Additive Manufacturing of Resected Oral and Oropharyngeal Tissue: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Care
2.3. Additive Manufacturing
2.4. Multidisciplinary Tumor Board Meeting
3. Results
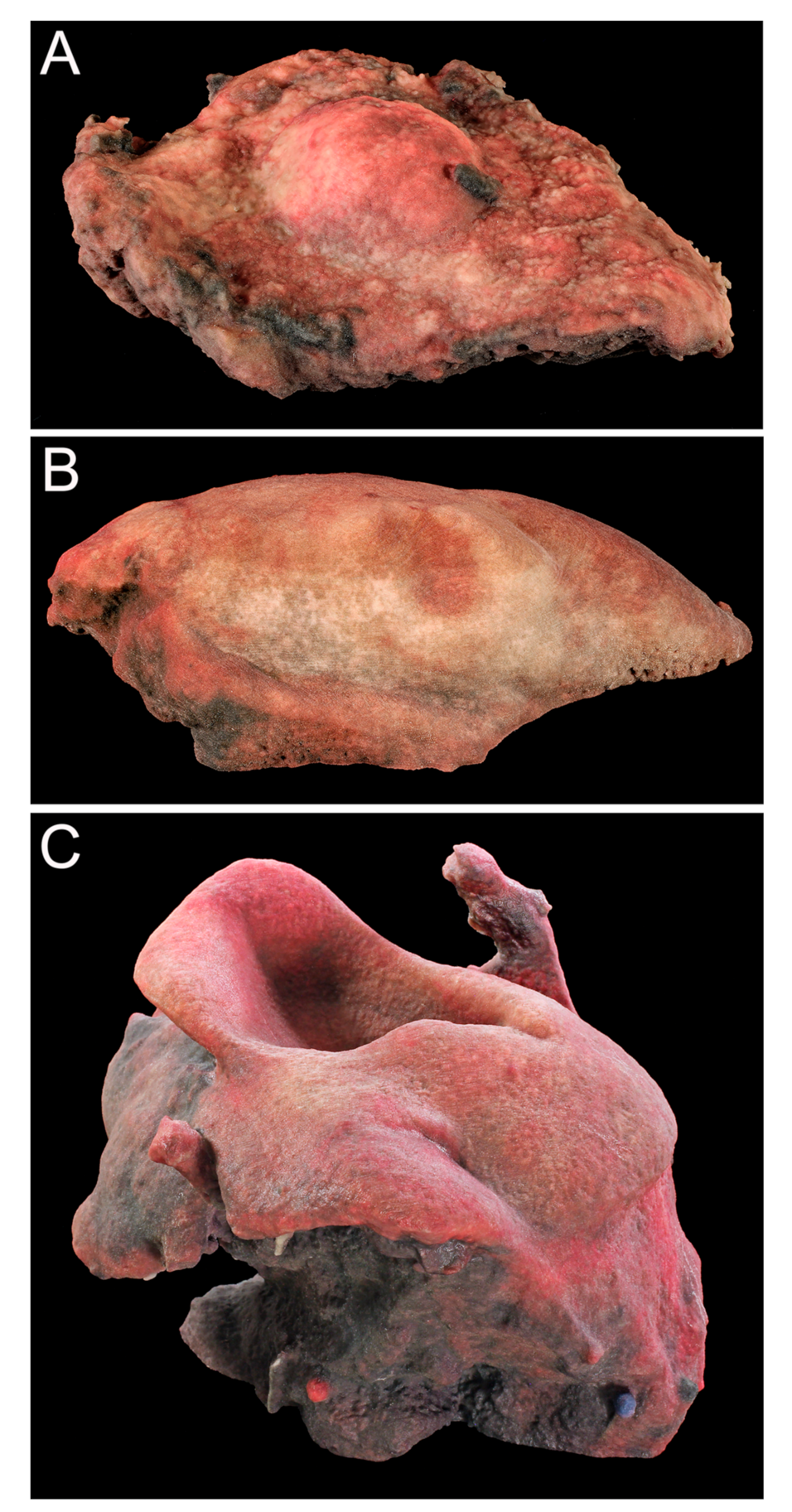
3.1. Case 1
3.2. Case 2
3.3. Case 3
3.4. Digital Reconstruction and Additive Manufacturing Process
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witowski, J.S.; Pędziwiatr, M.; Major, P.; Budzyński, A. Cost-effective, personalized, 3D-printed liver model for preoperative planning before laparoscopic liver hemihepatectomy for colorectal cancer metastases. Int. J. CARS 2017, 12, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Skewes, J.; Woodruff, M.A.; Dasgupta, P.; Rukin, N.J. Multi-colour extrusion fused deposition modelling: A low-cost 3D printing method for anatomical prostate cancer models. Sci. Rep. 2020, 10, 10004. [Google Scholar] [CrossRef] [PubMed]
- Wake, N.; Rosenkrantz, A.B.; Huang, R.; Park, K.U.; Wysock, J.S.; Taneja, S.S.; Huang, W.C.; Daniel, L.; Chandarana, H. Patient-specific 3D printed and augmented reality kidney and prostate cancer models: Impact on patient education. 3D Print. Med. 2019, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.H.; Siewerdsen, J.H.; Vescan, A.; Daly, M.J.; Prisman, E.; Irish, J.C. 3D rapid prototyping for otolaryngology—Head and neck surgery: Applications in image-guidance, surgical simulation and patient-specific modeling. PLoS ONE 2015, 10, e0136370. [Google Scholar] [CrossRef]
- Subburaj, K.; Nair, C.; Rajesh, S.; Meshram, S.; Ravi, B. Rapid development of auricular prosthesis using CAD and rapid prototyping technologies. Int. J. Oral Maxillofac. Surg. 2007, 36, 938–943. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Y.; Li, Y.S.; Gui, L.; Mao, C.; Chen, Y.-N.; Zhao, J.-Z. Application of CTA and CAD\CAM techniques in mandible reconstruction with free fibula flap. Zhonghua Zheng Xing Wai Ke Za Zhi 2006, 22, 325–327. [Google Scholar]
- Abraham, J. Imaging for head and neck cancer. Surg. Oncol. Clin. N. Am. 2015, 24, 455–471. [Google Scholar] [CrossRef]
- Alberico, R.A.; Husain, S.H.; Sirotkin, I. Imaging in head and neck oncology. Surg. Oncol. Clin. N. Am. 2004, 13, 13–35. [Google Scholar] [CrossRef]
- Huotilainen, E.; Paloheimo, M.; Salmi, M.; Paloheimo, K.-S.; Björkstrand, R.; Tuomi, J.; Markkola, A.; Mäkitie, A.A. Imaging requirements for medical applications of additive manufacturing. Acta Radiol. 2014, 55, 78–85. [Google Scholar] [CrossRef]
