The Association between Neighborhood Disorder and Health: Exploring the Moderating Role of Genotype and Marriage
Abstract
1. Introduction
1.1. Neighborhood Disorder and Health
1.2. Biomarkers of Inflammatory Burden
1.3. Genetic Variation and Marital Status as Moderators
1.4. Summary of Research Hypotheses
2. Materials and Methods
2.1. Sample
2.2. Procedures
2.3. Measures
2.3.1. Dependent Variables
2.3.2. Independent Variable
2.3.3. Moderators
2.3.4. Control Variables
2.4. Analytic Approach
3. Results
3.1. Descriptive and Association Analysis
3.2. The Effect of Neighborhood Disorder on Self-Rated Poor Health Status
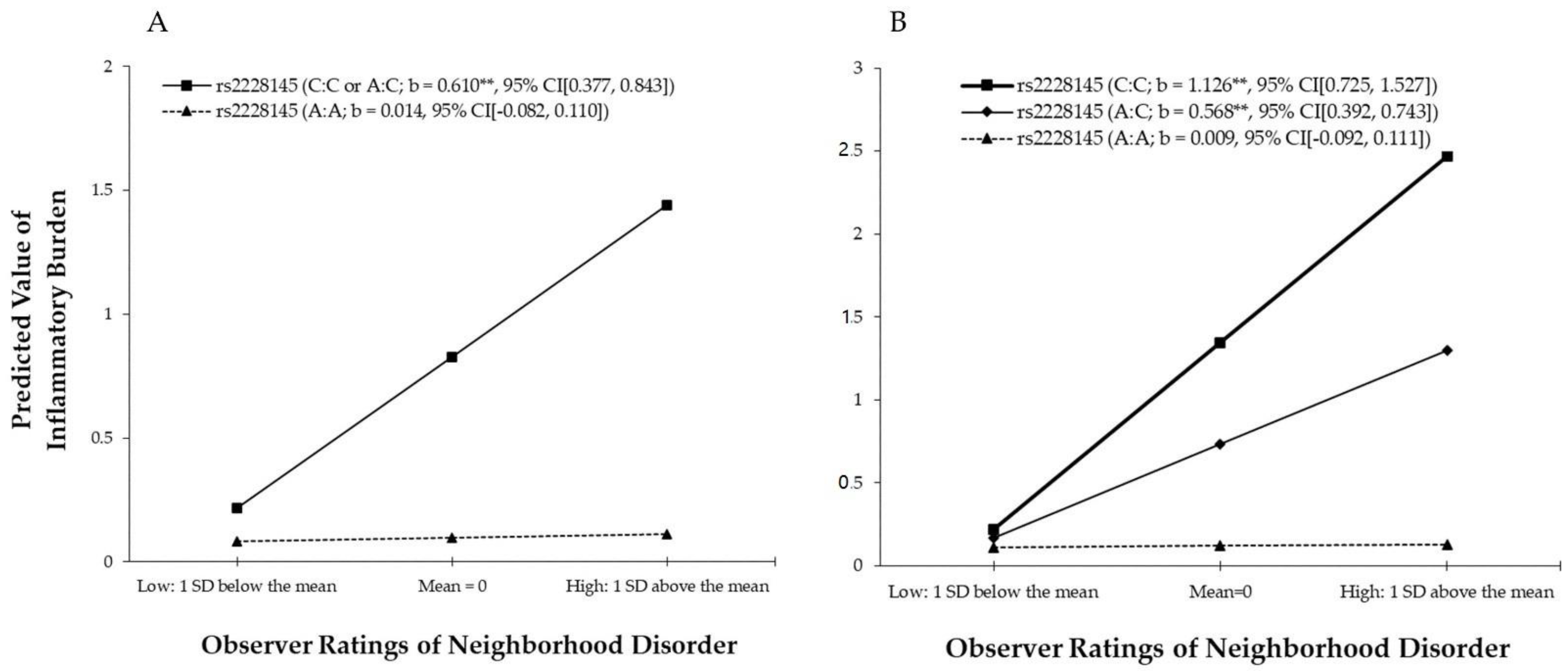
3.3. The Effect of G × E Interaction on Inflammatory Burden
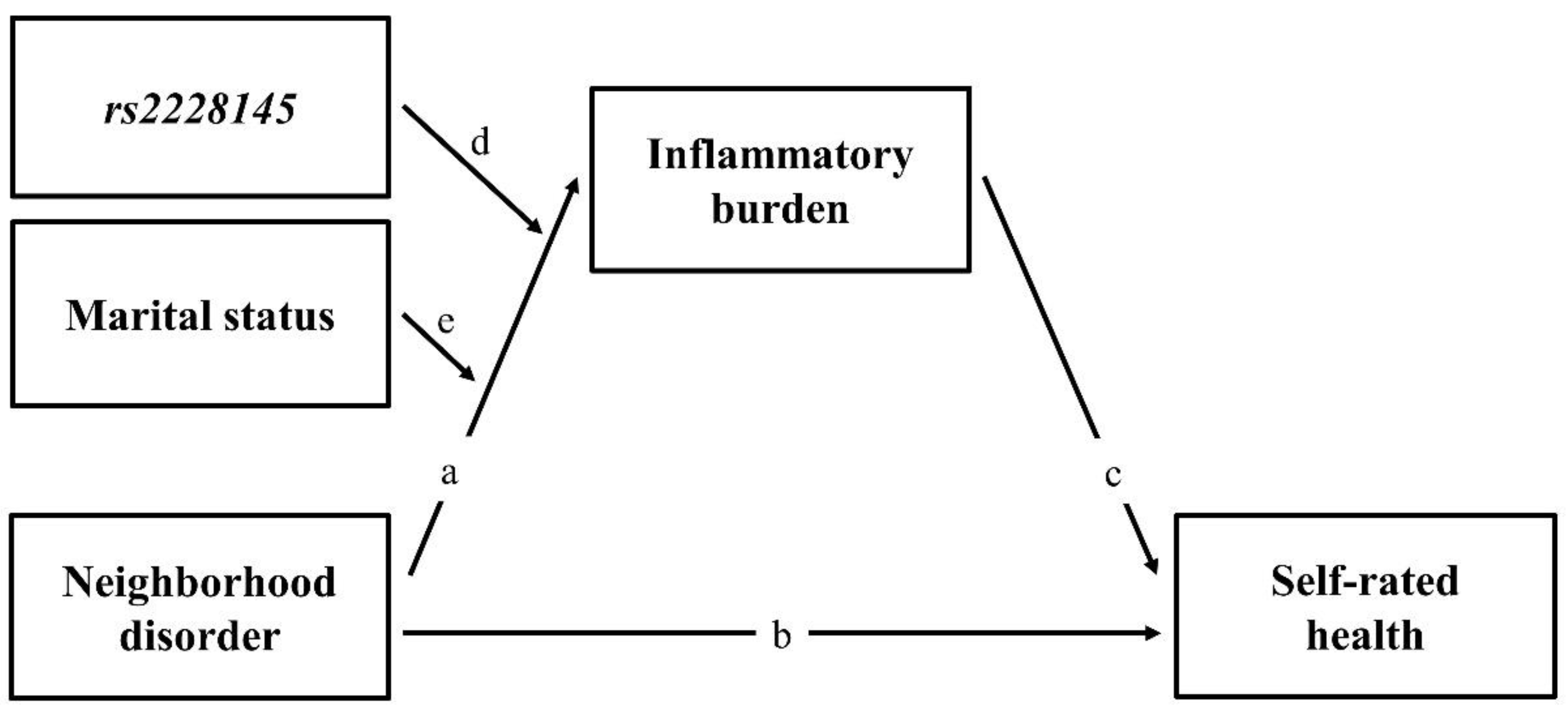
3.4. Mediated Moderation Effect of Inflammatory Burden
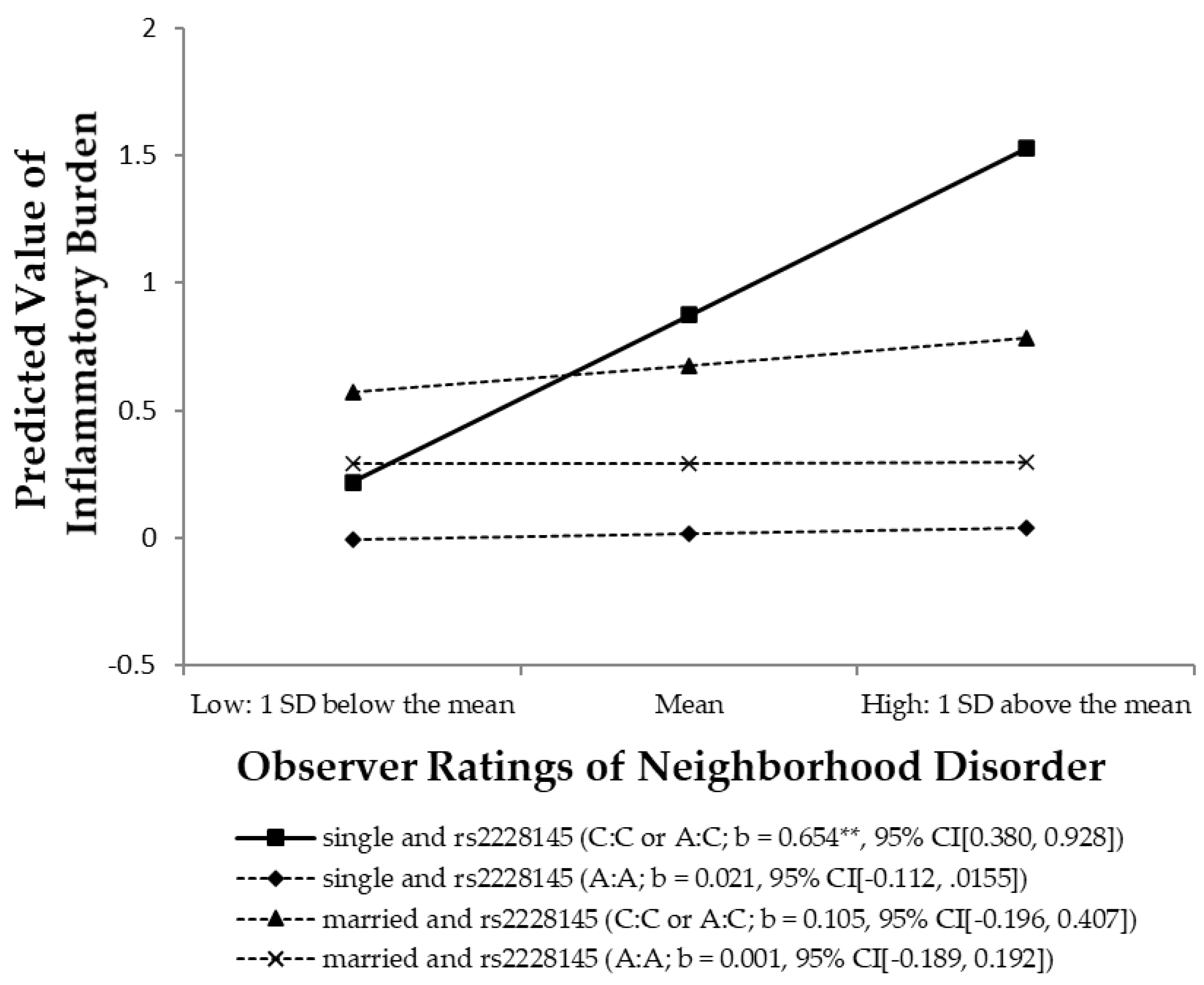
3.5. Test of the Stress-Buffering Hypothesis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, E.; Miller, G.E. Socioeconomic Status and Health: Mediating and Moderating Factors. Annu. Rev. Clin. Psychol. 2013, 9, 723–749. [Google Scholar] [CrossRef] [PubMed]
- Link, B.G.; Phelan, J. Social Conditions as Fundamental Causes of Disease. J. Health Soc. Behav. 1995, 55, 80–94. [Google Scholar] [CrossRef]
- Phelan, J.C.; Link, B.G.; Tehranifar, P. Social Conditions as Fundamental Causes of Health Inequalities Theory, Evidence, and Policy Implications. J. Health Soc. Behav. 2010, 51, S28–S40. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.A. Socioeconomic Position and Health: The Independent Contribution of Community Socioeconomic Context. Annu. Rev. Sociol. 1999, 25, 489–516. [Google Scholar] [CrossRef]
- Simons, R.L.; Lei, M.-K.; Klopack, E.; Beach, S.R.; Gibbons, F.X.; Philibert, R.A. The effects of social adversity, discrimination, and health risk behaviors on the accelerated aging of African Americans: Further support for the weathering hypothesis. Soc. Sci. Med. 2020, 113169, in press. [Google Scholar] [CrossRef]
- Lei, M.K.; Berg, M.T.; Simons, R.L.; Simons, L.G.; Beach, S.R. Childhood adversity and cardiovascular disease risk: An appraisal of recall methods with a focus on stress-buffering processes in childhood and adulthood. Soc. Sci. Med. 2020, 246, 112794. [Google Scholar] [CrossRef]
- Evans, G.W.; Kim, P.; Ting, A.H.; Tesher, H.B.; Shannis, D. Cumulative Risk, Maternal Responsiveness, and Allostatic Load among Young Adolescents. Dev. Psychol. 2007, 43, 341–351. [Google Scholar] [CrossRef]
- Fagundes, C.P.; Glaser, R.; Kiecolt-Glaser, J.K. Stressful Early Life Experiences and Immune Dysregulation across the Lifespan. Brainbehav. Immun. 2013, 27, 8–12. [Google Scholar] [CrossRef]
- Miller, G.E.; Chen, E.; Parker, K.J. Psychological Stress in Childhood and Susceptibility to the Chronic Diseases of Aging: Moving Toward a Model of Behavioral and Biological Mechanisms. Psychol. Bull. 2011, 137, 959–997. [Google Scholar] [CrossRef]
- Lei, M.-K.; Beach, S.R.; Simons, R.L. Childhood trauma, pubertal timing, and cardiovascular risk in adulthood. Health Psychol. 2018, 37, 613–617. [Google Scholar] [CrossRef]
- Aneshensel, C.S. Social Stress: Theory and Research. Annu. Rev. Sociol. 1992, 18, 15–38. [Google Scholar] [CrossRef]
- Hill, T.D.; Ross, C.E.; Angel, R.J. Neighborhood Disorder, Psychophysiological Distress, and Health. J. Health Soc. Behav. 2005, 46, 170–186. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.E.; Mirowsky, J. Neighborhood Disadvantage, Disorder, and Health. J. Health Soc. Behav. 2001, 42, 258–276. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.-K.; Simons, R.L.; Beach, S.R.; Philibert, R.A. Neighborhood disadvantage and biological aging: Using marginal structural models to assess the link between neighborhood census variables and epigenetic aging. J. Gerontol. Ser. B 2019, 74, e50–e59. [Google Scholar] [CrossRef]
- Lei, M.K.; Beach, S.R.; Simons, R.L. Biological embedding of neighborhood disadvantage and collective efficacy: Influences on chronic illness via accelerated cardiometabolic age. Dev. Psychopathol. 2018, 30, 1797–1815. [Google Scholar] [CrossRef]
