Abstract
In the field of rare diseases (RDs), the evidence standard is often lower than that required by health technology assessment (HTA) and payer authorities. In this commentary, we propose that appropriate economic evaluation for rare disease treatments should be initially informed by cost-of-illness (COI) studies conducted using a societal perspective. Such an approach contributes to improving countries’ understanding of RDs in their entirety as societal and not merely clinical, or product-specific issues. In order to exemplify how the disease burden’s distribution has changed over the last fifteen years, key COI studies for Hemophilia, Fragile X Syndrome, Cystic Fibrosis, and Juvenile Idiopathic Arthritis are examined. Evidence shows that, besides methodological variability and cross-country differences, the disease burden’s share represented by direct costs generally grows over time as novel treatments become available. Hence, to support effective decision-making processes, it seems necessary to assess the re-allocation of the burden produced by new medicinal products, and this approach requires identifying cost drivers through COI studies with robust design and standardized methodology.
1. Introduction
In recent years, the peculiarities of evaluations and decisions surrounding the pricing and reimbursement of therapies for rare diseases (RDs) have been in the spotlight of academic debate and discussions among clinicians, health economists, and policymakers. The quality of scientific evidence is often lower than that required or expected by regulatory, Health Technology Assessment (HTA) bodies and payer authorities. In the case of RDs, indeed, it might be impossible to conduct large randomized clinical trials, and economic evaluation techniques may be unable to adequately reflect preferences of patients and society [1]. At the same time, treatments (very often orphan medicinal product, OMPs) for RDs are in most cases offered at a price deemed high by national authorities (payers and providers), especially if compared to the apparently modest clinical benefit characterizing a substantial number of OMPs. In other words, by using the standard set of methods and decisional approaches, RD treatments (RDTs) are likely to appear as low value for money. Consequently, many countries have adopted integrative policies to mitigate the influence of strictly evidence-based decision-making approaches (i.e., standard HTA approaches). A recent review focused on 12 European countries and specifically on RDTs, reported that the most common policies include the creation of national plans, and setting-up disease or drug-based registries [2]. In addition, the national healthcare payers have been increasingly looking into innovative reimbursement approaches and applying value-based pricing. In this regard, the use of Managed Entry Agreements (MEA) is recognized in order to manage the clinical and economic uncertainties [3]. These interventions favor early diagnosis through newborn screening, enable early patient access to new OMPs, particularly those targeting an unmet medical need or addressing a major public health interest (e.g., compassionate use and new regulatory pathways like PRIME (PRIority MEdiciness) scheme or Adaptive pathway) and following dedicated HTA approaches. All these policies are likely to facilitate access to new therapies for RDs. However, they exogenously overcome the perception of “poor value for money” without providing credible reasons for why such therapies could also prove to be a reasonable investment from a health-economic standpoint. As a matter of fact, besides the arguments reported by Simoens [4], one of the main challenges for economic evaluation of therapies for RDs is the little number of active comparators, that is, most of these therapies are launched without any previous treatment indicated for the same condition. To this is added the use of surrogate endpoints not yet validated or currently being validated, which complicates the correlation of a possibly statistically significant endpoint, but not a clinically relevant outcome. In this perspective, economic evaluation would record in many cases a pure increase in the cost of therapies.
In this commentary paper, we would like to show how Cost of Illness (COI) studies (also known as Burden of Disease) conducted using a societal perspective can effectively inform appropriate pharmacoeconomic analysis for OMPs or RDTs. This approach is able to show the distribution of the disease burden (i.e., the consequence of health problems on a given population in terms of mortality, morbidity, quality of life, or financial costs, etc.) over the different actors impacted by the condition, i.e., the patient, her/his caregiver(s), the healthcare sector, pharmaceutical industry, other public institutions providing financial or organizational support, employers, etc.
COI should represent the status quo for budget impact analysis, extended to society perspective when new treatments are launched in the market. Specifically, this type of study shows a) how much the overall financial and economic burden is impacted by the new treatment and, b) to what extent does the new treatment reallocate that burden over the different actors (e.g., displacing it from patients and caregivers to the healthcare system).
Three arguments sustain our observation. First, when no indicated treatment is available, it is likely that the direct healthcare costs are low and, therefore, the launch of a new (and typically high-priced) medicinal-product (MP) heavily impacts the financial engagement of healthcare payers on each patient. In this light, evaluations conducted using the payer’s perspective will be easily biased towards the high delta cost, while the consideration of the overall burden would help to evaluate the extent of a shift in the burden from patients and other societal actors (e.g., social security systems, families or employers, etc.) to payers. Second, consideration of the overall burden of disease allows HTA agencies and payers to identify the real economic impact of a new high-priced MP on the healthcare system (thus anticipating the information of a budget impact analysis (BIA), but from the broader societal perspective), which in the case of RDs is generally modest [5]. Third, such an approach contributes to countries’ understanding of RDs in their entirety as societal and not merely clinical or product-specific issues, thus reframing the concept of value for money as a reduction of the overall burden of disease, irrespective of what actor bears the greatest share. In the examples provided below, even if not generated as systematic evaluations by policy-makers, the evolution of the burden’s allocation over time (when ad hoc new treatments become available) can clearly be seen.