- Fernandez, R.; Lau, R.; Yu, P.; Siu, I.; Chan, J.; Ng, C. Use of custom made 3-dimensional printed surgical guide for manubrio-sternal resection of solitary breast cancer metastasis: Case report. AME Case Rep. 2020, 4, 12. [Google Scholar] [CrossRef]
- Wu, Z.; Alzuhair, A.; Kim, H.; Lee, J.W.; Chung, I.Y.; Kim, J.; Lee, S.B.; Son, B.H.; Gong, G.; Kim, H.H.; et al. Magnetic resonance imaging based 3-dimensional printed breast surgical guide for breast-conserving surgery in ductal carcinoma in situ: A clinical trial. Sci. Rep. 2020, 10, 18534. [Google Scholar] [CrossRef] [PubMed]
- Hovens, M.C.; Lo, K.; Kerger, M.; Pedersen, J.; Nottle, T.; Kurganovs, N.; Ryan, A.; Peters, J.S.; Moon, D.; Costello, A.J.; et al. 3D modelling of radical prostatectomy specimens: Developing a method to quantify tumor morphometry for prostate cancer risk prediction. Pathol. Res. Pract. 2017, 213, 1523–1529. [Google Scholar] [CrossRef] [PubMed]
- Pischat, J.; Iglesias, J.E.; Yousry, T.; Ourselin, S.; Modat, M. A survey of methods for 3D histology reconstruction. Med. Image Anal. 2018, 46, 73–105. [Google Scholar] [CrossRef]
- Pawlaczyk-Kamieńska, T.; Winiarska, H.; Kulczyk, T.; Cofta, S. Dental Anomalies in Rare, Genetic Ciliopathic Disorder-A Case Report and Review of Literature. Int. J. Environ. Res. Public Health 2020, 17, 4337. [Google Scholar] [CrossRef] [PubMed]
- Pawlaczyk-Kamieńska, T.; Kulczyk, T.; Pawlaczyk-Wróblewska, E.; Borysewicz-Lewicka, M.; Niedziela, M. Limited Mandibular Movements as a Consequence of Unilateral or Asymmetrical Temporomandibular Joint Involvement in Juvenile Idiopathic Arthritis Patients. J. Clin. Med. 2020, 9, 2576. [Google Scholar] [CrossRef]
- Mäkitie, A.A.; Salmi, M.; Lindford, A.; Tuomi, J.; Lassus, P. Three-dimensional printing for restoration of the donor face: A new digital technique tested and used in the first facial allotransplantation patient in Finland. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 1648–1652. [Google Scholar] [CrossRef]
- Ranzuglia, G.; Callieri, M.; Dellepiane, M.; Cignoni, P.; Scopigno, R. MeshLab as a Complete Tool for the Integration of Photos and Color with High Resolution 3D Geometry Data. Paper Presented at the 40th Conference in Computer Applications and Quantitative Methods in Archaeology, Southampton, UK, March 2012; Available online: http://vcg.isti.cnr.it/Publications/2013/RCDCS13/MeshLab_Color.pdf (accessed on 1 August 2018).