- Ross, C.E.; Mirowsky, J. Neighborhood Disorder, Subjective Alienation, and Distress. J. Health Soc. Behav. 2009, 50, 49–64. [Google Scholar] [CrossRef]
- Podsakoff, P.M.; MacKenzie, S.B.; Lee, J.-Y.; Podsakoff, N.P. Common Method Biases in Behavioral Research: A Critical Review of the Literature and Recommended Remedies. J. Appl. Psychol. 2003, 88, 879–903. [Google Scholar] [CrossRef]
- Pearlin, L.I. The Sociological Study of Stress. J. Health Soc. Behav. 1989, 30, 241–256. [Google Scholar] [CrossRef]
- Pearlin, L.I. The Stress Process Revisited: Reflections on Concepts and their Interrelationships. In Handbook of the Sociology of Mental Health; Aneshensel, C.S., Phelan, J.C., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; pp. 395–416. [Google Scholar]
- Thoits, P.A. Stress and Health Major Findings and Policy Implications. J. Health Soc. Behav. 2010, 51, S41–S53. [Google Scholar] [CrossRef]
- Yang, Y.C.; McClintock, M.K.; Kozloski, M.; Li, T. Social Isolation and Adult Mortality: The Role of Chronic Inflammation and Sex Differences. J. Health Soc. Behav. 2013, 54, 183–203. [Google Scholar] [CrossRef]
- Simons, R.L.; Lei, M.-K.; Beach, S.R.; Simons, L.G.; Barr, A.B.; Gibbons, F.X.; Philiber, R.A. Testing Life Course Models Whereby Juvenile and Adult Adversity Combine to Influence Speed of Biological Aging. J. Health Soc. Behav. 2019, 60, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; Guralnik, J.M.; Longo, D.L.; Ferrucci, L. Interleukin-6 in Aging and Chronic Disease: A Magnificent Pathway. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.M.; Schorpp, K.M. Integrating biomarkers in social stratification and health research. Annu. Rev. Sociol. 2018, 44, 361–386. [Google Scholar] [CrossRef] [PubMed]
- Christian, L.M.; Glaser, R.; Porter, K.; Malarkey, W.B.; Beversdorf, D.; Kiecolt-Glaser, J.K. Poorer Self-Rated Health is Associated with Elevated Inflammatory Markers among Older Adults. Psychoneuroendocrinology 2011, 36, 1495–1504. [Google Scholar] [CrossRef]
- Aneshensel, C.S.; Sucoff, C.A. The Neighborhood Context of Adolescent Mental Health. J. Health Soc. Behav. 1996, 37, 293–310. [Google Scholar] [CrossRef]
- Boardman, J.D. Stress and Physical Health: The Role of Neighborhoods as Mediating and Moderating Mechanisms. Soc. Sci. Med. 2004, 58, 2473–2483. [Google Scholar] [CrossRef]
- Browning, C.R.; Cagney, K.A.; Iveniuk, J. Neighborhood Stressors and Cardiovascular Health: Crime and C-reactive Protein in Dallas, USA. Soc. Sci. Med. 2012, 75, 1271–1279. [Google Scholar] [CrossRef]
- Ford, E.S.; Giles, W.H.; Myers, G.L.; Rifai, N.; Ridker, P.M.; Mannino, D.M. C-reactive Protein Concentration Distribution among US Children and Young Adults: Findings from the National Health and Nutrition Examination Survey, 1999–2000. Clin. Chem. 2003, 49, 1353–1357. [Google Scholar] [CrossRef][Green Version]
- Khera, A.; McGuire, D.K.; Murphy, S.A.; Stanek, H.G.; Das, S.R.; Vongpatanasin, W.; Wians, F.H.; Grundy, S.M.; Lemos, J.A.d. Race and Gender Differences in C-reactive Protein Levels. J. Am. Coll. Cardiol. 2005, 46, 464–469. [Google Scholar] [CrossRef]
- Stafford, M.; Cummins, S.; Macintyre, S.; Ellaway, A.; Marmot, M. Gender Differences in the Associations between Health and Neighbourhood Environment. Soc. Sci. Med. 2005, 60, 1681–1692. [Google Scholar] [CrossRef]
- Peterson, R.D.; Krivo, L.J. Divergent Social Worlds: Neighborhood Crime and the Racial-Spatial Divide; Russell Sage Foundation: New York, NY, USA, 2010. [Google Scholar]
- Geronimus, A.T. Deep integration: Letting the epigenome out of the bottle without losing sight of the structural origins of population health. Am. J. Public Health 2013, 103, S56–S63. [Google Scholar] [CrossRef] [PubMed]
- Aneshensel, C.S.; Harig, F.; Wight, R.G. Aging, neighborhoods, and the built environment. In Handbook of Aging and the Social Sciences; Gorge, L.K., Ferraro, K.F., Eds.; Elsevier: San Diego, CA, USA, 2016; pp. 315–335. [Google Scholar]
