The Intersection of Rural Residence and Minority Race/Ethnicity in Cancer Disparities in the United States
Abstract
:1. Introduction
2. A Conceptual Framework
3. Social Determinants of Health and Their Role in Rural and Racial/Ethnic Disparities in Cancer
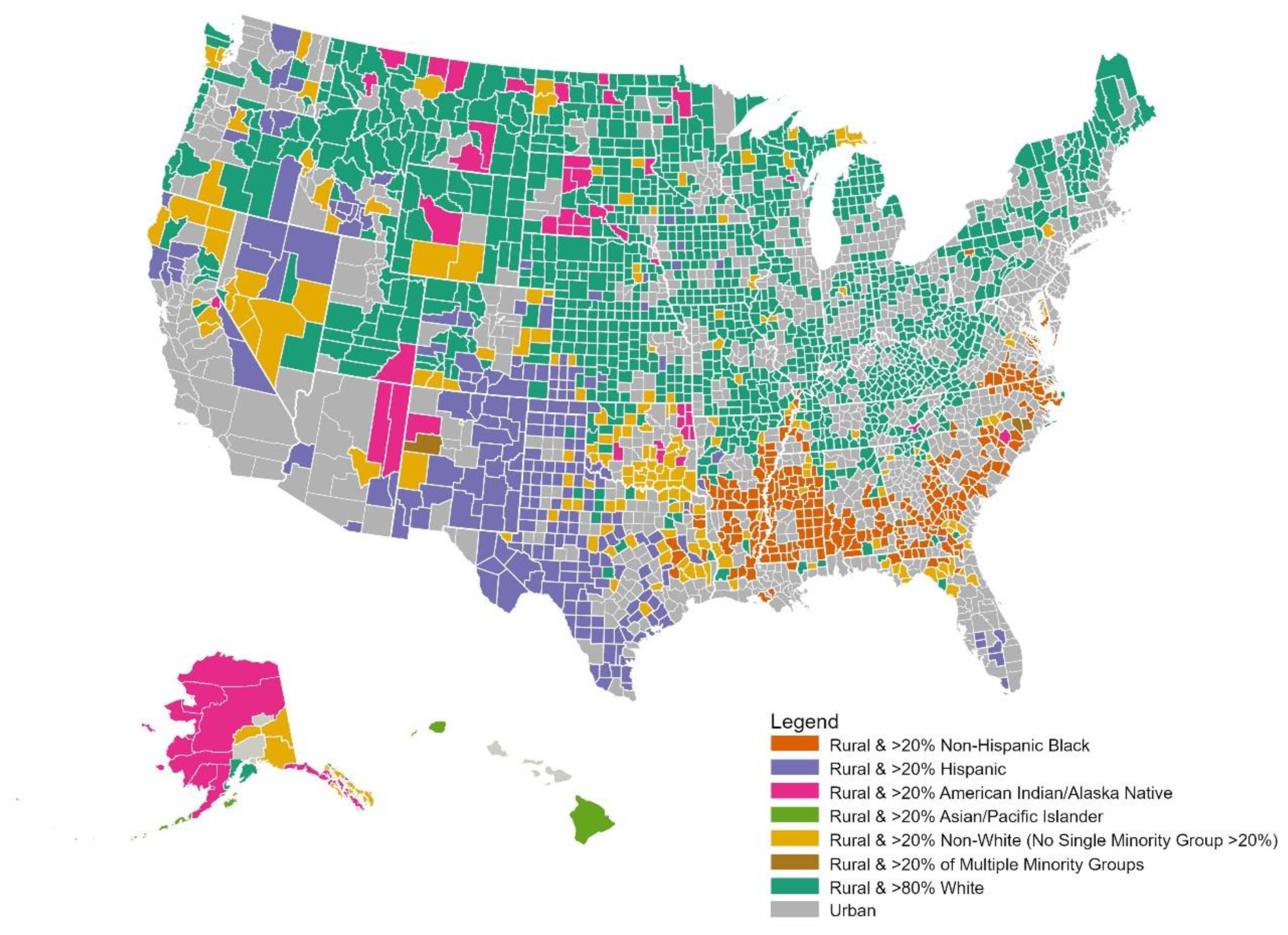
3.1. Structural Racism and Residential Segregation
3.2. Access to Healthcare Services
3.3. Socioeconomic Status, Educational Attainment, Housing, and Infrastructure
3.4. Environmental Factors
4. Rural and Racial/Ethnic Disparities in Cancer Across the Continuum
4.1. Risk Factors and Primary Prevention
4.2. Cancer Screening
4.3. Cancer Incidence and Staging
4.4. Cancer Treatment
4.5. Cancer Survivorship
4.6. Cancer Mortality and Survival
5. Implications/Recommendations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, K.J.; Borders, T.F.; Holmes, G.M.; Kozhimannil, K.B.; Ziller, E. What Is Rural? Challenges And Implications Of Definitions That Inadequately Encompass Rural People And Places. Health Aff. (Proj. Hope) 2019, 38, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Doogan, N.J.; Roberts, M.E.; Wewers, M.E.; Stanton, C.A.; Keith, D.R.; Gaalema, D.E.; Kurti, A.N.; Redner, R.; Cepeda-Benito, A.; Bunn, J.Y.; et al. A growing geographic disparity: Rural and urban cigarette smoking trends in the United States. Prev. Med. 2017, 104, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Croft, J.B.; Liu, Y.; Lu, H.; Kanny, D.; Wheaton, A.G.; Cunningham, T.J.; Khan, L.K.; Caraballo, R.S.; Holt, J.B.; et al. Health-Related Behaviors by Urban-Rural County Classification—United States, 2013. Mmwr. Surveill Summ. 2017, 66, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Walker, T.Y.; Elam-Evans, L.D.; Yankey, D.; Fredua, B.; Saraiya, M.; Stokley, S. Factors associated with not receiving HPV vaccine among adolescents by metropolitan statistical area status, United States, National Immunization Survey–Teen, 2016–2017. Hum. Vaccines Immunother. 2020, 16, 562–572. [Google Scholar] [CrossRef]
- Zahnd, W.E.; James, A.S.; Jenkins, W.D.; Izadi, S.R.; Fogleman, A.J.; Steward, D.E.; Colditz, G.A.; Brard, L. Rural-Urban Differences in Cancer Incidence and Trends in the United States. Cancer Epidemiol. Biomark. Prev. 2018, 27, 1265–1274. [Google Scholar] [CrossRef] [Green Version]
- Henley, S.J.; Anderson, R.N.; Thomas, C.C.; Massetti, G.M.; Peaker, B.; Richardson, L.C. Invasive Cancer Incidence, 2004–2013, and Deaths, 2006–2015, in Nonmetropolitan and Metropolitan Counties—United States. Mmwr. Surveill Summ. 2017, 66, 1–13. [Google Scholar] [CrossRef]
- Cole, A.M.; Jackson, J.E.; Doescher, M. Colorectal cancer screening disparities for rural minorities in the United States. J. Prim. Care Community Health 2013, 4, 106–111. [Google Scholar] [CrossRef]
- Atkins, G.T.; Kim, T.; Munson, J. Residence in Rural Areas of the United States and Lung Cancer Mortality. Disease Incidence, Treatment Disparities, and Stage-Specific Survival. Ann. Am. Thorac. Soc. 2017, 14, 403–411. [Google Scholar] [CrossRef]
- Alvidrez, J.; Castille, D.; Laude-Sharp, M.; Rosario, A.; Tabor, D. The National Institute on Minority Health and Health Disparities Research Framework. Am. J. Public Health 2019, 109, S16–S20. [Google Scholar] [CrossRef]
- Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity|The White House. Available online: https://obamawhitehouse.archives.gov/omb/fedreg_1997standards (accessed on 27 September 2020).