2. Methods
In order to exemplify how the burden’s distribution has changed over the last fifteen years, we identified four examples of RDs to support our observation: Hemophilia, Fragile X syndrome, Cystic Fibrosis, and Juvenile Idiopathic Arthritis, representing a broad spectrum of RDs. In selecting these diseases, we take advantage of previous studies carried out by the Social Economic Burden and Health-Related Quality of Life in Patients with Rare Diseases in Europe Project—BURQOL-RD. Therefore, we applied the BURQOL project’s same criteria (genetic origin, age at onset during adulthood or childhood, physical impairment and/or mental impairment, availability of effective therapies) in identifying effective examples of RDs for our analysis [6]. Hence, key articles focused on the socioeconomic evaluation of RDs, in particular applying a societal perspective, were selected. Figure 1 shows the structure of a COI study and how this is not a comparative approach, as Cost Effective Analysis (CEA) and Cost Utility Analysis (CUA) are, and it adopts a societal perspective, as CEA and CUA usually do not. In turn, this wide perspective of society allows us to effectively identify which actors hold in the greater extent of the disease burden, but it also requires a wider range of data than CEA and CUA.
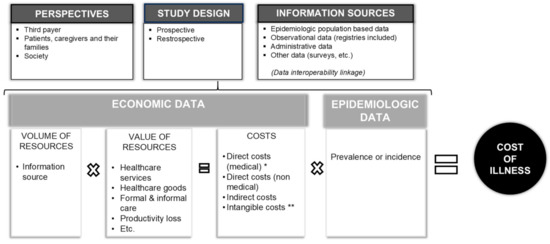
Figure 1.
The key factors in determining the cost of illness. * For drugs: Eventual managed entry agreements (label use) or early access use. ** For correctness, let us also mention intangible costs, even if we did not mention them in this commentary because they are not easily estimated and used in the scientific literature.
Even if our goal is not a systematic review of the literature or a meta-analysis, we considered those items of the PRISMA checklist concerning data items and their source, collection, measurement, and processing in all studies published during the period 2004–2019 focused on socioeconomic evaluation of RD examples, applying a societal perspective of cost of illness methods [7]. Additional inclusion criteria were used for study selection concerning: (i) Detailed description of the data source; (ii) reported data collection procedures; (iii) use of appropriate measures of costs; (iv) methods for cost data processing described in adequate detail; (v) provided estimates of indirect or direct costs for each disease. Lastly, we excluded reviews of existing economic studies relating to specific diseases and studies with partial estimation of costs.
The research was conducted searching the PubMed database and using the following terms: (i) Economic burden and (ii) costs in combination with the names of the four selected RDs.
Specifically, items considered for description of each study were:
- name of RD;
- publication year;
- country;
- study population (target and size);
- perspective (i.e., societal, third payer, patients, and families);
- study methodology;
- data source;
- annual average direct health care cost per patient (including all types of healthcare costs directly related to the studied disease from diagnosis and treatment to continuing care and rehabilitation);
- annual average direct formal non-healthcare cost per patient (including costs of transportation);
- annual average direct informal non-healthcare cost per patient (including informal care by non-professional caregivers such as family members or friends, etc.);
- annual average indirect cost per patient (productivity losses);
- total annual average cost per patient;
- costs were reported in terms of absolute values and percentage distribution referring to the total costs.
The search identified 49 citations (12 papers for Hemophilia; 7 papers for X Fragile Syndrome; 25 papers for Cystic Fibrosis; and 5 papers for Juvenile Idiopathic Arthritis). All articles were screened by title and abstracts to identify those that fit the above mentioned inclusion criteria. After excluding 34 papers that did not meet inclusion criteria, a total of 15 papers were included for full text analysis. The papers were assessed independently for inclusion by two authors.
Table 1 provides information regarding the main study characteristics and types of costs included, such as the direct health costs, direct non-health care costs (formal and informal), and indirect costs. While Table 2 shows the cost items most frequently considered in the analyzed COI studies.

Table 1.
Samples of cost of illness studies concerning the selected rare diseases.

Table 2.
Summary of cost items considered by the samples of cost of illness studies.