- Geis, K.J.; Ross, C.E. A New Look at Urban Alienation: The Effect of Neighborhood Disorder on Perceived Powerlessness. Soc. Psychol. Q. 1998, 61, 232–246. [Google Scholar] [CrossRef]
- Sampson, R.J.; Raudenbush, S.W. Systematic Social Observation of Public Spaces: A New Look at Disorder in Urban Neighborhoods. Am. J. Sociol. 1999, 105, 603–651. [Google Scholar] [CrossRef]
- Roux, D.V.A.; Mair, C. Neighborhoods and Health. Ann. N. Y. Acad. Sci. 2010, 1186, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Simons, R.L.; Man-Kit, L.; Carter, S.; Beach, S.R.; Gibbons, F.X.; Gerrard, M.; Philibert, R.A. Inflammation Mediates the Effect of Discrimination, Religiosity, and Friendship Network on Expression of the Tp53 Cancer Suppressor Gene. Ssm-Popul. Health 2019, 7, 100389. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.E.; Chen, E.; Fok, A.K.; Walker, H.; Lim, A.; Nicholls, E.F.; Cole, S.; Kobor, M.S. Low Early-Life Social Class Leaves a Biological Residue Manifested by Decreased Glucocorticoid and Increased Proinflammatory Signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 14716–14721. [Google Scholar] [CrossRef]
- Broyles, S.T.; Staiano, A.E.; Drazba, K.T.; Gupta, A.K.; Sothern, M.; Katzmarzyk, P.T. Elevated C-Reactive Protein in Children from Risky Neighborhoods: Evidence for a Stress Pathway Linking Neighborhoods and Inflammation in children. PLoS ONE 2012, 7, e45419. [Google Scholar] [CrossRef]
- Danesh, J.; Whincup, P.; Walker, M.; Lennon, L.; Thomson, A.; Appleby, P.; Gallimore, J.R.; Pepys, M.B. Low Grade Inflammation and Coronary Heart Disease: Prospective Study and Updated Meta-Analyses. Br. Med. J. 2000, 321, 199–204. [Google Scholar] [CrossRef]
- Morley, J.E.; Baumgartner, R.N. Cytokine-Related Aging Process. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, M924–M929. [Google Scholar] [CrossRef]
- Jones, S.A.; Rose-John, S. The Role of Soluble Receptors in Cytokine Biology: The Agonistic Properties of the sIL-6R/IL-6 Complex. Biochim. Biophys. Acta Mol. Cell Res. 2002, 1592, 251–263. [Google Scholar] [CrossRef]
- Shariat, S.F.; Andrews, B.; Kattan, M.W.; Kim, J.H.; Wheeler, T.M.; Slawin, K.M. Plasma Levels of Interleukin-6 and Its Soluble Receptor are Associated with Prostate Cancer Progression and Metastasis. Urology 2001, 58, 1008–1015. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Michalakeas, C.A.; Parissis, J.; Paraskevaidis, I.; Ntai, K.; Papadakis, I.; Anastasiou-Nana, M.; Lekakis, J. Inflammatory Markers in Coronary Artery Disease. Biofactors 2012, 38, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Pollitt, R.A.; Kaufman, J.S.; Rose, K.M.; Diez-Roux, A.V.; Zeng, D.; Heiss, G. Early-Life and Adult Socioeconomic Status and Inflammatory Risk Markers in Adulthood. Eur. J. Epidemiol. 2007, 22, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Gallo, L.C.; Fortmann, A.L.; Monteros, K.E.D.L.; Mills, P.J.; Barrett-Connor, E.; Roesch, S.C.; Matthews, K.A. Individual and Neighborhood Socioeconomic Status and Inflammation in Mexican American Women: What is the Role of Obesity? Psychosom. Med. 2012, 74, 535–542. [Google Scholar] [CrossRef]
- Holmes, L.M.; Marcelli, E.A. Neighborhoods and Systemic Inflammation: High CRP among Legal and Unauthorized Brazilian Migrants. Health Place 2012, 18, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Nazmi, A.; Roux, A.D.; Ranjit, N.; Seeman, T.E.; Jenny, N.S. Cross-Sectional and Longitudinal Associations of Neighborhood Characteristics with Inflammatory Markers: Findings from the Multi-Ethnic Study of Atherosclerosis. Health Place 2010, 16, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Sutin, A.R.; Costa, P.T.C., Jr.; Wethington, E.; Eaton, W. Perceptions of Stressful Life Events as Turning Points are associated with Self-Rated Health and Psychological Distress. Anxietystresscoping 2010, 23, 479–492. [Google Scholar] [CrossRef]