- Guijarro-Martinez, R.; Gellrich, N.C.; Witte, J.; Tapioles, D.; Von Briel, C.; Kolotas, C.; Achinger, J.; Hailemariam, S.; Schulte, H.; Rohner, D.; et al. Optimization of the interface between radiology, surgery, radiotherapy, and pathology in head and neck tumor surgery: A navigation-assisted multidisciplinary network. Int. J. Oral Maxillofac. Surg. 2014, 43, 156–162. [Google Scholar] [CrossRef]
- Spiro, R.H.; Guillamondegui, O.; Paulino, A.F.; Huvos, A.G. Pattern of invasion and margin assessment in patients with oral tongue cancer. Head Neck 1999, 21, 408–413. [Google Scholar] [CrossRef]
- Sutton, D.N.; Brown, J.S.; Rogers, S.N.; Vaughan, E.; Woolgar, J. The prognostic implications of the surgical margin in oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2003, 32, 30–34. [Google Scholar] [CrossRef]
- Binahmed, A.; Nason, R.W.; Abdoh, A.A. The clinical significance of the positive surgical margin in oral cancer. Oral Oncol. 2007, 43, 780–784. [Google Scholar] [CrossRef]
- Slootweg, P.J.; Hordijk, G.J.; Schade, Y.; Van Es, R.J.; Koole, R. Treatment failure and margin status in head and neck cancer: A critical view on the potential value of molecular pathology. Oral Oncol. 2002, 38, 500–503. [Google Scholar] [CrossRef]
- Trivedi, H.; Turkbey, B.; Rastinehad, A.R.; Benjamin, C.J.; Bernardo, M.; Pohida, T.J.; Shah, V.; Merino, M.J.; Wood, B.J.; Linehan, W.M.; et al. Use of patient-specific MRI-based prostate mold for validation of multiparametric MRI in localization of prostate cancer. Urology 2012, 79, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Pohida, T.; Turkbey, B.; Mani, H.; Merino, M.; Pinto, P.A.; Choyke, P.; Bernardo, M. A method for correlating in vivo prostate magnetic resonance imaging and histopathology using individualized magnetic resonance-based molds. Rev. Sci. Instrum. 2009, 80, 104301. [Google Scholar] [CrossRef]
- Kiessling, F.; Le-Huu, M.; Kunert, T.; Thorn, M.; Vosseler, S.; Schmidt, K.; Hoffend, J.; Meinzer, H.-P.; Fusenig, N.E.; Semmler, W. Improved correlation of histological data with DCE MRI parameter maps by 3D reconstruction, reslicing and parameterization of the histological images. Eur. Radiol. 2005, 15, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.N.; Chatzinoff, Y.; Passoni, N.M.; Kapur, P.; Roehrborn, C.G.; Xi, Y.; Rofsky, N.M.; Torrealba, J.; Francis, F.; Futch, C.; et al. Improved magnetic resonance imaging-pathology correlation with imaging-derived, 3Dprinted, patient-specific whole-mount molds of the prostate. Investig. Radiol. 2017, 52, 507–513. [Google Scholar] [CrossRef]
- Koivuholma, A.; Aro, K.; Mäkitie, A.; Salmi, M.; Mirtti, T.; Hagström, J.; Atula, T. Three-Dimensional Presentation of Tumor Histopathology: A Model Using Tongue Squamous Cell Carcinoma. Diagnostics 2021, 11, 109. [Google Scholar] [CrossRef]
- Xiao, J.; Huang, W.; Yang, X.; Yan, W.-J.; Song, D.-W.; Wei, H.-F.; Liu, T.-L.; Wu, Z.-P.; Yang, C. En Bloc Resection of Primary Malignant Bone Tumor in the Cervical Spine Based on 3-Dimensional Printing Technology. Orthop. Surg. 2016, 8, 171–178. [Google Scholar] [CrossRef]