3. Results
An overview of the COI approach and the cost items most frequently considered in the analyzed COI studies is provided by Table 2, and a more detailed description of results for each sampled RD follows.
Hemophilia A is a rare, X-linked bleeding disorder that affects approximately 1 of every 5000 to 10,000 live-born males [23]. Hemophilia B is much less common than A, with an incidence of approximately 1 in 25,000 births [24]. A definitive diagnosis of hemophilia A or B is typically made based on an established family history and/or patients’ presentation of a bleeding event that has been confirmed by laboratory tests to be the result of coagulation factor deficiency. The clinical presentations of the two conditions are indistinguishable, and the bleeding tendencies associated with each disorder tend to correlate directly with plasma concentrations of factor VIII (FVIII) and factor IX (FIX), respectively. Recurrent bleeding into the joint and muscles leads to irreversible bone and cartilage damage, culminating in disabling hemophilic arthropathy. Treatment for hemophilia A or B involves routine administration of exogenous coagulation factors to replace the missing/deficient endogenous FVIII or FIX, respectively [25]: This treatment implementation has changed continuously through the years in terms of products used (i.e., by-pass agents for patients with inhibitors, recombinant and monoclonal antibody drugs replacing Factor VIII) and administration method (on demand and prophylaxis administration) [25]. The overall cost of treatment remains high, particularly among patients with more severe forms of hemophilia [24]. Clotting factors have been estimated to account for approximately 90% of the direct health care costs for hemophilia management, and costs varied based on the treatment approach, patient characteristics in terms of age or comorbidities, and disease severity [26,27].
The COI analyses focusing on hemophilia covered several European countries, including two cross-countries studies [8,9], while one considers the hemophilia burden in the USA [10]. Due to the increasing availability of ad hoc treatments [25], direct costs are the main cost driver and range from 77% to 97% of total costs. In some cases, the included cost items consider only those drugs and healthcare services strictly related to the hemophilia treatment [11]; in other cases, direct cost items address a wider range of healthcare services providing the perspective of the whole process of care (i.e., primary care visits or dental care) [15]. Direct formal non-healthcare costs refer to patient transport expenses, and one study also considers informal care [8,10]. Last, the human capital approach seems to prevail in these instances, as sick leave and absenteeism along with early retirement are considered.
Fragile X Syndrome (FXS) is a chromosome X-linked genetic condition that leads to intellectual disability, learning and behavioral challenges, and physical characteristics [28]. This neuro-developmental disorder occurs in both sexes; about 1.4 per 10,000 males and 0.9 per 10,000 females have FXS, and European Union (EU) prevalence is estimated to be 32 per 100,000 inhabitants [29]. FXS is on average diagnosed around age 3, when cognitive and developmental disabilities become apparent. The severe comorbid profile of individuals with FXS contributes to high healthcare costs and utilization of medical specialist visits, tests or procedures, and pharmacotherapy management. The burden of FXS is not only on the patient; similar to patients with other developmental disabilities (DD), family caregivers of patients with FXS face multiple challenges. The caregiver burden from FXS is mostly related to managing behavioral symptoms associated with the disease [30]. Studies have found that families affected by FXS report an increased financial burden, and over 60% stated that they had to change work hours or quit jobs because of having a child with FXS [31]. Multidisciplinary treatment, including speech and language therapy, occupational therapy, physical therapy, special education, behavioral interventions, and genetic counselling, are needed for patients affected with FXS.
Unlike hemophilia, few studies analyze the social and economic burden of the Fragile X Syndrome from the societal perspective. To date, FXS is generally treated with selective serotonin reuptake inhibitors along with several long-term, non-medical interventions, and no ad hoc, specific treatment has been made available yet. Table 1 provides two instances of COI studies: The first includes not only direct healthcare costs and indirect costs, but also direct formal and informal non-healthcare costs, showing how these two items are relevant to this type of disease (i.e., 40% and 48%, respectively). Moreover, indirect costs show the impact of informal care on parents’ working condition [13]. The second study adopts a third payer perspective and then focuses just on healthcare services and goods consumed by FXS patients [14].
Cystic fibrosis (CF) is a rare genetic (autosomal recessive), multi-systemic, fatal disease that starts at birth and progresses over time to lung failure. CF is caused by a reduced quantity and/or impaired function of the CF transmembrane conductance regulator (CFTR) protein due to mutations in the CFTR gene. CF is mainly characterized by progressive lung function decline. This decline in lung function increases the risk of pulmonary exacerbations [32], which further exacerbates lung function decline and increases the risk of death [33,34]. Moreover, patients may experience extensive damage in multiple organs before presenting with symptoms or a decline in ppFEV1. Clinical presentation and progression vary with genotype. CF substantially impacts patients’ health-related quality of life and results in early death, regardless of genotype.