- Jones, D.S. The persistence of American Indian health disparities. Am. J. Public Health 2006, 96, 2122–2134. [Google Scholar] [CrossRef]
- Philbin, M.M.; Flake, M.; Hatzenbuehler, M.L.; Hirsch, J.S. State-level immigration and immigrant-focused policies as drivers of Latino health disparities in the United States. Soc. Sci. Med. 2018, 199, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Findling, M.G.; Bleich, S.N.; Casey, L.S.; Blendon, R.J.; Benson, J.M.; Sayde, J.M.; Miller, C. Discrimination in the United States: Experiences of Latinos. Health Serv. Res. 2019, 54, 1409–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichter, D.T.; Johnson, K.M. A Demographic Lifeline? Immigration and Hispanic Population Growth in Rural America. Popul. Res. Policy Rev. 2020, 39, 785–803. [Google Scholar] [CrossRef]
- Gee, G.C.; Ro, A.; Shariff-Marco, S.; Chae, D. Racial discrimination and health among asian americans: Evidence, assessment, and directions for future research. Epidemiol. Rev. 2009, 31, 130–151. [Google Scholar] [CrossRef]
- McMurtry, C.L.; Findling, M.G.; Casey, L.S.; Blendon, R.J.; Benson, J.M.; Sayde, J.M.; Miller, C. Discrimination in the United States: Experiences of Asian Americans. Health Serv. Res. 2019, 54, 1419–1430. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, J.T.; Ford, C.L.; Wallace, S.P.; Wang, M.C.; Takahashi, L.M. Racial and ethnic residential segregation and access to health care in rural areas. Health Place 2017, 43, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Shariff-Marco, S.; Klassen, A.C.; Bowie, J.V. Racial/ethnic differences in self-reported racism and Its association with cancer-related health behaviors. Am. J. Public Health 2010, 100, 364–374. [Google Scholar] [CrossRef]
- Morello-Frosch, R.; Jesdale, B.M. Separate and unequal: Residential segregation and estimated cancer risks associated with ambient air toxins in U.S. metropolitan areas. Environ. Health Perspect. 2006, 114, 386–393. [Google Scholar] [CrossRef] [Green Version]
- Fang, P.; He, W.; Gomez, D.; Hoffman, K.E.; Smith, B.D.; Giordano, S.H.; Jagsi, R.; Smith, G.L. Racial disparities in guideline-concordant cancer care and mortality in the United States. Adv. Radiat Oncol. 2018, 3, 221–229. [Google Scholar] [CrossRef]
- Landrine, H.; Corrall, I.; Lee, J.G.L.; Efird, J.; Hall, M.B.; Bess, J.J. Residential Segregation and Racial Cancer Disparities: A Systematic Review. J. Racial Ethn. Health Disparities 2017, 4, 1195–1205. [Google Scholar] [CrossRef]
- Pruitt, S.L.; Lee, S.J.; Tiro, J.A.; Xuan, L.; Ruiz, J.M.; Inrig, S. Residential racial segregation and mortality among black, white, and Hispanic urban breast cancer patients in Texas, 1995 to 2009. Cancer 2015, 121, 1845–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, E.T.; Gomez, S.L. Impact of neighborhood racial composition and metropolitan residential segregation on disparities in breast cancer stage at diagnosis and survival between black and white women in California. J. Community Health 2010, 35, 398–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, D.R.; Kontos, E.Z.; Viswanath, K.; Haas, J.S.; Lathan, C.S.; MacConaill, L.E.; Chen, J.; Ayanian, J.Z. Integrating Multiple Social Statuses in Health Disparities Research: The Case of Lung Cancer. Health Serv. Res. 2012, 47, 1255–1277. [Google Scholar] [CrossRef] [PubMed]
- Zavala, V.A.; Bracci, P.M.; Carethers, J.M.; Carvajal-Carmona, L.; Coggins, N.B.; Cruz-Correa, M.R.; Davis, M.; de Smith, A.J.; Dutil, J.; Figueiredo, J.C.; et al. Cancer health disparities in racial/ethnic minorities in the United States. Br. J. Cancer 2020, 124, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.T.; Ford, C.L.; Wallace, S.P.; Wang, M.C.; Takahashi, L.M. Intersection of Living in a Rural Versus Urban Area and Race/Ethnicity in Explaining Access to Health Care in the United States. Am. J. Public Health 2016, 106, 1463–1469. [Google Scholar] [CrossRef]
- Green, M.A.; Evans, C.R.; Subramanian, S.V. Can intersectionality theory enrich population health research? Soc. Sci. Med. 2017, 178, 214–216. [Google Scholar] [CrossRef]
- Pender, J.; Hertz, T.; Cromartie, J.; Farrigan, T. Rural America at a Glance, 2019 Edition. Economic Information Bulletin No. (EIB-212). Economic Research Service, United States Department of Agriculture. Available online: https://www.ers.usda.gov/publications/pub-details/?pubid=95340 (accessed on 9 December 2020).
- Lichter, D.T. Immigration and the New Racial Diversity in Rural America. Rural Sociol. 2012, 77, 3–35. [Google Scholar] [CrossRef] [Green Version]
- USDA ERS—Rural Classifications. Available online: https://www.ers.usda.gov/topics/rural-economy-population/rural-classifications/ (accessed on 9 December 2020).
- U.S. Census Bureau. Population Estimates Program. Available online: https://www2.census.gov/programs-surveys/popest/datasets/2010-2018/ (accessed on 9 December 2020).
- Foutz, J.; Artiga, S.; Garfield, R. The Role of Medicaid in Rural America. 25 April 2017. Available online: https://www.kff.org/medicaid/issue-brief/the-role-of-medicaid-in-rural-america/ (accessed on 9 December 2020).
- Hendryx, M.; Luo, J. Increased Cancer Screening for Low-income Adults Under the Affordable Care Act Medicaid Expansion. Med. Care 2018, 56, 944–949. [Google Scholar] [CrossRef]
- Sineshaw, H.M.; Ellis, M.A.; Yabroff, K.R.; Han, X.; Jemal, A.; Day, T.A.; Graboyes, E.M. Association of Medicaid Expansion Under the Affordable Care Act With Stage at Diagnosis and Time to Treatment Initiation for Patients With Head and Neck Squamous Cell Carcinoma. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 247–255. [Google Scholar] [CrossRef]
- Gee, G.C.; Ford, C.L. Structural racism and health inequities: Old Issues, New Directions. Du Bois Rev. 2011, 8, 115–132. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.R.; Lawrence, J.A.; Davis, B.A. Racism and Health: Evidence and Needed Research. Annu. Rev. Public Health 2019, 40, 105–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahnd, W.E.; McLafferty, S.L.; Eberth, J.M. Multilevel analysis in rural cancer control: A conceptual framework and methodological implications. Prev. Med. 2019, 129, 105835. [Google Scholar] [CrossRef] [PubMed]
- Wingo, P.A.; Howe, H.L.; Thun, M.J.; Ballard-Barbash, R.; Ward, E.; Brown, M.L.; Sylvester, J.; Friedell, G.H.; Alley, L.; Rowland, J.H.; et al. A national framework for cancer surveillance in the United States. Cancer Causes Control. 2005, 16, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Probst, J.C.; Bellinger, J.D.; Walsemann, K.M.; Hardin, J.; Glover, S.H. Higher risk of death in rural blacks and whites than urbanites is related to lower incomes, education, and health coverage. Health Aff. 2011, 30, 1872–1879. [Google Scholar] [CrossRef]
- Probst, J.C.; Moore, C.G.; Glover, S.H.; Samuels, M.E. Person and place: The compounding effects of race/ethnicity and rurality on health. Am. J. Public Health 2004, 94, 1695–1703. [Google Scholar] [CrossRef]
- Probst, J.C.; Zahnd, W.E.; Hung, P.; Eberth, J.M.; Crouch, E.L.; Merrell, M.A. Rural-Urban Mortality Disparities: Variations Across Causes of Death and Race/Ethnicity, 2013–2017. Am. J. Public Health 2020, 110, 1325–1327. [Google Scholar] [CrossRef]
- Feagin, J.; Bennefield, Z. Systemic racism and U.S. health care. Soc. Sci. Med. 2014, 103, 7–14. [Google Scholar] [CrossRef]
- Williams, D.R.; Neighbors, H.W.; Jackson, J.S. Racial/ethnic discrimination and health: Findings from community studies. Am. J. Public Health 2003, 93, 200–208. [Google Scholar] [CrossRef]
- Acevedo-Garcia, D.L.K. Residential Segregation and Health. In Neighborhoods and Health; Kawachi, I., Berkman, L.F., Eds.; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Acevedo-Garcia, D.; Lochner, K.A.; Osypuk, T.L.; Subramanian, S.V. Future directions in residential segregation and health research: A multilevel approach. Am. J. Public Health 2003, 93, 215–221. [Google Scholar] [CrossRef]
- Krieger, N.; Jahn, J.L.; Waterman, P.D.; Chen, J.T. Breast Cancer Estrogen Receptor Status According to Biological Generation: US Black and White Women Born 1915–1979. Am. J. Epidemiol. 2018, 187, 960–970. [Google Scholar] [CrossRef]
- Krieger, N.; Wright, E.; Chen, J.T.; Waterman, P.D.; Huntley, E.R.; Arcaya, M. Cancer Stage at Diagnosis, Historical Redlining, and Current Neighborhood Characteristics: Breast, Cervical, Lung, and Colorectal Cancers, Massachusetts, 2001–2015. Am. J. Epidemiol. 2020, 189, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Massey, D.S.; Denton, N.A. Hypersegregation in U.S. Metropolitan Areas: Black and Hispanic Segregation along Five Dimensions. Demography 1989, 26, 373–391. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Wilson, R.F. Social determinants of health among African Americans in a rural community in the Deep South: An ecological exploration. Rural Remote Health 2011, 11, 196. [Google Scholar] [CrossRef]
- Menon, N.M.; Leslie, T.F.; Frankenfeld, C.L. Cancer-related diagnostic and treatment capabilities of hospitals in the context of racial residential segregation. Public Health 2020, 182, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.S.; Earle, C.C.; Orav, J.E.; Brawarsky, P.; Neville, B.A.; Williams, D.R. Racial segregation and disparities in cancer stage for seniors. J. Gen. Intern. Med. 2008, 23, 699–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, Y.; Landrine, H.; Smith, T.; Kaw, C.; Corral, I.; Stein, K. Residential Segregation and Disparities in Health-Related Quality of Life Among Black and White Cancer Survivors. Health Psychol. 2011, 30, 137–144. [Google Scholar] [CrossRef]
- Hayanga, A.J.; Zeliadt, S.B.; Backhus, L.M. Residential segregation and lung cancer mortality in the United States. Arch. Surg. 2013, 148, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Moss, J.L.; Ehrenkranz, R.; Perez, L.G.; Hair, B.Y.; Julian, A.K. Geographic disparities in cancer screening and fatalism among a nationally representative sample of US adults. J. Epidemiol. Community Health 2019, 73, 1128–1135. [Google Scholar] [CrossRef]
- James, C.V.; Moonesinghe, R.; Wilson-Frederick, S.M.; Hall, J.E.; Penman-Aguilar, A.; Bouye, K. Racial/ethnic health disparities among rural adults—United States, 2012–2015. Mmwr. Surveill. Summ. 2017, 66, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Penchansky, R.; Thomas, J.W. The concept of access: Definition and relationship to consumer satisfaction. Med. Care 1981, 19, 127–140. [Google Scholar] [CrossRef]
- Largent, E.A. Public Health, Racism, and the Lasting Impact of Hospital Segregation. Public Health Rep. 2018, 133, 715–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Government Accountability Office. Rural Hospitals: Federal Efforts Should Target Areas Where Closures Would Threaten Access to Care. Available online: https://www.gao.gov/assets/160/150195.pdf (accessed on 29 January 2021).