- Benyamini, Y.; Idler, E.L. Community Studies Reporting association between Self-Rated Health and Mortality Additional Studies, 1995 to 1998. Res. Aging 1999, 21, 392–401. [Google Scholar] [CrossRef]
- Idler, E.L.; Benyamini, Y. Self-Rated Health and Mortality: A Review of Twenty-Seven Community Studies. J. Health Soc. Behav. 1997, 38, 21–37. [Google Scholar] [CrossRef]
- Foster, H.; Brooks-Gunn, J. Neighborhood Influences on Antisocial Behavior during Childhood and Adolescence. In Handbook of Life-Course Criminology: Emerging Trends and Directions for Future Research; Gibson, C.L., Krohn, M.D., Eds.; Springer: New York, NY, USA, 2013; pp. 69–90. [Google Scholar]
- Lei, M.-K.; Simons, R.L. A Multilevel Structural Resilience Model: When and for Whom do Neighbourhoods Really Matter? Int. Sociol. Assoc. Esymposium Sociol. 2020, 30, 1–12. [Google Scholar]
- Lei, M.K.; Beach, S.R. Can We Uncouple Neighborhood Disadvantage and Delinquent Behaviors? An Experimental Test of Family Resilience Guided by the Social Disorganization Theory of Delinquent Behaviors. Fam. Process 2020. [Google Scholar] [CrossRef] [PubMed]
- Caspi, A.; Moffitt, T.E. Gene–Environment Interactions in Psychiatry: Joining Forces with Neuroscience. Nat. Rev. Neurosci. 2006, 7, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.-K.; Simons, R.L.; Edmond, M.B.; Simons, L.G.; Cutrona, C.E. The Effect of Neighborhood Disadvantage, Social Ties, and Genetic Variation on the Antisocial Behavior of African American Women: A Multilevel Analysis. Dev. Psychopathol. 2014, 26, 1113–1128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freese, J. Genetics and the Social Science Explanation of Individual Outcomes. Am. J. Sociol. 2008, 114, S1–S35. [Google Scholar] [CrossRef]
- Guo, G.; Roettger, M.E.; Cai, T. The Integration of Genetic Propensities into Social-Control Models of Delinquency and Violence among Male Youths. Am. Sociol. Rev. 2008, 73, 543–568. [Google Scholar] [CrossRef]
- Simons, R.L.; Lei, M.K.; Beach, S.R.H.; Brody, G.H.; Philibert, R.A.; Gibbons, F.X. Social Environment, Genes, and Aggression Evidence Supporting the Differential Susceptibility Perspective. Am. Sociol. Rev. 2011, 76, 883–912. [Google Scholar] [CrossRef]
- Brody, G.H.; Yu, T.; Chen, Y.-F.; Kogan, S.M.; Evans, G.W.; Windle, M.; Gerrard, M.; Gibbons, F.X.; Simons, R.L.; Philibert., R.A. Supportive Family Environments, Genes that Confer Sensitivity, and Allostatic Load among Rural African American Emerging Adults: A Prospective Analysis. J. Fam. Psychol. 2013, 27, 22–29. [Google Scholar] [CrossRef]
- Caspi, A.; Hariri, A.R.; Holmes, A.; Uher, R.; Moffitt, T.E. Genetic Sensitivity to the Environment: The Case of the Serotonin Transporter Gene and its Implications for Studying Complex Diseases and Traits. Am. J. Psychiatry 2010, 167, 509–527. [Google Scholar] [CrossRef]
- Cutrona, C.E.; Russell, D.W.; Hessling, R.M.; Brown, P.A.; Murry, V. Direct and Moderating Effects of Community Context on the Psychological Well-Being of African American Women. J. Personal. Soc. Psychol. 2000, 79, 1088–1101. [Google Scholar] [CrossRef]
- Simons, R.L.; Simons, L.G.; Burt, C.H.; Brody, G.H.; Cutrona, C. Collective Efficacy, Authoritative Parenting and Delinquency: A Longitudinal Test of a Model Integrating Community and Family Level Processes. Criminology 2005, 43, 989–1029. [Google Scholar] [CrossRef]
- Taylor, S.E.; Way, B.M.; Seeman, T.E. Early Adversity and Adult Health Outcomes. Dev. Psychopathol. 2011, 23, 939–954. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.P.; Zheng, D.; Palmen, J.; Pang, D.X.; Woo, P.; Humphries, S.E. Effects of Genetic Variation on Chromatin Structure and the Transcriptional Machinery: Analysis of the IL6 Gene Locus. Genes Immun. 2012, 13, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Galicia, J.C.; Tai, H.; Komatsu, Y.; Shimada, Y.; Akazawa, K.; Yoshie, H. Polymorphisms in the IL-6 Receptor (IL-6R) Gene: Strong Evidence that Serum Levels of Soluble IL-6R are Genetically Influenced. Genes Immun. 2004, 5, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Rifai, N.; Hu, F.B. Interleukin-6 Receptor Gene Variations, Plasma Interleukin-6 Levels, and Type 2 Diabetes in US Women. Diabetes 2007, 56, 3075–3081. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Pare, G.; Parker, A.; Zee, R.Y.; Danik, J.S.; Buring, J.E.; Kwiatkowski, D.; Cook, N.R.; Miletich, J.P.; Chasman, D.I. Loci Related to Metabolic-Syndrome Pathways Including LEPR, HNF1A, IL6R, and GCKR Associate with Plasma C-Reactive Protein: The Women’s Genome Health Study. Am. J. Hum. Genet. 2008, 82, 1185–1192. [Google Scholar] [CrossRef]