Prenatal diagnostics, newborn screening, and new treatment algorithms are changing the incidence and the prevalence of the disease. CF has an estimated prevalence of approximately 0.737 per 10,000 inhabitants in Europe [35]. Although progress in early diagnosis and new therapeutic strategies are also substantially improving the prognosis of CF and, currently, average life expectancy of patients exceeds 40 years. Only recently (from 2012), novel therapies (CTFR modulators) [15] that directly correct errors caused by mutations of the CFTR gene are becoming available in the EU for patients with certain mutations in CFTR protein.
Existing treatments for CF can be broadly classified in two groups: (1) Therapies managing the symptoms, complications, and comorbidities of the disease (e.g., antibiotics, mucolytics, pancreatic enzyme replacement therapy) and (2) CFTR modulators (i.e., correctors and potentiators), which target the underlying cause of the disease. Regarding the latter, four medicinal products (with orphan designation) have been approved by the European Commission [15]. Concomitant administration of these two groups is recommended to maintain and improve lung function, reduce the risk of infections and exacerbations, and improve quality of life. CFTR modulators, targeted at the underlying cause of CF rather than symptoms, have improved survival with short- and long-term improvements in clinical outcomes, but they have also increased costs causing difficulties in their availability for many countries. Therefore, given changing life expectancy and high cost of novel drugs, resources dedicated to CF care are very likely to have substantially increased in recent years [36,37,38].
The socioeconomic literature on CF provides effective instances of the treatments’ impact on disease cost. The studies described in Table 1 [15,16,17,18] show how much treatment costs may impact on the balance of a disease with an ad hoc therapy, and how the inclusion of a wide perspective of indirect costs [15,17,18] and informal care [17] can cast a different light on high direct healthcare costs. For instance, the direct medical costs in Germany [33] are mainly driven by high drug prices since the introduction of very expensive CF mutation-specific drugs in 2012; even if, as the authors also reported, costs increase with disease severity and related complications. The two German studies [15,16] have different evaluation perspectives: Heimeshoff et al. adopt a societal perspective [15], noting that the relevance of indirect costs is likely to increase in the future as life expectancy of CF patients increases. While Frey et al. assume a payer perspective [16] and contend that CF has a constant and wide-ranging economic impact on payers, with considerable differences in the distribution of costs and service utilization between younger and older patients, as well as mild vs. severe patients. Hence, they remind us how pharmaceutical expenses will increase in the future as causative treatment gains importance.
Juvenile Idiopathic Arthritis (JIA) is currently grouped in multiple categories, some of which have counterparts in the more frequent adult diseases of rheumatoid arthritis, axial spondyloarthritis, and psoriatic arthritis, though with considerable differences in phenotype at different ages. The etiology of JIA is unknown and the pathogenesis unclear, but it is likely multifactorial [39]. JIA is the most common chronic rheumatic disease in childhood, with an incidence of 10–19/100,000 children below the age of 16 years, and it is also one of the major causes of acquired disability and impairment of quality of life in childhood [29]. Early and aggressive control of arthritis is essential to prevent long-term disability [40]. JIA has an overall prevalence estimate in the EU of 16-150 cases per 100,000 inhabitants [41]. Without appropriate treatment, JIA may result in devastating consequences. Children may experience permanent disability from joint destruction, growth deformities, or blindness. Drug therapy covers a broad spectrum of medicines, e.g., non-steroidal anti-inflammatory agents, intra-articular corticosteroid injections, disease-modifying anti-rheumatic drugs, anti-interleukin therapy, and biologicals. The choice of medication depends on the subtype of JIA. However, treatment still needs to be developed. The hope is that recent changes in treatment approaches will result in marked improvement in long-term functional outcomes of patients with JIA.
JIA literature considers a wide range of healthcare services, aids, and goods representing direct healthcare cost items. Direct non-healthcare costs include formal care in two papers [19,21], while only one considers also informal care [21]; moreover, the included items effectively represent the burden on families in terms of transport, home alterations and professional care, etc. A good example of the extent of variability in this type of analysis is the indirect costs estimation by Minden et al. in 2004 and 2009 in Germany [19,22]. Indeed, Minden et al. [22] in 2004 adopt a human capital approach including both loss of productivity in terms of sick leave and work disability, implying an entitlement to a social security pension; while Minden et al. [19] in 2009 define loss of productivity as lost working days by parents.