- Thomas, S.R.; Holmes, G.M.; Pink, G.H. To What Extent do Community Characteristics Explain Differences in Closure among Financially Distressed Rural Hospitals? J. Health Care Poor Underserved 2016, 27, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Hung, P.; Deng, S.; Zahnd, W.E.; Adams, S.A.; Olatosi, B.; Crouch, E.L.; Eberth, J.M. Geographic disparities in residential proximity to colorectal and cervical cancer care providers. Cancer 2019, 126, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Onega, T.; Alford-Teaster, J.; Wang, F. Population-based geographic access to parent and satellite National Cancer Institute Cancer Center Facilities. Cancer 2017, 123, 3305–3311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grogan, C.M.; Park, S.E. The racial divide in state medicaid expansions. J. Health Politics Policy Law 2017, 42, 539–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hastert, T.A.; Banegas, M.P.; Hamel, L.M.; Reed, A.R.; Baird, T.; Beebe-Dimmer, J.L.; Schwartz, A.G. Race, financial hardship, and limiting care due to cost in a diverse cohort of cancer survivors. J. Cancer Surviv. 2019, 13, 429–437. [Google Scholar] [CrossRef]
- Prather, C.; Fuller, T.R.; Jeffries, W.L.; Marshall, K.J.; Howell, A.V.; Belyue-Umole, A.; King, W. Racism, African American Women, and Their Sexual and Reproductive Health: A Review of Historical and Contemporary Evidence and Implications for Health Equity. Health Equity 2018, 2, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, E.A.; Mendenhall, E.; McAlearney, A.S.; Rolle, I.; Whitaker, E.E.; Warnecke, R.; Ferrans, C.E. An exploratory study of how trust in health care institutions varies across African American, Hispanic and white populations. Commun. Med. 2011, 8, 89–98. [Google Scholar] [CrossRef]
- Hoffman, K.M.; Trawalter, S.; Axt, J.R.; Oliver, M.N. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc. Natl. Acad. Sci. USA 2016, 113, 4296–4301. [Google Scholar] [CrossRef] [Green Version]
- Takeshita, J.; Wang, S.; Loren, A.W.; Mitra, N.; Shults, J.; Shin, D.B.; Sawinski, D.L. Association of Racial/Ethnic and Gender Concordance Between Patients and Physicians With Patient Experience Ratings. JAMA Netw. Open 2020, 3, e2024583. [Google Scholar] [CrossRef]
- American Association of Medical Colleges. Diversity in Medicine: Facts and Figures 2019. Available online: https://www.aamc.org/data-reports/workforce/interactive-data/figure-13-percentage-us-medical-school-graduates-race/ethnicity-alone-academic-year-2018-2019 (accessed on 26 January 2021).
- Butkus, R.; Rapp, K.; Cooney, T.G.; Engel, L.S. Envisioning a Better U.S. Health Care System for All: Reducing Barriers to Care and Addressing Social Determinants of Health. Ann. Intern. Med. 2020, 172, S50–S59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labor Force Characteristics by Race and Ethnicity, 2018: BLS Reports: U.S. Bureau of Labor Statistics. Available online: https://www.bls.gov/opub/reports/race-and-ethnicity/2018/home.htm (accessed on 28 November 2020).
- Charlton, M.; Schlichting, J.; Chioreso, C.; Ward, M.; Vikas, P. Challenges of Rural Cancer Care in the United States. Oncol. (Williston Park) 2015, 29, 633–640. [Google Scholar]
- Probst, J.; Eberth, J.M.; Crouch, E. Structural Urbanism Contributes to Poorer Health Outcomes For Rural America. Health Aff. (Proj. Hope) 2019, 38, 1976–1984. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.K.; Daus, G.P.; Allender, M.; Ramey, C.T.; Martin, E.K.; Perry, C.; Reyes, A.A.L.; Vedamuthu, I.P. Social Determinants of Health in the United States: Addressing Major Health Inequality Trends for the Nation, 1935–2016. Int. J. Mch. Aids 2017, 6, 139–164. [Google Scholar] [CrossRef] [PubMed]
- Hicken, M.T.; Burnside, L.; Edwards, D.L.; Lee, H. Black–White Health Inequalities by Intentional Design: The Lasting Health Impact of Racial Residential Segregation. In Racism: Science & Tools for the Public Health Professional; Ford, C., Ed.; APHA Press: Washington, DC, USA, 2019; pp. 117–132. [Google Scholar]
- Parisi, D.; Grice, S.M.; Taquino, M.; Gill, D.A. Community Concentration of Poverty and Its Consequences on Nonmetro persistence of poverty in Mississippi. Sociol. Spectrum. 2005, 5, 469–483. [Google Scholar] [CrossRef]
- Quillian, L. Does Segregation Create Winners and Losers? Residential Segregation and Inequality in Educational Attainment. Soc. Probl. 2014, 61, 402–426. [Google Scholar] [CrossRef]
- Probst, J.C.; Ajmal, F. Social Determinants of Health among Rural Asian and Pacific Islander Populations. Policy Brief. July 2019. Available online: https://www.ruralhealthresearch.org/publications/1271 (accessed on 9 December 2020).
- Probst, J.C.; Ajmal, F. Social Determinants of Health among the Rural Hispanic Population. Policy Brief. July 2019. Available online: https://www.ruralhealthresearch.org/publications/1269 (accessed on 9 December 2020).
- Probst, J.C.; Ajmal, F. Social Determinants of Health among the Rural African American population. Policy Brief. July 2019. Available online: https://www.ruralhealthresearch.org/publications/1268 (accessed on 9 December 2020).
- Probst, J.C.; Ajmal, F. Social Determinants of Health among Rural American Indian and Alaska Native Populations. Policy Brief. July 2019. Available online: https://www.ruralhealthresearch.org/publications/1270 (accessed on 9 December 2020).