- Sarwar, N.; Butterworth, A.; Freitag, D.F.; Gregson, J.; Willeit, P.; Gorman, D.N.; Gao, P.; Saleheen, D.; Rendon, A.; Nelson, C.P.; et al. Interleukin-6 Receptor Pathways in Coronary Heart Disease: A Collaborative Meta-Analysis of 82 Studies. Lancet 2012, 379, 1205–1213. [Google Scholar]
- Turner, R.J.; Marino, F. Social Support and Social Structure: A Descriptive Epidemiology. J. Health Soc. Behav. 1994, 35, 193–212. [Google Scholar] [CrossRef]
- Cohen, S. Psychosocial Models of the Role of Social Support in the Etiology of Physical Disease. Health Psychol. 1988, 7, 269–297. [Google Scholar] [CrossRef]
- Lin, N.; Ensel, W.M. Life Stress and Health: Stressors and Resources. Am. Sociol. Rev. 1989, 54, 382–399. [Google Scholar] [CrossRef]
- Waldron, I.; Hughes, M.E.; Brooks, T.L. Marriage Protection and Marriage Selection—Prospective Evidence for Reciprocal Effects of Marital Status and Health. Soc. Sci. Med. 1996, 43, 113–123. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, K.J.; Newton, T.L. Marriage and Health: His and Hers. Psychol. Bull. 2001, 127, 472–503. [Google Scholar] [CrossRef] [PubMed]
- Soons, J.P.M.; Liefbroer., A.C. Together is Better? Effects of Relationship Status and Resources on Young Adults’ Well-Being. J. Soc. Pers. Relatsh. 2008, 25, 603–624. [Google Scholar] [CrossRef]
- Hughes, M.E.; Waite, L.J. Marital Biography and Health at Mid-Life. J. Health Soc. Behav. 2009, 50, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.M.; Kronick, R.G. Marital Status and Longevity in the United States Population. J. Epidemiol. Community Health 2006, 60, 760–765. [Google Scholar] [CrossRef]
- Heckman, J.J. Sample Selection Bias as a Specification Error. Econom. J. Econom. Soc. 1979, 47, 153–161. [Google Scholar] [CrossRef]
- Singer, B.; Ryff, C.D.; Seeman, T. Operationalizing Allostatic Load. In Allostasis, Homeostasis, and the Costs of Physiological Adaptation; Schulkin, J., Ed.; Cambridge University Press: New York, NY, USA, 2004; pp. 113–149. [Google Scholar]
- Idler, E.L.; Russell, L.B.; Davis, D. Survival, Functional Limitations, and Self-Rated Health in the NHANES I Epidemiologic Follow-up Study, 1992. Am. J. Epidemiol. 2000, 152, 874–883. [Google Scholar] [CrossRef]
- Sampson, R.J.; Morenoff, J.D.; Gannon-Rowley, T. Assessing Neighborhood Effects: Social Processes and New Directions in Research. Annu. Rev. Sociol. 2002, 28, 443–478. [Google Scholar] [CrossRef]
- Millar, R.J. Neighborhood cohesion, disorder, and physical function in older adults: An examination of racial/ethnic differences. J. Aging Health 2020, 32, 1133–1144. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, Criteria, and Rules of Thumb for Evaluating Normed and Standardized Assessment Instruments in Psychology. Psychol. Assess. 1994, 64, 284–290. [Google Scholar] [CrossRef]
- Lewis, C.M. Genetic Association Studies: Design, Analysis and Interpretation. Brief. Bioinform. 2002, 3, 146–153. [Google Scholar] [CrossRef]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of Population Structure Using Multilocus Genotype Data: Dominant Markers and Null Alleles. Mol. Ecol. Notes 2007, 7, 574–578. [Google Scholar] [CrossRef]