4. Discussion
RDs have been an increasing area of focus, as three effects have converged in recent years: (i) Continuing innovation stemming from the genomic revolution, (ii) regulatory and financial incentives for RDTs, and (iii) the increasingly mobilized, coordinated, and sophisticated patient community. The cost of RDs, and by corollary the opportunity for savings, continues to increase.
We posit that COI provides critical information needed by policymakers when dealing with new RDTs. To show the type of evidence made available so far, we analyzed examples of COI papers published in the last decade on four selected RDs. These results showed significant heterogeneity in identifying all cost items associated with the selected RDs, consistent with results from Angelis et al. [42], suggesting the following reflections. First, the existence of a pharmaceutical treatment is positively correlated with the availability of cost analyses; second, indirect costs and direct non-healthcare costs account for a relevant portion of total costs when there is no specific indicated therapy (e.g., FXS), but only symptom treatment.
There are several characteristics related to COI studies requiring attention (Table 3). First, it is the need of reliable datasets addressing all information related to all types of costs of rare or ultra-rare conditions, often unavailable or not well-structured, which constitutes a problem for drug-developers, regulators, and particularly for HTA bodies and payers. Second, the four disease examples addressed in this paper demonstrate the high variability of costs depending on different perspectives. This variability, strongly linked with disease characteristics, is correlated with the level of innovation in treatments for the specific rare therapeutic area. Innovation can modify the course of the disease and in some cases—when there is an added clinical value—change the natural history of the disease. Hence, it is almost mandatory to trace this flow of transformation over time and to monitor both clinical aspects and associated costs. Third, COI studies are still relatively scarce compared with economic evaluations in general and epidemiological studies: The reasons for this scarcity might be in part the difficulty of linking diagnoses and clinical outcomes to resource utilization and in identifying costs outside the healthcare system.

Table 3.
“Pros” and “cons” of cost of illness (COI) analyses.
However, from a positive perspective, the category of COI covers several key aspects ranging from the incidence or prevalence of disease to its effect on longevity, morbidity, health status, and quality of life, and also considers related financial aspects, including direct and indirect expenditures resulting from premature death, disability, or injury due to corresponding disease and/or its comorbidities [43]. Moreover, this wide range of perspectives provides detailed indications regarding which actor is bearing the social economic and care burden, and where it is shifting as a new treatment is introduced.
Therefore, COI studies are necessary for the decision-making process at national and local levels: From a payer perspective, it is essential that the COI be a structuring part of the national dossiers for drug price negotiating. Alongside the assessment of unmet medical needs (mostly maximum level for the rare condition), the added clinical value of a new treatment (the most challenging criteria) and the robustness of clinical trials (often low or very-low), COI studies would help to better complete the overall rare disease picture. This would also help the payer or decision maker identify cost areas (not necessarily only related to the RDs) and additionally to start planning any shifts of his pharmaceutical budget from one area to another more promising one (in terms of clinical benefit) or priority (public health needs). In particular for RDs, this approach would help not only in the case of treatments approved by regulatory authority (label indication), but also to evaluate an eventual early access program planned to be used in an RD context (e.g., Italian 648/96 Law or French ATU procedures) [44,45].
It should be highlighted that the burden of disease (or COI) is an element of uncertainty, and, like all the other uncertainties that characterize a new product upon introduction in a specific therapeutic area, COI should also be managed [46]. For several years now, various payers have been applying the MEAs [3] as a set of tools aimed at facilitating access to new medicines in order to manage clinical (performance-based risk-sharing agreement) and financial (budget impact) uncertainties (See Figure 1). Patient registries are also used to verify appropriateness and implement Managed Entry Agreements [47,48].
In the last years, some initiatives have been developed to deal with economic challenges of rare diseases: BURQOL-RD [49,50], a multinational study, recently evaluated the burden of ten rare diseases in Europe, using a prevalence-based method with a bottom-up approach to quantify resources from a societal perspective, which is the mostly used methodology for these studies in rare diseases. Several programs at the European level (i.e., EUnetHTA [51], FP7 Advance HTA [52], IMPACT HTA [53]) are addressing HTA use and dissemination even in the field of RDs, improving and strengthening the methodological tools and practices.
5. Conclusions
We believe that COI studies are useful to inform policy-makers in evaluating the full impact of proposed new treatments for RDs, but methodological heterogeneity makes available studies difficult to compare. To effectively support decision-making processes, it is necessary to assess re-allocation of the burden of disease produced by new treatments, requiring identification of cost drivers through COI studies with robust design and standardized methodology.