- Greenberg-Worisek, A.J.; Kurani, S.; Finney Rutten, L.J.; Blake, K.D.; Moser, R.P.; Hesse, B.W. Tracking healthy people 2020 Internet, broadband, and mobile device access goals: An update using data from the health information national trends survey. J. Med. Internet Res. 2019, 21, e13300. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Beydoun, H.A.; Mode, N.; Dore, G.A.; Canas, J.A.; Eid, S.M.; Zonderman, A.B. Racial disparities in adult all-cause and cause-specific mortality among us adults: Mediating and moderating factors. BMC Public Health 2016, 16, 1113. [Google Scholar] [CrossRef] [Green Version]
- Fairfield, K.M.; Black, A.W.; Lucas, F.L.; Murray, K.; Ziller, E.; Korsen, N.; Waterston, L.B.; Han, P.K.J. Association between Rurality and Lung Cancer Treatment Characteristics and Timeliness. J. Rural Health 2019, 35, 560–565. [Google Scholar] [CrossRef]
- Boscoe, F.P.; Henry, K.A.; Sherman, R.L.; Johnson, C.J. The relationship between cancer incidence, stage, and poverty in the United States. Int. J. Cancer 2016, 139, 607–612. [Google Scholar] [CrossRef] [Green Version]
- Heiney, S.P.; Truman, S.; Babatunde, O.A.; Felder, T.M.; Eberth, J.M.; Crouch, E.; Wickersham, K.E.; Adams, S.A. Racial and Geographic Disparities in Endocrine Therapy Adherence among Younger Breast Cancer Survivors. Am. J. Clin. Oncol. 2020, 43, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Desmond, R.A.; Jackson, B.E.; Waterbor, J.W. Disparities in Cancer Survivorship Indicators in the Deep South Based on BRFSS Data: Recommendations for Survivorship Care Plans. South. Med. J. 2017, 110, 181–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strosnider, H.; Kennedy, C.; Monti, M.; Yip, F. Rural and Urban Differences in Air Quality, 2008–2012, and Community Drinking Water Quality, 2010–2015—United States. Mmwr. Surveill. Summ. 2017, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zahnd, W.E.; Mueller-Luckey, G.S.; Ratnapradipa, K.; Smith, T. Predictors and Spatial Variation of Radon Testing in Illinois, 2005–2012. J. Public Health Manag. Pr. 2018, 24, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Baris, D.; Waddell, R.; Beane Freeman, L.E.; Schwenn, M.; Colt, J.S.; Ayotte, J.D.; Ward, M.H.; Nuckols, J.; Schned, A.; Jackson, B.; et al. Elevated Bladder Cancer in Northern New England: The Role of Drinking Water and Arsenic. J. Natl. Cancer Inst. 2016, 108, djw099. [Google Scholar] [CrossRef]
- National Institutes of Health. About|Agricultural Health Study. Available online: https://aghealth.nih.gov/about/index.html (accessed on 3 October 2020).
- Tyson, F.L.; Midwife, K.C.; Gavin, J.; Gaylord, C.E.; Lee, C.; Setlow, V.P.; Wilson, S. Cancer, the environment, and environmental justice? Cancer 2000, 83, 1784–1792. [Google Scholar] [CrossRef]
- James, W.; Jia, C.; Kedia, S. Uneven magnitude of disparities in cancer risks from air toxics. Int. J. Environ. Res. Public Health 2012, 9, 4365–4385. [Google Scholar] [CrossRef] [Green Version]
- Gearhart-Serna, L.M.; Hoffman, K.; Devi, G.R. Environmental Quality and Invasive Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1920–1928. [Google Scholar] [CrossRef] [Green Version]
- Madia, F.; Worth, A.; Whelan, M.; Corvi, R. Carcinogenicity assessment: Addressing the challenges of cancer and chemicals in the environment. Environ. Int. 2019, 128, 417–429. [Google Scholar] [CrossRef]
- Patel, C.J.; Kerr, J.; Thomas, D.C.; Mukherjee, B.; Ritz, B.; Chatterjee, N.; Jankowska, M.; Madan, J.; Karagas, M.R.; Mcallister, K.A.; et al. Opportunities and challenges for environmental exposure assessment in population-based studies. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1370–1380. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, J.K.; Ritchey, J.; Solomon, T.A.; Cordova, F.M. Cigarette Use Among American Indians and Alaska Natives in Metropolitan Areas, Rural Areas, and Tribal Lands. J. Public Health Manag. Pract. 2019, 25, S11–S19. [Google Scholar] [CrossRef] [PubMed]
- Koller, K.R.; Flanagan, C.A.; Day, G.E.; Patten, C.; Umans, J.G.; Austin, M.A.; Hopkins, S.E.; Raindl, C.; Boyer, B.B. High tobacco use prevalence with significant regional and sex differences in smokeless tobacco use among western alaska native people: The WATCH study. Int. J. Circumpolar Health 2017, 76, 1398009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. Health Reports of the Surgeon General. In The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General; Centers for Disease Control and Prevention (US): Atlanta, GA, USA, 2014. [Google Scholar]
- Doescher, M.P.; Jackson, J.E.; Jerant, A.; Gary Hart, L. Prevalence and trends in smoking: A national rural study. J. Rural Health 2006, 22, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T.; Levy, D.T.; Meza, R. Trends and factors related to smokeless Tobacco use in the United States. Nicotine Tob. Res. 2016, 18, 1740–1748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilley, J.A.; Peterson, E.; Bobo, M.; Pickle, K.E.; Rohde, K. Tobacco use prevalence—Disentangling associations between Alaska Native race, low socio-economic status and rural disparities. Int. J. Circumpolar Health 2013, 72, 21582. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.A.; Arcury, T.A.; Chen, H.; Anderson, A.M.; Savoca, M.R.; Kohrman, T.; Quandt, S.A. Use of tobacco products among rural older adults: Prevalence of ever use and cumulative lifetime use. Addict. Behav. 2009, 34, 662–667. [Google Scholar] [CrossRef] [Green Version]
- Chaffee, B.W.; Couch, E.T.; Urata, J.; Gansky, S.A.; Essex, G.; Cheng, J. Predictors of Smokeless Tobacco Susceptibility, Initiation, and Progression Over Time Among Adolescents in a Rural Cohort. Subst. Use Misuse 2019, 54, 1154–1166. [Google Scholar] [CrossRef]
- Boffetta, P.; Hashibe, M. Alcohol and cancer. Lancet Oncol. 2006, 7, 149–156. [Google Scholar] [CrossRef]
- Dixon, M.A.; Chartier, K.G. Alcohol use patterns among urban and rural residents: Demographic and social influences. Alcohol Res. Curr. Rev. 2016, 38, 69–77. [Google Scholar]
- Arcury, T.A.; Talton, J.W.; Summers, P.; Chen, H.; Laurienti, P.J.; Quandt, S.A. Alcohol Consumption and Risk for Dependence Among Male Latino Migrant Farmworkers Compared to Latino Nonfarmworkers in North Carolina. Alcohol. Clin. Exp. Res. 2016, 40, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Steck, S.E.; Murphy, E.A. Dietary patterns and cancer risk. Nat. Rev. Cancer 2020, 20, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Dutko, P.; Ver Ploeg, M.; Farrigan, T. Characteristics and Influential Factors of Food Deserts. United States Department of Agriculture, Economic Research Service, Economic Research Report Number 140; August 2012. Available online: https://www.ers.usda.gov/webdocs/publications/45014/30940_err140.pdf (accessed on 9 December 2020).