- Al Jabbari, O.; Saleh, A.; Patel, A.P.; Igo, S.R.; Reardon, M.J. Use of three-dimensional models to assist in the resection of malignant cardiac tumors. J. Card. Surg. 2016, 31, 581–583. [Google Scholar] [CrossRef]
- Kim, M.P.; Ta, A.H.; Ellsworth, W.A.; Marco, R.A.; Gaur, P.; Miller, J.S. Three-dimensional model for surgical planning in resection of thoracic tumors. Int. J. Surg. Case Rep. 2015, 16, 127–129. [Google Scholar] [CrossRef][Green Version]
- Yang, Y.; Zhou, Z.; Liu, R.; Chen, L.; Xiang, H.; Chen, N. Application of 3D visualization and 3D printing technology on ERCP for patients with hilar cholangiocarcinoma. Exp. Ther. Med. 2018, 15, 3259–3264. [Google Scholar] [CrossRef]
- Huotilainen, E.; Salmi, M.; Lindahl, J. Three-dimensional printed surgical templates for fresh cadaveric osteochondral allograft surgery with dimension verification by multivariate computed tomography analysis. Knee 2019, 26, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, A.B.V.; Salmi, M.; Vallittu, P.; Serlo, W.; Tuomi, J.; Mäkitie, A.A. Main Clinical Use of Additive Manufacturing (Three-Dimensional Printing) in Finland Restricted to the Head and Neck Area in 2016–2017. Scand. J. Surg. 2019. [Google Scholar] [CrossRef] [PubMed]







| Case | Photographs Aligned/Total | Stereophotogrammetry Processing Time (h) | Scaling Factor | Print Time (h:min) | Print Volume/Material Consumption (cm3) |
|---|---|---|---|---|---|
| 1 | 46/63 | 7 | 2.5 | 2:01 | 139.92 |
| 2 | 111/117 | 16 | 2.0 | 3:39 | 227.63 |
| 3 | 194/195 | 24 | 1.5 | 9:09 | 976.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irace, A.L.; Koivuholma, A.; Huotilainen, E.; Hagström, J.; Aro, K.; Salmi, M.; Markkola, A.; Sistonen, H.; Atula, T.; Mäkitie, A.A. Additive Manufacturing of Resected Oral and Oropharyngeal Tissue: A Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 911. https://doi.org/10.3390/ijerph18030911
Irace AL, Koivuholma A, Huotilainen E, Hagström J, Aro K, Salmi M, Markkola A, Sistonen H, Atula T, Mäkitie AA. Additive Manufacturing of Resected Oral and Oropharyngeal Tissue: A Pilot Study. International Journal of Environmental Research and Public Health. 2021; 18(3):911. https://doi.org/10.3390/ijerph18030911
Chicago/Turabian StyleIrace, Alexandria L., Anne Koivuholma, Eero Huotilainen, Jaana Hagström, Katri Aro, Mika Salmi, Antti Markkola, Heli Sistonen, Timo Atula, and Antti A. Mäkitie. 2021. "Additive Manufacturing of Resected Oral and Oropharyngeal Tissue: A Pilot Study" International Journal of Environmental Research and Public Health 18, no. 3: 911. https://doi.org/10.3390/ijerph18030911
APA StyleIrace, A. L., Koivuholma, A., Huotilainen, E., Hagström, J., Aro, K., Salmi, M., Markkola, A., Sistonen, H., Atula, T., & Mäkitie, A. A. (2021). Additive Manufacturing of Resected Oral and Oropharyngeal Tissue: A Pilot Study. International Journal of Environmental Research and Public Health, 18(3), 911. https://doi.org/10.3390/ijerph18030911