Author Contributions
Conceptualization, P.A. and E.X.; methodology, P.A., M.C., E.X., and Y.K.; formal analysis, M.C., Y.K., and D.T.; resources, Y.K.; data curation, M.C.; writing—original draft preparation, P.A., M.C., E.X., and Y.K.; writing—review and editing, E.X. and M.C.; supervision, Y.K and D.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Drummond, M.; Evans, B.; LeLorier, J.; Karakiewicz, P.; Martin, D.; Tugwell, P.; MacLeod, S. Evidence and values: Requirements for public reimbursement of drugs for rare diseases—A case study in oncology. Can. J. Clin. Pharmacol. 2009, 16, e273–e281. [Google Scholar] [PubMed]
- Czech, M.; Baran-Kooiker, A.; Atikeler, K.; Demirtshyan, M.; Gaitova, K.; Holownia-Voloskova, M.; Turcu-Stiolica, A.; Kooiker, C.; Piniazhko, O.; Konstandyan, N.; et al. A review of rare disease policies and orphan drug reimbursement systems in 12 Eurasian countries. Front. Public Health 2020, 7, 416. [Google Scholar] [CrossRef] [PubMed]
- Morel, T.; Arickx, F.; Befrits, G.; Siviero, P.; van der Meijden, C.; Xoxi, E.; Simoens, S. Reconciling uncertainty of costs and outcomes with the need for access to orphan medicinal products: A comparative study of managed entry agreements across seven European countries. Orphanet J. Rare Dis. 2013, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Simoens, S. Public health and prevention in Europe: Is it cost-effective? J. Pharm. Health Serv. Res. 2011, 2, 151–155. [Google Scholar] [CrossRef]
- Schlander, M.; Dintsios, C.M.; Gandjour, A. Budgetary Impact and Cost Drivers of Drugs for Rare and Ultrarare Diseases. Value Health 2018, 21, 525–531. [Google Scholar] [CrossRef]
- Linertová, R.; Serrano-Aguilar, P.; Posada-de-la-Paz, M.; Hens-Pérez, M.; Kanavos, P.; Taruscio, D.; Schieppati, A.; Stefanov, R.; Péntek, M.; Delgado, C.; et al. Delphi approach to select rare diseases for a European representative survey. The BURQOL-RD study. Health Policy 2012, 108, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Cavazza, M.; Kodra, Y.; Armeni, P.; De Santis, M.; López-Bastida, J.; Linertová, R.; Oliva-Moreno, J.; Serrano-Aguilar, P.; Posada-de-la-Paz, M.; Taruscio, D.; et al. Social/economic costs and quality of life in patients with haemophilia in Europe. Eur. J. Health Econ. 2016, 17, 53–65. [Google Scholar] [CrossRef]
- O’Hara, J.; Hughes, D.; Camp, C.; Burke, T.; Carroll, L.; Diego, D.G. The cost of severe haemophilia in Europe: The CHESS study. Orphanet J. Rare Dis. 2017, 31, 12. [Google Scholar] [CrossRef]
- Chen, C.X.; Baker, J.R.; Nichol, M.B. Economic Burden of Illness among Persons with Hemophilia B from HUGS Vb: Examining the Association of Severity and Treatment Regimens with Costs and Annual Bleed Rates. Value Health 2017, 20, 1074–1082. [Google Scholar] [CrossRef]
- Café, A.; Carvalho, M.; Crato, M.; Faria, M.; Kjollerstrom, P.; Oliveira, C.; Pinto, P.R.; Salvado, R.; Dos Santos, A.A.; Silva, C. Haemophilia A: Health and economic burden of a rare disease in Portugal. Orphanet J. Rare Dis. 2019, 4, 211. [Google Scholar] [CrossRef] [PubMed]
- Henrard, S.; Devleesschauwer, B.; Beutels, P.; Callens, M.; De Smet, F.; Hermans, C.; Speybroeck, N. The health and economic burden of haemophilia in Belgium: A rare, expensive and challenging disease. Orphanet J. Rare Dis. 2014, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Chevreul, K.; Gandré, C.; Brigham, K.B.; López-Bastida, J.; Linertová, R.; Oliva-Moreno, J.; Serrano-Aguilar, P.; Posada-de-la-Paz, M.; Taruscio, D.; Schieppati, A.; et al. Social/economic costs and health-related quality of life in patients with fragile X syndrome in Europe. Eur. J. Health Econ. 2016, 17, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Sacco, P.; Capkun-Niggli, G.; Zhang, X.; Rosemary, J. The Economic Burden of Fragile X Syndrome: Healthcare Resource Utilization in the United States. Am. Health Drug Benefits 2013, 6, 73–83, PMID: 24991348; PMCID: PMC4031705. [Google Scholar]