- Quandt, S.A.; Groeschel-Johnson, A.; Kinzer, H.T.; Jensen, A.; Miles, K.; O’Hara, H.M.; Chen, H.; Arcury, T.A. Migrant Farmworker Nutritional Strategies: Implications for Diabetes Management. J. Agromedicine 2018, 23, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, M.A.; Warren, A.C.; Vanwassenhove-Paetzold, J.; George, C.; Carroll, D.S.; Becenti, L.J.; Martinez, A.; Jones, B.; Bachman-Carter, K.; Begay, M.G.; et al. Implementation of the Navajo fruit and vegetable prescription programme to improve access to healthy foods in a rural food desert. Public Health Nutr. 2020, 23, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Savoca, M.R.; Arcury, T.A.; Leng, X.; Bell, R.A.; Chen, H.; Anderson, A.; Kohrman, T.; Quandt, S.A. The Diet Quality of Rural Older Adults in the South as Measured by Healthy Eating Index-2005 Varies by Ethnicity. J. Am. Diet. Assoc. 2009, 109, 2063–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Champagne, C.M.; Bogle, M.L.; McGee, B.B.; Yadrick, K.; Allen, H.R.; Kramer, T.R.; Simpson, P.; Gossett, J.; Weber, J. Dietary intake in the lower Mississippi delta region: Results from the foods of our delta study. J. Am. Diet. Assoc. 2004, 104, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Befort, C.A.; Nazir, N.; Perri, M.G. Prevalence of obesity among adults from rural and urban areas of the United States: Findings from NHANES (2005–2008). J. Rural Health 2012, 28, 392–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, M.; Zhang, X.; Harris, C.D.; Holt, J.B.; Croft, J.B. Spatial Disparities in the Distribution of Parks and Green Spaces in the USA. Ann. Behav. Med. 2013, 45, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Kaczynski, A.T.; Eberth, J.M.; Stowe, E.W.; Wende, M.E.; Liese, A.D.; McLain, A.C.; Breneman, C.B.; Josey, M.J. Development of a national childhood obesogenic environment index in the United States: Differences by region and rurality. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 83. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Graubard, B.I.; Broutian, T.; Pickard, R.K.L.; Tong, Z.Y.; Xiao, W.; Kahle, L.; Gillison, M.L. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J. Clin. Oncol. 2018, 36, 262–267. [Google Scholar] [CrossRef]
- Zahnd, W.E.; Jenkins, W.D.; Mueller-Luckey, G.S. Cancer mortality in the mississippi delta region: Descriptive epidemiology and needed future research and interventions. J. Health Care Poor Underserved 2017, 28, 315–328. [Google Scholar] [CrossRef]
- Walker, T.Y.; Elam-Evans, L.D.; Williams, C.L.; Fredua, B.; Yankey, D.; Markowitz, L.E.; Stokley, S. Trends in human papillomavirus (HPV) vaccination initiation among adolescents aged 13–17 by metropolitan statistical area (MSA) status, National Immunization Survey–Teen, 2013–2017. Hum. Vaccines Immunother. 2020, 16, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Luque, J.S.; Raychowdhury, S.; Weaver, M. Health care provider challenges for reaching Hispanic immigrants with HPV vaccination in rural Georgia. Rural Remote Health 2012, 12, 168. [Google Scholar]
- Cates, J.R.; Brewer, N.T.; Fazekas, K.I.; Mitchell, C.E.; Smith, J.S. Racial differences in HPV knowledge, HPV vaccine acceptability, and related beliefs among rural, Southern women. J. Rural Health 2009, 25, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Landrine, H.; Klonoff, E.A. Racial segregation and cigarette smoking among Blacks: Findings at the individual level. J. Health Psychol. 2000, 5, 211–219. [Google Scholar] [CrossRef]
- Havewala, F. The dynamics between the food environment and residential segregation: An analysis of metropolitan areas. Food Policy 2020. [Google Scholar] [CrossRef]
- Lichter, D.T.; Parisi, D.; Grice, S.M.; Taquino, M.C. National estimates of racial segregation in rural and small-town America. Demography 2007, 44, 563–581. [Google Scholar] [CrossRef]
- Anderson, A.E.; Henry, K.A.; Samadder, N.J.; Merrill, R.M.; Kinney, A.Y. Rural vs urban residence affects risk-appropriate colorectal cancer screening. Clin. Gastroenterol. Hepatol. 2013, 11, 526–533. [Google Scholar] [CrossRef] [Green Version]
- Bennett, K.J.; Pumkam, C.; Bellinger, J.D.; Probst, J.C. Cancer screening delivery in persistent poverty rural counties. J. Prim. Care Community Health 2011, 2, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Khan-Gates, J.A.; Ersek, J.L.; Eberth, J.M.; Adams, S.A.; Pruitt, S.L. Geographic Access to Mammography and Its Relationship to Breast Cancer Screening and Stage at Diagnosis: A Systematic Review. Womens Health Issues 2015, 25, 482–493. [Google Scholar] [CrossRef] [Green Version]
- Duggan, C.; Molina, Y.; Carosso, E.; Ibarra, G.; Thompson, B. County of residence and screening practices among Latinas and non-Latina whites in two rural communities. Ethn. Dis. 2019, 29, 31–38. [Google Scholar] [CrossRef]
- Nuno, T.; Gerald, J.K.; Harris, R.; Martinez, M.E.; Estrada, A.; Garcia, F.; Nuño, T.; Gerald, J.K.; Harris, R.; Martinez, M.E.; et al. Comparison of breast and cervical cancer screening utilization among rural and urban Hispanic and American Indian women in the Southwestern United States. Cancer Causes Control. 2012, 23, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.J.; Probst, J.C.; Bellinger, J.D. Receipt of cancer screening services: Surprising results for some rural minorities. J. Rural Health 2012, 28, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Towne, S.D., Jr.; Smith, M.L.; Ory, M.G. Geographic variations in access and utilization of cancer screening services: Examining disparities among American Indian and Alaska Native Elders. Int. J. Health Geogr. 2014, 13, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, A.; Doria-Rose, V.P.; Silvestri, G.A.; Yabroff, K.R. Evaluating Lung Cancer Screening Uptake, Outcomes, and Costs in the United States: Challenges With Existing Data and Recommendations for Improvement. JNCI J. Natl. Cancer Inst. 2019, 111, 342–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odahowski, C.L.; Zahnd, W.E.; Eberth, M.J. Challenges and Opportunities for Lung Cancer Screening in Rural America. J. Am. Coll. Radiol. 2019, 16, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Fedewa, S.A. Lung Cancer Screening With Low-Dose Computed Tomography in the United States-2010 to 2015. Jama Oncol. 2017, 3, 1278–1281. [Google Scholar] [CrossRef]
- Zgodic, A.; Zahnd, W.E.; Miller, D.P.; Studts, J.L.; Eberth, J.M. Predictors of Lung Cancer Screening Utilization in a Population-Based Survey. J. Am. Coll. Radiol. 2020. [Google Scholar] [CrossRef]
- Wang, H.; Roy, S.; Kim, J.; Farazi, P.A.; Siashpush, M.; Su, D. Barriers of colorectal cancer screening in rural USA: A systematic review. Rural Remote Health 2019, 19, 5181. [Google Scholar] [CrossRef]
- Katz, M.L.; James, A.S.; Pignone, M.P.; Hudson, M.A.; Jackson, E.; Oates, V.; Campbell, M.K. Colorectal cancer screening among African American church members: A qualitative and quantitative study of patient-provider communication. BMC Public Health 2004, 4, 62. [Google Scholar] [CrossRef]
- Surveillance Epidemiology and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence-SEER Research Limited Field Data. 21 Registries. Nov 2019 Sub (2000–2017)-Linked to County Attributes-Time Dependent (1990–2017)/Income-Rurality, 1969–2018 Counties, National Cancer Institute. DCCPS, Surveillance Research Program, Released April 2020. Based upon the November 2019 Submission. Available online: https://seer.cancer.gov/data/citation.html (accessed on 3 February 2021).