- Bowling, A.; Stafford, M. How do Objective and Subjective Assessments of Neighbourhood Influence Social and Physical Functioning in Older Age? Findings from a British Survey of Ageing. Soc. Sci. Med. 2007, 64, 2533–2549. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.L.; Marsland, A.L.; Flory, J.; Votruba-Drzal, E.; Muldoon, M.F.; Manuck, S.B. Community Socioeconomic Status is Associated with Circulating Interleukin-6 and C-Reactive Protein. Psychosom. Med. 2008, 70, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Sampson, R.J.; Raudenbush, S.W.; Earls, F. Neighborhoods and Violent Crime: A Multilevel Study of Collective Efficacy. Science 1997, 277, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Davis, C.G.; Kendler, K.S. Childhood Adversity and Adult Psychiatric Disorder in the US National Comorbidity Survey. Psychol. Med. 1997, 27, 1101–1119. [Google Scholar] [CrossRef]
- Muthén, B.O.; Satorra, A. Complex Sample Data in Structural Equation Modeling. Sociol. Methodol. 1995, 25, 267–316. [Google Scholar] [CrossRef]
- Muthén, L.K.; Muthén, B.O. Mplus 8.0 User’s Guide; Muthén and Muthén: Los Angeles, CA, USA, 2017. [Google Scholar]
- MacKinnon, P.D.; Lockwood, C.M.; Hoffman, J.M.; West, S.G.; Sheets, V. A Comparison of Methods to Test Mediation and Other Intervening Variable Effects. Psychol. Methods 2002, 7, 83–104. [Google Scholar] [CrossRef]
- Dawson, J.F.; Richter, A.W. Probing Three-Way Interactions in Moderated Multiple Regression: Development and Application of a Slope Difference Test. J. Appl. Psychol. 2006, 91, 917–926. [Google Scholar] [CrossRef]
- Roisman, G.I.; Newmana, D.A.; Fraley, R.C.; Haltigan, J.D.; Groh, A.M.; Haydon, K.C. Distinguishing Differential Susceptibility from Diathesis-Stress: Recommendations for Evaluating Interaction Effects. Dev. Psychopathol. 2012, 24, 389–409. [Google Scholar] [CrossRef]
- Preacher, K.J.; Rucker, D.D.; Hayes, A.F. Addressing Moderated Mediation Hypotheses: Theory, Methods, and Prescriptions. Multivar. Behav. Res. 2007, 42, 185–227. [Google Scholar] [CrossRef]
- Cherlin, A.J. Demographic trends in the United States: A review of research in the 2000s. J. Marriage Fam. 2010, 72, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Musick, K.; Brand, J.E.; Davis, D. Variation in the relationship between education and marriage: Marriage market mismatch? J. Marriage Fam. 2012, 74, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhao, H. Sample Size Needed to Detect Gene-Gene Interactions using Association Designs. Am. J. Epidemiol. 2003, 158, 899–914. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Biblarz, T.J.; Gottainer, G. Family Structure and Children’s Success: A Comparison of Widowed and Divorced Single-Mother Families. J. Marriage Fam. 2000, 62, 533–548. [Google Scholar] [CrossRef]
- Boardman, J.D.; Onge, J.M.S.; Rogers, R.G.; Denney, J.T. Race Differentials in Obesity: The Impact of Place. J. Health Soc. Behav. 2005, 46, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Browning, C.R.; Cagney, K.A. Moving beyond Poverty: Neighborhood Structure, Social Processes, and Health. J. Health Soc. Behav. 2003, 44, 552–571. [Google Scholar] [CrossRef]
- Barr, A.B.; Culatta, E.; Simons, R.L. Romantic Relationships and Health among African American Young Adults Linking Patterns of Relationship Quality over Time to Changes in Physical and Mental Health. J. Health Soc. Behav. 2013, 54, 369–385. [Google Scholar] [CrossRef]
- Janicka, I. Sense of security in single women and women in lasting relationships. Health Psychol. Rep. 2015, 3, 158–166. [Google Scholar] [CrossRef]
- Mesch, G.S. Perceptions of risk, lifestyle activities, and fear of crime. Deviant Behav. 2000, 21, 47–62. [Google Scholar] [CrossRef]
| Variables | Mean (SD) | % |
|---|---|---|
| Self-rated poor health | 1.95 (0.99) | |
| Inflammatory burden | −0.05 (1.45) | |
| Neighborhood disorder | 0.00 (1.00) | |
| rs2228145 (1 = C:C or A:C), % | 22.50% | |
| Married, % | 30.50% | |
| Childhood adversity | 1.46 (1.24) | |
| Family socioeconomic status | 0.00 (0.78) | |
| Age | 46.17 (6.10) | |
| South, % | 67.40% | |
| Length of residence | 8.52 (8.59) | |
| Neighborhood cohesion | −0.38 (9.61) | |
| Neighborhood disadvantage | 0.08 (1.04) | |
| Residential stability | 0.04 (1.00) |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Self-rated poor health | ⸻ | ||||||||||||