- Heimeshoff, M.; Hollmeyer, H.; Schreyögg, J.; Tiemann, O.; Staab, D. Cost of Illness of Cystic Fibrosis in Germany. Pharm. Econ. 2012, 30, 763–777. [Google Scholar] [CrossRef]
- Frey, S.; Stargardt, T.; Schneider, U.; Schreyögg, J. The Economic Burden of Cystic Fibrosis in Germany from a Payer Perspective. Pharm. Econ. 2019, 37, 1029–1039. [Google Scholar] [CrossRef]
- Chevreul, K.; Berg Brigham, K.; Michel, M.; Rault, G.; BURQOL-RD Research Network. Costs and health-related quality of life of patients with cystic fibrosis and their carers in France. J. Cyst. Fibros. 2015, 4, 384–391. [Google Scholar] [CrossRef]
- Kopciuch, D.; Zaprutko, T.; Paczkowska, A.; Nowakowska, E. Costs of treatment of adult patients with cystic fibrosis in Poland and internationally. Public Health 2017, 148, 49–55. [Google Scholar] [CrossRef]
- Minden, K.; Niewerth, M.; Listing, J.; Möbius, D.; Thon, A.; Ganser, G.; Ermisch-Omran, B.; Zink, A. The economic burden of juvenile idiopathic arthritis-results from the German paediatric rheumatologic database. Clin. Exp. Rheumatol. 2009, 27, 863–869. [Google Scholar]
- Yucel, I.K.; Seyahi, E.; Kasapcopur, O.; Arisoy, N. Economic impact of juvenile idiopathic arthritis and familial Mediterranean fever. Rheumatol. Int. 2012, 32, 1955–1962. [Google Scholar] [CrossRef]
- Angelis, A.; Kanavos, P.; López-Bastida, J.; Linertová, R.; Serrano-Aguilar, P.; BURQOL-RD Research Network. Socioeconomic costs and health-related quality of life in juvenile idiopathic arthritis: A cost-of-illness study in the United Kingdom. BMC Musculoskelet. Disord. 2016, 17, 321. [Google Scholar] [CrossRef] [PubMed]
- Minden, K.; Niewerth, M.; Listing, J.; Biedermann, T.; Schöntube, M.; Zink, A. Burden and cost of illness in patients with juvenile idiopathic arthritis. Ann. Rheum. Dis. 2004, 63, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.; Recht, M. Current Options and New Developments in the Treatment of Haemophilia. Drugs 2011, 71, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.R.; Key, N.S.; Escobar, M.A.; Hemophilia, A.; Hemophilia, B. Williams Hematology, 8th ed.; Kaushansky, K., Lichtman, M., Beutler, E., Eds.; McGraw Hill: New York, NY, USA, 2010; ISBN -10. [Google Scholar]
- Aledort, L.; Mannucci, P.M.; Schramm, W.; Tarantino, M. Factor VIII replacement is still the standard of care in haemophilia A. Blood Transfus. 2019, 17, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Colombo, G.L.; Di Matteo, S.; Mancuso, M.E.; Santagostino, E. Cost-utility analysis of prophylaxis versus treatment on demand in severe hemophilia A. Clinicoecon. Outcomes Res. 2011, 3, 55–61. [Google Scholar] [CrossRef]
- Thorat, T.; Neumann, P.J.; Chambers, J.D. Hemophilia Burden of Disease: A Systematic Review of the Cost-Utility Literature for Hemophilia. J. Manag. Care Spec. Pharm. 2018, 24, 632–642. [Google Scholar] [CrossRef]
- Saldarriaga, W.; Tassone, F.; González-Teshima, L.Y.; Forero-Forero, J.V.; Ayala-Zapata, S.; Hagerman, R. Fragile X Syndrome. Colomb. Med. 2014, 45, 190–198. [Google Scholar] [CrossRef]
- Orphanet Report Series—Prevalence of Rare Diseases: Bibliographic Data 2020 1. Available online: http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf (accessed on 26 September 2020).