- Zahnd, W.E.; Gomez, S.L.; Steck, S.E.; Brown, M.J.; Ganai, S.; Zhang, J.; Arp Adams, S.; Berger, F.G.; Eberth, J.M. Rural-urban and racial/ethnic trends and disparities in early-onset and average-onset colorectal cancer. Cancer 2020. [Google Scholar] [CrossRef]
- Singh, G.K. Rural-urban trends and patterns in cervical cancer mortality, incidence, stage, and survival in the United States, 1950–2008. J. Community Health 2012, 37, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Houston, K.A.; Mitchell, K.A.; King, J.; White, A.; Ryan, B.M. Histologic Lung Cancer Incidence Rates and Trends Vary by Race/Ethnicity and Residential County. J. Thorac. Oncol. 2018, 13, 497–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rovito, M.J.; Taylor, S.; Lockwood, R.; Adams, W.B.; Craycraft, M. Testicular Cancer Incidence and Mortality Within Rural and Urban Regions. J. Adolesc. Young Adult Oncol. 2020, 9, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Zahnd, W.E.; Fogleman, A.J.; Jenkins, W.D. Rural-Urban Disparities in Stage of Diagnosis Among Cancers With Preventive Opportunities. Am. J. Prev. Med. 2018, 54, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Sabatino, S.A.; White, M.C. Rural-urban and racial/ethnic disparities in invasive cervical cancer incidence in the United States, 2010–2014. Prev. Chronic Dis. 2019, 16, E70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camacho, F.T.; Tan, X.; Alcala, H.E.; Shah, S.; Anderson, R.T.; Balkrishnan, R. Impact of patient race and geographical factors on initiation and adherence to adjuvant endocrine therapy in medicare breast cancer survivors. Medicine (Baltim.) 2017, 96, e7147. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, C.I. Racial disparities in breast cancer diagnosis and treatment by hormone receptor and HER2 status. Cancer Epidemiol Biomark. Prev. 2015, 24, 1666–1672. [Google Scholar] [CrossRef] [Green Version]
- Steele, C.B.; Pisu, M.; Richardson, L.C. Urban/rural patterns in receipt of treatment for non-small cell lung cancer among black and white medicare beneficiaries, 2000–2003. J. Natl. Med. Assoc. 2011, 103, 711–718. [Google Scholar] [CrossRef]
- Zahnd, W.E.; Hyon, K.S.; Diaz-Sylvester, P.; Izadi, S.R.; Colditz, G.A.; Brard, L. Rural-urban differences in surgical treatment, regional lymph node examination, and survival in endometrial cancer patients. Cancer Causes Control. 2018, 29, 221–232. [Google Scholar] [CrossRef]
- AlNasser, M.; Schneider, E.B.; Gearhart, S.L.; Wick, E.C.; Fang, S.H.; Haider, A.H.; Efron, J.E. National disparities in laparoscopic colorectal procedures for colon cancer. Surg. Endosc. 2014, 28, 49–57. [Google Scholar] [CrossRef]
- Thomas, P.S.; Class, C.A.; Gandhi, T.R.; Bambhroliya, A.; Do, K.A.; Brewster, A.M. Demographic, clinical, and geographical factors associated with lack of receipt of physician recommended chemotherapy in women with breast cancer in Texas. Cancer Causes Control. 2019, 30, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Des Bordes, J.K.A.; Lopez, D.S.; Swartz, M.D.; Volk, R.J. Sociodemographic Disparities in Cure-Intended Treatment in Localized Prostate Cancer. J. Racial Ethn. Health Disparities 2018, 5, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Bregar, A.J.; Melamed, A.; Diver, E.; Clemmer, J.T.; Uppal, S.; Schorge, J.O.; Rice, L.W.; del Carmen, M.G.; Rauh-Hain, J.A. Minimally Invasive Staging Surgery in Women with Early-Stage Endometrial Cancer: Analysis of the National Cancer Data Base. Ann. Surg. Oncol. 2017, 24, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, E.; Thirunavukarasu, P.; Al-Sukhni, E.; Attwood, K.; Nurkin, S.J. National disparities in minimally invasive surgery for rectal cancer. Surg. Endosc. 2016, 30, 1060–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsoniadis, E.G.; Fan, Y.; Jarosek, S.; Gaertner, W.B.; Melton, G.B.; Madoff, R.D.; Kwaan, M.R. Decreased Use of Sphincter-Preserving Procedures Among African Americans with Rectal Cancer. Ann. Surg. Oncol. 2018, 25, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Lairson, D.R.; Swartz, M.D.; Du, X.L. Racial, Socioeconomic, and Geographic Disparities in the Receipt, Timing to Initiation, and Duration of Adjuvant Androgen Deprivation Therapy in Men with Prostate Cancer. J. Racial Ethn. Health Disparities 2019, 6, 133–142. [Google Scholar] [CrossRef]
- Mollica, M.A.; Weaver, K.E.; McNeel, T.S.; Kent, E.E. Examining urban and rural differences in perceived timeliness of care among cancer patients: A SEER-CAHPS study. Cancer 2018, 124, 3257–3265. [Google Scholar] [CrossRef] [Green Version]
- Kong, A.L.; Yen, T.W.F.; Pezzin, L.E.; Miao, H.; Sparapani, R.A.; Laud, P.W.; Nattinger, A.B. Socioeconomic and racial differences in treatment for breast cancer at a low-volume hospital. Ann. Surg. Oncol. 2011, 18, 3220–3227. [Google Scholar] [CrossRef] [Green Version]
- Hughes, R.G.; Hunt, S.S.; Luft, H.S. Effects of surgeon volume and hospital volume on quality of care in hospitals. Med. Care 1987, 25, 489–503. [Google Scholar] [CrossRef]
- Whitaker, R.G.; Holmes, G.M.; Pink, G.H. The Impact of the Low Volume Hospital (LVH) Program on the Viability of Small, Rural Hospitals. NC Rural Health Research Program, Findings Brief. October 2016. Available online: https://www.shepscenter.unc.edu/wp-content/uploads/dlm_uploads/2016/10/Impact-of-LVH.pdf (accessed on 9 December 2020).
- Esnaola, N.F.; Ford, M.E. Racial Differences and Disparities in Cancer Care and Outcomes: Where’s the Rub? Surg. Oncol. Clin. N. Am. 2012, 21, 417. [Google Scholar] [CrossRef] [Green Version]
- Jessup, J.M.; Stewart, A.; Greene, F.L.; Minsky, B.D. Adjuvant chemotherapy for stage III colon cancer: Implications of race/ethnicity, age, and differentiation. JAMA 2005, 29, 2703–2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandelblatt, J.S.; Kerner, J.F.; Hadley, J.; Hwang, Y.T.; Eggert, L.; Johnson, L.E.; Gold, K.; OPTIONS. Variations in breast carcinoma treatment in older medicare beneficiaries: Is it black or white. Cancer 2002, 95, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Cykert, S.; Dilworth-Anderson, P.; Monroe, M.H.; Walker, P.; McGuire, F.R.; Corbie-Smith, G.; Edwards, L.J.; Bunton, A.J. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. JAMA 2010, 303, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Definition of survivorship—NCI Dictionary of Cancer Terms—National Cancer Institute. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/survivorship (accessed on 15 October 2020).
- Khan, S.; Hicks, V.; Rancilio, D.; Langston, M.; Richardson, K.; Drake, B.F. Predictors of Follow-Up Visits Post Radical Prostatectomy. Am. J. Men’s Health 2018, 12, 760–765. [Google Scholar] [CrossRef] [Green Version]
- Weaver, K.E.; Geiger, A.M.; Lu, L.; Case, L.D. Rural-urban disparities in health status among US cancer survivors. Cancer 2013, 119, 1050–1057. [Google Scholar] [CrossRef] [Green Version]
- Spencer, J.C.; Reeve, B.B.; Troester, M.A.; Wheeler, S.B. Factors Associated with Endocrine Therapy Non-Adherence in Breast Cancer Survivors. Psycho Oncol. 2020, 29, 647–654. [Google Scholar] [CrossRef]
- Schootman, M.; Homan, S.; Weaver, K.E.; Jeffe, D.B.; Yun, S. The health and welfare of rural and urban cancer survivors in Missouri. Prev. Chronic Dis. 2013, 10, E152. [Google Scholar] [CrossRef] [Green Version]
- Andrykowski, M.A.; Steffens, R.F.; Bush, H.M.; Tucker, T.C. Disparities in mental health outcomes among lung cancer survivors associated with ruralness of residence. Psychooncology 2014, 23, 428–436. [Google Scholar] [CrossRef]
- Weaver, K.E.; Palmer, N.; Lu, L.; Case, L.D.; Geiger, A.M. Rural-urban differences in health behaviors and implications for health status among US cancer survivors. Cancer Causes Control. 2013, 24, 1481–1490. [Google Scholar] [CrossRef] [Green Version]
- Odahowski, C.L.; Zahnd, W.E.; Zgodic, A.; Edward, J.S.; Hill, L.N.; Davis, M.M.; Perry, C.K.; Shannon, J.; Wheeler, S.B.; Vanderpool, R.C.; et al. Financial hardship among rural cancer survivors: An analysis of the Medical Expenditure Panel Survey. Prev. Med. 2019, 105881. [Google Scholar] [CrossRef]
- Zahnd, W.E.; Davis, M.M.; Rotter, J.S.; Vanderpool, R.C.; Perry, C.K.; Shannon, J.; Ko, L.K.; Wheeler, S.B.; Odahowski, C.L.; Farris, P.E.; et al. Rural-urban differences in financial burden among cancer survivors: An analysis of a nationally representative survey. Supportive Care Cancer 2019, 27, 4779–4786. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.E.; Rowland, J.H.; Bellizzi, K.M.; Aziz, N.M. Forgoing medical care because of cost: Assessing disparities in healthcare access among cancer survivors living in the United States. Cancer 2010, 116, 3493–3504. [Google Scholar] [CrossRef] [PubMed]
- Yabroff, K.R.; Dowling, E.C.; Guy, G.P., Jr.; Banegas, M.P.; Davidoff, A.; Han, X.; Virgo, K.S.; McNeel, T.S.; Chawla, N.; Blanch-Hartigan, D.; et al. Financial Hardship Associated With Cancer in the United States: Findings From a Population-Based Sample of Adult Cancer Survivors. J. Clin. Oncol. 2016, 34, 259–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, J.C.; Rotter, J.S.; Eberth, J.M.; Zahnd, W.E.; Vanderpool, R.C.; Ko, L.K.; Davis, M.M.; Troester, M.A.; Olshan, A.F.; Wheeler, S.B. Employment Changes Following Breast Cancer Diagnosis: The Effects of Race and Place. J. Natl. Cancer Inst. 2020, 112, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Guadagnolo, B.A.; Petereit, D.G.; Coleman, C.N. Cancer Care Access and Outcomes for American Indian Populations in the United States: Challenges and Models for Progress. Semin. Radiat. Oncol. 2017, 27, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention, National Center for Health Statistics. Underlying Cause of Death “ ” 1999–2019 on CDC WONDER Online Database, released in 2020. Data are from the Multiple Cause of Death Files, 1999–2019, as “ ” compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Available online: http://wonder.cdc.gov/ucd-icd10.html (accessed on 25 January 2021).