| 2. Inflammatory burden | 0.11 * | ⸻ | |||||||||||
| 3. Neighborhood disorder | 0.11 * | 0.11 * | ⸻ | ||||||||||
| 4. rs2228145 (1 = C:C or A:C) | −0.07 | 0.22 ** | −0.01 | ⸻ | |||||||||
| 5. Married | −0.12 * | 0.11 † | −0.04 | 0.06 | ⸻ | ||||||||
| 6. Childhood adversity | 0.08 | −0.01 | −0.06 | 0.03 | −0.03 | ⸻ | |||||||
| 7. FSES | −0.17 ** | 0.11 * | −0.03 | 0.03 | 0.31 ** | 0.03 | ⸻ | ||||||
| 8. Age | 0.10 † | 0.14 * | −0.03 | 0.01 | 0.01 | 0.03 | 0.04 | ⸻ | |||||
| 9. South | −0.02 | −0.13 * | −0.16 ** | −0.02 | −0.01 | −0.05 | −0.07 | −0.10 † | ⸻ | ||||
| 10. Length of residence | 0.01 | 0.07 | 0.05 | −0.02 | 0.09 † | −0.11 † | 0.10 † | 0.32 ** | 0.14 * | ⸻ | |||
| 11. Neighborhood cohesion | −0.04 | 0.06 | −0.10 * | 0.02 | 0.05 | −0.15 ** | 0.10 † | 0.11 * | 0.16 ** | 0.22 ** | ⸻ | ||
| 12. ND a | 0.10 † | −0.10 | 0.15 ** | −0.03 | −0.14 * | 0.03 | −0.24 ** | −0.14 * | −0.19 | −0.07 | −0.20 ** | ⸻ | |
| 13. Residential stability a | −0.07 | 0.13 † | −0.01 | 0.01 | 0.14 ** | −0.13 * | 0.10 | 0.09 | 0.17 | 0.25 ** | 0.14 ** | −0.25 | ⸻ |
| Dependent variable: | Model 1 (ND) | Model 2 (ND, IB) | ||
| Self-rated poor health status (0–4) | Coeff. (SE) | Odds Ratio | Coeff. (SE) | Odds Ratio |
| Independent variable | ||||
| Observer ratings of neighborhood disorder (ND) | 0.123 * | 1.131 | 0.109 † | 1.115 |
| (0.058) | (0.058) | |||
| Mediator | ||||
| Inflammatory burden (IB) | 0.094 ** | 1.099 | ||
| (0.037) | ||||
| Control variables | ||||
| Childhood adversity | 0.074 † | 1.077 | 0.073 † | 1.076 |
| (0.041) | (0.041) | |||
| Family SES | −0.215 ** | 0.807 | −0.228 ** | 0.796 |
| (0.072) | (0.076) | |||
| Age | 0.023 * | 1.023 | 0.021 † | 1.021 |
| (0.011) | (0.011) | |||
| South | 0.045 | 1.046 | 0.086 | 1.090 |
| (0.173) | (0.177) | |||
| Length of residence | −0.001 | 0.999 | −0.001 | 0.999 |
| (0.009) | (0.009) | |||
| Neighborhood cohesion | 0.000 | 1.000 | −0.001 | 0.999 |
| (0.006) | (0.007) | |||
| Concentrated disadvantage | 0.050 | 1.051 | 0.060 | 1.062 |
| (0.054) | (0.055) | |||
| Residential stability | −0.047 | 0.954 | −0.063 | 0.939 |
| (0.058) | (0.063) | |||
| Thresholds | ||||
| Threshold parameter 0–1 | −1.434 ** | 0.238 | −1.424 ** | 0.241 |
| (0.178) | (0.180) | |||
| Threshold parameter 1–2 | −0.325 * | 0.723 | −0.306 † | 0.736 |
| (0.158) | (0.158) | |||
| Threshold parameter 2–3 | 0.590 ** | 1.804 | 0.619 ** | 1.857 |
| (0.155) | (0.157) | |||
| Threshold parameter 3–4 | 1.976 ** | 7.214 | 2.015 ** | 7.501 |
| (0.178) | (0.177) | |||
| R-Square | 0.072 | 0.088 * | ||
| ∆R-Square | 0.016 ** | |||
| Dependent variable: | Model 1 (ND, Gene) | Model 2 (ND × Gene) | Model 3 (ND, Gene) | Model 4 (ND × Gene) | ||||
| Log-transformed Inflammatory burden | b | β | b | β | b | β | b | β |
| Environment and Genetic Variables | ||||||||
| Observer ratings of neighborhood disorder (ND) | 0.164 * | 0.114 | 0.014 | 0.010 | 0.163 * | 0.112 | 0.009 | 0.006 |
| (0.074) | (0.049) | (0.075) | (0.052) | |||||
| rs2228145 (1 = C:C or A:C) (Gene) | 0.727 ** | 0.210 | 0.731 ** | 0.211 | ||||
| (0.197) | (0.182) | |||||||
| rs2228145 (2 = C:C; 1 = A:C; 0 = A:A) (Gene) | 0.633 ** | 0.213 | 0.612 ** | 0.206 | ||||
| (0.225) | (0.217) | |||||||
| Gene-Environment Interaction | ||||||||
| Neighborhood disorder | 0.596 ** | 0.212 | ||||||
| × rs2228145 (1 = C:C or A:C) | (0.143) | |||||||
| Neighborhood disorder | 0.558 ** | 0.223 | ||||||
| × rs2228145 (2 = C:C; 1 = A:C; 0 = A:A) | (0.119) | |||||||
| Control Variables | ||||||||
| Childhood adversity | 0.012 | 0.011 | −0.003 | −0.002 | 0.008 | 0.007 | −0.003 | −0.003 |
| (0.063) | (0.059) | (0.064) | (0.060) | |||||
| Family SES | 0.115 | 0.061 | 0.140 † | 0.075 | 0.112 | 0.060 | 0.139 † | 0.075 |
| (0.081) | (0.083) | (0.082) | (0.084) | |||||
| Age | 0.023 † | 0.097 | 0.024 † | 0.102 | 0.022 † | 0.092 | 0.024 † | 0.102 |
| (0.013) | (0.013) | (0.013) | (0.013) | |||||
| South | −0.420 * | −0.136 | −0.404 * | −0.131 | −0.402 * | −0.130 | −0.394 * | −0.128 |
