health 2019, 16, 5152. [Google Scholar] [CrossRef]
- Morcillo, P.; Esteban, M.A.; Cuesta, A. Mercury and its toxic effects on fish. AIMS Environ. Sci. 2017, 4, 386–402. [Google Scholar] [CrossRef]
- Mieiro, C.L.; Pereira, M.E.; Duarte, A.C.; Pacheco, M. Brain as a critical target of mercury in environmentally exposed fish (Dicentrarchus labrax)-Bioaccumulation and oxidative stress profiles. Aquat. Toxicol. 2011, 103, 233–240. [Google Scholar] [CrossRef]
- Farina, M.; Avila, D.S.; Da Rocha, J.B.T.; Aschner, M. Metals, oxidative stress and neurodegeneration: A focus on iron, manganese and mercury. Neurochem. Int. 2013, 62, 575–594. [Google Scholar] [CrossRef]
- Pereira, P.; Puga, S.; Cardoso, V.; Pinto-Ribeiro, F.; Raimundo, J.; Barata, M.; Pousão-Ferreira, P.; Pacheco, M.; Almeida, A. Inorganic mercury accumulation in brain following waterborne exposure elicits a deficit on the number of brain cells and impairs swimming behavior in fish (white seabream—Diplodus sargus). Aquat. Toxicol. 2016, 170, 400–412. [Google Scholar] [CrossRef]
- Cardoso, T.P.; Mársico, E.T.; Medeiros, R.J.; Tortelly, R.; Sobreiro, L.G. Mercury level and histopathologic analysis of muscle, kidney and brain of largehead hairtail (Trichiurus lepturus) collected in Itaipu beach-Niterói, Rio de Janeiro, Brazil. Cienc. Rural 2009, 39, 540–546. [Google Scholar] [CrossRef]
- Núnez-Nogueira, G. Concentration of essential and non-essential metals in two shark species commonly caught in Mexican (Gulf of Mexico) coastline. In Contaminación e Impacto Ambiental: Diagnóstico y Tendencias, 2nd ed.; Botello, A.V., Rendón-von Osten, J., Gold-Bouchot, G., Agraz-Hernandéz, C., Eds.; Instituto Nacional de Ecología Univ. Autón. de Campeche, Univ.: Campeche, México; Nal. Autón. de México: Tlacopac, Mexico, 2005; Volume 26, pp. 451–474. [Google Scholar]
- Vahter, M.E.; Mottet, N.K.; Friberg, L.T.; Lind, S.B.; Charleston, J.S.; Burbacher, T.M. Demethylation of methyl mercury in different brain sites of Macaca fascicularis monkeys during long-term subclinical methyl mercury exposure. Toxicol. Appl. Pharmacol. 1995, 134, 273–284. [Google Scholar] [CrossRef]
- Allen, J.W.; Shanker, G.; Tan, K.H.; Aschner, M. The consequences of methylmercury exposure on interactive functions between astrocytes and neurons. Neurotoxicology 2002, 23, 755–759. [Google Scholar] [CrossRef]
- Wang, X.; Wu, F.; Wang, W.X. In vivo mercury demethylation in a marine fish (Acanthopagrus schlegeli). Environ. Sci. Technol. 2017, 51, 6441–6451. [Google Scholar] [CrossRef] [PubMed]
- Shalini, R.; Jeyasekaran, G.; Shakila, R.J.; Arisekar, U. Trace element concentrations in the organs of fish along the southeast coast of India. Mar. Pollut. Bull. 2021, 162, 111817. [Google Scholar] [CrossRef]
- Ugarte, A.; Abrego, Z.; Unceta, N.; Goicolea, M.A.; Barrio, R.J. Evaluation of the bioaccumulation of trace elements in tuna species by correlation analysis between their concentrations in muscle and first dorsal spine using microwave-assisted digestion and ICP-MS. Intern. J. Environ. Anal. Chem. 2012, 92, 1761–1775. [Google Scholar] [CrossRef]
- Perugini, M.; Visciano, P.; Manera, M.; Zaccaroni, A.; Vincenzo, O.; Amorena, M. Heavy metal (As, Cd, Hg, Pb, Cu, Zn, Se) concentrations in muscle and bone of four commercial fish caught in the central Adriatic Sea, Italy. Environ. Monit. Assess. 2014, 186, 2205–2213. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.X. Physiologically based pharmacokinetic model for inorganic and methylmercury in a marine fish. Environ. Sci. Technol. 2015, 49, 10173–10181. [Google Scholar] [CrossRef] [PubMed]
- Bastos, W.R.; Dórea, J.G.; Bernardi, J.V.E.; Manzatto, A.G.; Mussy, M.H.; Lauthartte, L.C.; Lacerda, L.D.; Malm, O. Sex-related mercury bioaccumulation in fish from the Madeira River, Amazon. Environ. Res. 2016, 144, 73–80. [Google Scholar] [CrossRef]
- Madenjian, C.P.; Chipps, S.R.; Blanchfield, P.J. Time to refine mercury mass balance models for fish. FACETS 2021, 6, 272–286. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).