- Singh, G.K.; Williams, S.D.; Siahpush, M.; Mulhollen, A. Socioeconomic, Rural-Urban, and Racial Inequalities in US Cancer Mortality: Part I-All Cancers and Lung Cancer and Part II-Colorectal, Prostate, Breast, and Cervical Cancers. J. Cancer Epidemiol. 2011, 2011, 107497. [Google Scholar] [CrossRef]
- Singh, G.K.; Siahpush, M. Widening rural-urban disparities in all-cause mortality and mortality from major causes of death in the USA, 1969–2009. J. Urban. Health 2014, 91, 272–292. [Google Scholar] [CrossRef]
- Johnson, A.M.; Johnson, A.; Hines, R.B.; Mohammadi, R. Neighborhood context and non-small cell lung cancer outcomes in Florida non-elderly patients by race/ethnicity. Lung Cancer 2020, 142, 20–27. [Google Scholar] [CrossRef]
- Higginbotham, J.C.; Moulder, J.; Currier, M. Rural v. urban aspects of cancer: First-year data from the Mississippi Central Cancer Registry. Fam. Community Health 2001, 24, 1–9. [Google Scholar] [CrossRef]
- Hines, R.; Markossian, T.; Johnson, A.; Dong, F.; Bayakly, R. Geographic residency status and census tract socioeconomic status as determinants of colorectal cancer outcomes. Am. J. Public Health 2014, 104, e63–e71. [Google Scholar] [CrossRef]
- Thompson, B.; Hohl, S.D.; Molina, Y.; Paskett, E.D.; Fisher, J.L.; Baltic, R.D.; Washington, C.M. Breast Cancer Disparities Among Women in Underserved Communities in the USA. Curr. Breast Cancer Rep. 2018, 10, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Puechl, A.M.; Chino, F.; Havrilesky, L.J.; Davidson, B.A.; Chino, J.P. Place of death by region and urbanization among gynecologic cancer patients: 2006–2016. Gynecol. Oncol. 2019, 155, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Ward, E.; Wu, X.; Martin, H.J.; McLaughlin, C.C.; Thun, M.J. Geographic patterns of prostate cancer mortality and variations in access to medical care in the United States. Cancer Epidemiol. Biomark. Prev. 2005, 14, 590–595. [Google Scholar] [CrossRef] [Green Version]
- Jacobs-Wingo, J.L.; Espey, D.K.; Groom, A.V.; Phillips, L.E.; Haverkamp, D.S.; Stanley, S.L. Causes and disparities in death rates among Urban American Indian and Alaska native populations, 1999–2009. Am. J. Public Health 2016, 106, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Lindley, L.C.; Oyana, T.J. Geographic Variation in Mortality Among Children and Adolescents Diagnosed With Cancer in Tennessee: Does Race Matter? J. Pediatric Oncol. Nurs. 2016, 33, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hines, R.B.; Markossian, T.W. Differences in Late-Stage Diagnosis, Treatment, and Colorectal Cancer-Related Death Between Rural and Urban African Americans and Whites in Georgia. J. Rural Health 2012, 28, 296–305. [Google Scholar] [CrossRef]
- Delavar, A.; Feng, Q.; Johnson, K.J. Rural/urban residence and childhood and adolescent cancer survival in the United States. Cancer 2019, 125, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Clarke, J.A.; Despotis, A.M.; Ramirez, R.J.; Zevallos, J.P.; Mazul, A.L. Head and Neck Cancer Survival Disparities by Race and Rural–Urban Context. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1955–1961. [Google Scholar] [CrossRef]
- Rogers, C.R.; Blackburn, B.E.; Huntington, M.; Curtin, K.; Thorpe, R.J.; Rowe, K.; Snyder, J.; Deshmukh, V.; Newman, M.; Fraser, A.; et al. Rural–urban disparities in colorectal cancer survival and risk among men in Utah: A statewide population-based study. Cancer Causes Control. 2020, 31, 241–253. [Google Scholar] [CrossRef]
- Patel, M.I.; Lopez, A.M.; Blackstock, W.; Reeder-Hayes, K.; Allyn Moushey, E.; Phillips, J.; Tap, W. Cancer disparities and health equity: A policy statement from the american society of clinical oncology. J. Clin. Oncol. 2020, 38, 3439–3448. [Google Scholar] [CrossRef]
- Bouye, K.E.; McCleary, K.J.; Williams, K.B. Increasing Diversity in the Health Professions: Reflections on Student Pipeline Programs. J. Healthc. Sci. Humanit 2016, 6, 67–79. [Google Scholar] [PubMed]
- Oklahoma State University. OSU Ceremony Opens First Tribally Affiliated Medical School. Available online: https://news.okstate.edu/articles/communications/2020/osu-ceremony-opens-first-tribally-affiliated-medical-school.html/ (accessed on 25 January 2021).
- Davidoff, A.J.; Hill, S.C.; Bernard, D.; Yabroff, K.R. The Affordable Care Act and Expanded Insurance Eligibility among Nonelderly Adult Cancer Survivors. Jnci J. Natl. Cancer Inst. 2018, 107, djv181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, T.; Sinner, H.F.; Walling, S.C.; Chen, Q.; Huang, B.; Tucker, T.C.; Patel, J.A.; Evers, B.M.; Bhakta, A.S. Impact of the Affordable Care Act on Colorectal Cancer Screening, Incidence, and Survival in Kentucky. J. Am. Coll. Surg. 2019, 228, 342–353.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yankey, D.; Elam-Evans, L.D.; Bish, C.L.; Stokley, S.K. Human papillomavirus vaccination estimates among adolescents in the Mississippi delta region: National immunization survey-teen, 2015–2017. Prev. Chronic Dis. 2020, 17, E31. [Google Scholar] [CrossRef] [PubMed]
- Gennuso, K.P.; Jovaag, A.; Catlin, B.B.; Rodock, M.; Park, H. Assessment of Factors Contributing to Health Outcomes in the Eight States of the Mississippi Delta Region. Prev. Chronic Dis. 2016, 13, E33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokdad, A.H.; Dwyer-Lindgren, L.; Fitzmaurice, C.; Stubbs, R.W.; Bertozzi-Villa, A.; Morozoff, C.; Charara, R.; Allen, C.; Naghavi, M.; Murray, C.J. Trends and Patterns of Disparities in Cancer Mortality Among US Counties, 1980–2014. JAMA 2017, 317, 388–406. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Sahar, L.; Robbins, A.; Jemal, A. Where can colorectal cancer screening interventions have the most impact? Cancer Epidemiol. Biomark. Prev. 2015, 24, 1151–1156. [Google Scholar] [CrossRef] [Green Version]
- Grants and Contracts—Appalachian Regional Commission. Available online: https://www.arc.gov/grants-and-contracts/ (accessed on 1 December 2020).
- DRA: FY 2021 Close-Out Budget Justification. 2019. Available online: https://dra.gov/images/uploads/content_files/FY_2021_Close_Out_Justification_FINAL.pdf (accessed on 1 December 2020).
- Blewett, L.A.; Call, K.T.; Turner, J.; Hest, R. Data Resources for Conducting Health Services and Policy Research. Annu. Rev. Public Health 2018, 39, 437–452. [Google Scholar] [CrossRef] [Green Version]
- Zahnd, W.E.; Askelson, N.; Vanderpool, R.C.; Stradtman, L.; Edward, J.; Farris, P.E.; Petermann, V.; Eberth, J.M. Challenges of using nationally representative, population-based surveys to assess rural cancer disparities. Prev. Med. 2019, 129, 105812. [Google Scholar] [CrossRef]
- Becker, T.; Babey, S.H.; Shimkhada, R.; Scheitler, A.J.; Ponce, N.A. Limited access to health data on American Indian and Alaska Natives impedes population health insights. Policy Brief. 16 November 2020. Available online: https://healthpolicy.ucla.edu/publications/search/pages/detail.aspx?PubID=2004 (accessed on 9 December 2020).