| (0.174) | (.0180) | (0.172) | (0.178) | |||||
| Length of residence | 0.001 | 0.006 | −0.001 | −0.004 | −0.001 | −0.001 | −0.003 | −0.018 |
| (0.008) | (0.007) | (0.008) | (0.007) | |||||
| Neighborhood cohesion | 0.006 | 0.043 | 0.011 | 0.076 | 0.006 | 0.043 | 0.012 | 0.078 |
| (0.009) | (0.009) | (0.009) | (0.009) | |||||
| Concentrated disadvantage | −0.104 | −0.075 | −0.107 | −0.077 | −0.104 | −0.074 | −0.103 | −0.074 |
| (0.100) | (0.103) | (0.099) | (0.101) | |||||
| Residential stability | 0.169 † | 0.117 | 0.172 † | 0.119 | 0.174 † | 0.120 | 0.179 † | 0.123 |
| (0.094) | (0.094) | (0.094) | (0.094) | |||||
| Constant | 0.065 | 0.045 | 0.098 | 0.068 | 0.074 | 0.051 | 0.122 | 0.084 |
| (0.160) | (0.161) | (0.164) | (0.168) | |||||
| R-Square | 0.122 | 0.153 | 0.123 | 0.159 | ||||
| ∆R-Square | 0.031 ** | 0.036 ** | ||||||
| Dependent variable: | Model 1 (ND, married) | Model 2 (ND × married) | Model 3 (ND × married × Gene) | |||
| Log-transformed Inflammatory burden | b | β | b | β | b | β |
| Environment and Genetic Variables | ||||||
| Observer ratings of neighborhood disorder (ND) | 0.167 * | 0.115 | 0.215 * | 0.148 | 0.021 | 0.015 |
| (0.085) | (0.089) | (0.068) | ||||
| People married or single (1 = married) | 0.216 | 0.069 | 0.212 | 0.067 | 0.277 | 0.088 |
| (0.169) | (0.177) | (0.207) | ||||
| rs2228145 (1 = C:C or A:C) | 0.856 ** | 0.247 | ||||
| (0.292) | ||||||
| Gene-Environment Interaction | ||||||
| Neighborhood disorder × Married | −0.220 | −0.072 | −0.020 | −0.006 | ||
| (0.186) | (0.135) | |||||
| Neighborhood disorder × rs2228145 | 0.633 ** | 0.225 | ||||
| (0.167) | ||||||
| People married or single × rs2228145 | −0.472 | −0.089 | ||||
| (0.385) | ||||||
| Neighborhood disorder × Married × rs2228145 | −0.529 * | −0.066 | ||||
| (0.224) | ||||||
| Control Variables | ||||||
| Childhood adversity | 0.020 | 0.017 | 0.016 | 0.014 | 0.001 | 0.001 |
| (0.067) | (0.064) | (0.058) | ||||
| Family SES | 0.085 | 0.046 | 0.079 | 0.043 | 0.097 | 0.052 |
| (0.079) | (0.108) | (0.090) | ||||
| Age | 0.023 † | 0.099 | 0.024 † | 0.101 | 0.024 † | 0.100 |
| (0.013) | (0.014) | (0.013) | ||||
| South | −0.428 * | −0.139 | −0.437 * | −0.142 | −0.428 * | −0.139 |
| (0.184) | (0.176) | (0.186) | ||||
| Length of residence | −0.001 | −0.002 | −0.001 | −0.004 | 0.001 | 0.004 |
| (0.008) | (0.010) | (0.008) | ||||
| Neighborhood cohesion | 0.007 | 0.047 | 0.007 | 0.043 | 0.010 | 0.067 |
| (0.009) | (0.009) | (0.009) | ||||
| Concentrated disadvantage | −0.106 | −0.076 | −0.108 | −0.077 | −0.104 | −0.075 |
| (0.104) | (0.082) | (0.103) | ||||
| Residential stability | 0.163 † | 0.113 | 0.157 | 0.109 | 0.165 † | 0.114 |
| (0.096) | (0.083) | (0.093) | ||||
| Constant | 0.169 | 0.117 | 0.182 | 0.126 | 0.017 | 0.012 |
| (0.179) | (0.194) | (0.188) | ||||
| R-Square | 0.082 | 0.086 | 0.163 | |||
| ∆R-Square | 0.004 | 0.077 ** | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, M.-K.; Simons, R.L. The Association between Neighborhood Disorder and Health: Exploring the Moderating Role of Genotype and Marriage. Int. J. Environ. Res. Public Health 2021, 18, 898. https://doi.org/10.3390/ijerph18030898
Lei M-K, Simons RL. The Association between Neighborhood Disorder and Health: Exploring the Moderating Role of Genotype and Marriage. International Journal of Environmental Research and Public Health. 2021; 18(3):898. https://doi.org/10.3390/ijerph18030898
Chicago/Turabian StyleLei, Man-Kit, and Ronald L. Simons. 2021. "The Association between Neighborhood Disorder and Health: Exploring the Moderating Role of Genotype and Marriage" International Journal of Environmental Research and Public Health 18, no. 3: 898. https://doi.org/10.3390/ijerph18030898
APA StyleLei, M.-K., & Simons, R. L. (2021). The Association between Neighborhood Disorder and Health: Exploring the Moderating Role of Genotype and Marriage. International Journal of Environmental Research and Public Health, 18(3), 898. https://doi.org/10.3390/ijerph18030898