- Bailey, D.B.; Raspa, M.; Bishop, E.; Holiday, D. No change in the age of diagnosis for fragile x syndrome: Findings from a national parent survey. Pediatrics 2009, 124, 527–533. [Google Scholar] [CrossRef]
- Ouyang, L.; Grosse, S.; Raspa, M.; Bailey, D. Employment impact and financial burden for families of children with fragile X syndrome: Findings from the National Fragile X Survey. J. Intel. Disab. Res. 2010, 54, 918–928. [Google Scholar] [CrossRef]
- Goss, C.H.; Burns, J.L. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax 2007, 62, 360–367. [Google Scholar] [CrossRef]
- De Boer, K.; Vandemheen, K.L.; Tullis, E.; Doucette, S.; Fergusson, D.; Freitag, A.; Paterson, N.; Jackson, M.; Lougheed, M.D.; Kumar, V.; et al. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax 2011, 66, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Wagener, J.S.; Rasouliyan, L.; VanDevanter, D.R.; Pasta, D.J.; Regelmann, W.E.; Morgan, W.J.; Konstan, M.W. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Oral, inhaled, and intravenous antibiotic choice for treating pulmonary exacerbations in cystic fibrosis. Pediatr. Pulmonol. 2013, 48, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.B.; Fink, A. Background and Epidemiology. Pediatr. Clin. N. Am. 2016, 63, 567–584. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.P.; Orenstein, D.M.; Milla, C.E. Pricing for orphan drugs: Will the market bear what society cannot? JAMA 2013, 310, 1343–1344. [Google Scholar] [CrossRef] [PubMed]
- Ferkol, T.; Quinton, P. Precision medicine: At what price? Am. J. Respir. Care Med. 2015, 192, 658–659. [Google Scholar] [CrossRef]
- Orestein, D.M.; O’Sullivan, B.P.; Quinton, P.M. Cystic fibrosis: Breakthrough drugs at break-the-bank prices. Glob. Adv. Health Med. 2015, 4, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Finch, S.L.; Rosenberg, A.M.; Vatanparast, H. Vitamin D and juvenile idiopathic arthritis. Pediatr. Rheumatol. Online J. 2018, 16, 34. [Google Scholar] [CrossRef]
- Gökçe, I.; Demĭrkaya, E. New Treatment Strategies in the Treatment of Juvenile Idiopathic Arthritis. Arch. Reumathol. 2011, 26, 71–85. [Google Scholar] [CrossRef]
- EMA—Committee for Medicinal Products for Human Use (CHMP), Guideline on Clinical Investigation of Medicinal Products for the Treatment of Rheumatoid Arthritis, 14 December 2017, CPMP/EWP/556/95 Rev 2. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-investigation-medicinal-products-treatment-rheumatoid-arthritis_en.pdf (accessed on 26 September 2020).
- Angelis, A.; Tordrup, D.; Kanavos, P. Socio-economic burden of rare diseases: A systematic review of cost of illness evidence. Health Policy 2015, 119, 964–979. [Google Scholar] [CrossRef]
- Genetic Alliance UK Hidden Costs Report. 2016. Available online: https://www.geneticalliance.org.uk/media/2502/hiddencosts-full-report_21916-v2-1.pdf (accessed on 26 September 2020).
- Italian Law December 23, 1996, n. 648. Available online: https://www.normattiva.it/uri-res/N2Ls?urn:nir:stato:legge:1996-12-23;648!vig= (accessed on 26 September 2020).
- Agence Nationale de Sécurité du Médicament et des Produits de Santé, Notice to Applicants for Marketing for Temporary Authorisation for Use (ATU), July 2015. Available online: https://ansm.sante.fr/var/ansm_site/storage/original/application/cadfbcf9594614d59c8915670853a28b.pdf (accessed on 26 September 2020).
- Jo, C. Cost-of-illness studies: Concepts, scopes, and methods. Clin. Mol. Hepatol. 2014, 20, 327–337. [Google Scholar] [CrossRef]
- Xoxi, E.; Tomino, C.; de Nigro, L.; Pani, L. The Italian post-marketing registries. Pharm. Program. 2012, 5, 57–60. [Google Scholar] [CrossRef]
- Montilla, S.; Xoxi, E.; Russo, P.; Cicchetti, A.; Pani, L. Monitoring registries at Italian Medicines Agency: Fostering access, guaranteeing sustainability. Int. J. Technol. Assess. Health Care 2015, 31, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Bastida, J.; Oliva-Moreno, J.; Linertova, R.; Pedro Serrano-Aguilar, P. Social/economic costs and health-related quality of life in patients with rare diseases in Europe. Eur. J. Health Econ. 2016, 17, S1–S5. [Google Scholar] [CrossRef] [PubMed]
- Eurordis—Rare Diseases in Europe, Social Economic Burden and Health-Related Quality of Life in Patients with Rare Diseases in Europe. Available online: https://www.eurordis.org/content/burqol-rd-project (accessed on 26 September 2020).
- EUnetHTA—European Network for Health Technology Assessment. Available online: https://www.eunethta.eu/ (accessed on 26 September 2020).
- EUFP7 Program—Advancing and Strengthening the Methodological Tools and Practices Relating to the Application and Implementation of Health Technology Assessment (HTA). Available online: http://www.advance-hta.eu/ (accessed on 26 September 2020).
- Impact HTA Program—Improved Methods and Actionable Tools for Enhancing HTA. Available online: https://www.impact-hta.eu/ (accessed on 26 September 2020).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).