- Haddad, D.N.; Sandler, K.L.; Henderson, L.M.; Rivera, M.P.; Aldrich, M.C. Disparities in lung cancer screening: A review. Ann. Am. Thorac. Soc. 2020, 17, 399–405. [Google Scholar] [CrossRef]
- Aldrich, M.C.; Mercaldo, S.F.; Sandler, K.L.; Blot, W.J.; Grogan, E.L.; Blume, J.D. Evaluation of USPSTF Lung Cancer Screening Guidelines among African American Adult Smokers. JAMA Oncol. 2019, 5, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- United States Preventive Services Taskforce. Recommendation: Lung Cancer: Screening. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/draft-update-summary/lung-cancer-screening1 (accessed on 1 December 2020).
- Huey, R.W.; Hawk, E.; Offodile, A.C. Mind the Gap: Precision Oncology and Its Potential to Widen Disparities. J. Oncol. Pract. 2019, 15, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.C.T.; Smieliauskas, F.; Geynisman, D.M.; Kelly, R.J.; Smith, T.J. Trends in the cost and use of targeted cancer therapies for the privately insured nonelderly: 2001 to 2011. J. Clin. Oncol. 2015, 33, 2190–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haslam, A.; Prasad, V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw. Open 2019, 2, e192535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institutes of Health. RFA-CA-20-051: Social and Behavioral Intervention Research to Address Modifiable Risk Factors for Cancer in Rural Populations (R01 Clinical Trial Required). Available online: https://grants.nih.gov/grants/guide/rfa-files/RFA-CA-20-051.html (accessed on 1 December 2020).
- Moonshot Blue Ribbon Panel Implementation Science Working Group. Accelerating Implementation of Evidence-Base Cancer Prevention and Screening Strategies. Available online: https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/blue-ribbon-panel/implementation-science-working-group-report.pdf (accessed on 9 December 2020).
- Weaver, S.J.; Blake, K.D.; Vanderpool, R.C.; Gardner, B.; Croyle, R.T.; Srinivasan, S. Advancing Rural Cancer Control Research: National Cancer Institute Efforts to Identify Gaps and Opportunities. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1515–1518. [Google Scholar] [CrossRef]
- Blake, K.D.; Moss, J.L.; Gaysynsky, A.; Srinivasan, S.; Croyle, R.T. Making the Case for Investment in Rural Cancer Control: An Analysis of Rural Cancer Incidence, Mortality, and Funding Trends. Cancer Epidemiol. Biomark. Prev. 2017, 26, 992–997. [Google Scholar] [CrossRef] [Green Version]
- National Cancer Institute, Division of Cancer Control and Population Sciences. Rural Cancer Control. Available online: https://cancercontrol.cancer.gov/research-emphasis/rural (accessed on 9 December 2020).
- Vanderpool, R.C.; Stradtman, L.R.; Brandt, H.M. Policy opportunities to increase HPV vaccination in rural communities. Hum. Vaccines Immunother. 2019, 15, 1527–1532. [Google Scholar] [CrossRef]
- HPV Vaccination Evidence-Based Programs Listing|Evidence-Based Cancer Control Programs (EBCCP). Available online: https://ebccp.cancercontrol.cancer.gov/topicPrograms.do?topicId=22626661&choice=default (accessed on 1 December 2020).
- Bragge, P.; Grimshaw, J.M.; Lokker, C.; Colquhoun, H.; Albrecht, L.; Baron, J.; Dadich, A.; Damschroder, L.; Danko, K.; Fernandez, M.E.; et al. AIMD—A validated, simplified framework of interventions to promote and integrate evidence into health practices, systems, and policies. BMC Med. Res. Methodol. 2017, 17, 38. [Google Scholar] [CrossRef] [Green Version]
- Pinnock, H.; Barwick, M.; Carpenter, C.R.; Eldridge, S.; Grandes, G.; Griffiths, C.J.; Rycroft-Malone, J.; Meissner, P.; Murray, E.; Patel, A.; et al. Standards for Reporting Implementation Studies (StaRI) Statement. BMJ 2017, 356. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, T.C.; Glasziou, P.P.; Boutron, I.; Milne, R.; Perera, R.; Moher, D.; Altman, D.G.; Barbour, V.; Macdonald, H.; Johnston, M.; et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Welch, V.A.; Norheim, O.F.; Jull, J.; Cookson, R.; Sommerfelt, H.; Tugwell, P. CONSORT-Equity 2017 extension and elaboration for better reporting of health equity in randomised trials. BMJ Clin. Res. 2017, 359, j5085. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Racial/Ethnic Group | Age-Adjusted Rural Incidence Rate per 100,000 | Age-Adjusted Urban Incidence Rate per 100,000 |
|---|---|---|
| All Cancers | ||
| Non-Hispanic White | 471.9 | 470.6 |
| Non-Hispanic Black | 455.9 | 454.1 |
| American Indian/Alaska Native | 338.5 | 337.8 |
| Asian/Pacific Islander | 320.3 * | 305.9 |
| Hispanic | 322.9 | 349.3 * |
| Lung Cancer | ||
| Non-Hispanic White | 71.6 * | 58.5 |
| Non-Hispanic Black | 68.1 * | 56.1 |
| American Indian/Alaska Native | 35.7 | 41.2 * |
| Asian/Pacific Islander | 40.5 * | 36.4 |
| Hispanic | 31.3 * | 28.9 |
| Colorectal Cancer | ||
| Non-Hispanic White | 43.6 * | 37.7 |
| Non-Hispanic Black | 52.7 * | 44.6 |
| American Indian/Alaska Native | 42.3 * | 33.1 |
| Asian/Pacific Islander | 34.8 | 32.2 |
| Hispanic | 35.3 | 33.7 |
| Prostate Cancer | ||
| Non-Hispanic White | 99.7 | 106.0 * |
| Non-Hispanic Black | 166.7 | 181.9 * |
| American Indian/Alaska Native | 64.3 | 64.0 |
| Asian/Pacific Islander | 61.2 | 57.2 |
| Hispanic | 69.6 | 93.0 * |
| Female Breast Cancer | ||
| Non-Hispanic White | 120.1 | 139.7 * |
| Non-Hispanic Black | 124.2 | 128.9 |
| American Indian/Alaska Native | 80.4 | 94.1 * |
| Asian/Pacific Islander | 109.4 | 104.3 |
| Hispanic | 89.7 | 99.4 * |
| Cervical Cancer | ||
| Non-Hispanic White | 8.6 * | 6.5 |
| Non-Hispanic Black | 10.8 * | 8.8 |
| American Indian/Alaska Native | 10.8 | 7.9 |
| Asian/Pacific Islander | 6.6 | 6.6 |
| Hispanic | 8.4 | 9.2 |
| Racial/Ethnic Group | Age-Adjusted Rural Mortality Rate per 100,000 | Age-Adjusted Urban Mortality Rate per 100,000 |
|---|---|---|
| All Cancers | ||
| Non-Hispanic White | 176.0 * | 160.1 |
| Non-Hispanic Black | 199.1 * | 184.4 |
| American Indian/Alaska Native | 161.2 * | 120.8 |
| Asian/Pacific Islander | 104.7 | 98.4 |
| Hispanic | 109.1 | 111.0 |
| Lung Cancer | ||
| Non-Hispanic White | 50.0 * | 41.8 |
| Non-Hispanic Black | 49.2 * | 42.6 |
| American Indian/Alaska Native | 40.0 * | 29.8 |
| Asian/Pacific Islander | 24.6 * | 22.0 |
| Hispanic | 48.5 * | 38.4 |
| Colorectal Cancer | ||
| Non-Hispanic White | 15.8 * | 13.3 |
| Non-Hispanic Black | 22.0 * | 18.5 |
| American Indian/Alaska Native | 18.6 * | 11.8 |
| Asian/Pacific Islander | 9.9 | 9.5 |
| Hispanic | 16.0 * | 13.4 |
| Prostate Cancer | ||
| Non-Hispanic White | 18.6 * | 17.8 |
| Non-Hispanic Black | 40.7 * | 37.9 |
| American Indian/Alaska Native | 20.7 * | 14.2 |
| Asian/Pacific Islander | 8.9 | 8.5 |
| Hispanic | 13.0 | 15.7 * |
| Female Breast Cancer | ||
| Non-Hispanic White | 20.5 | 20.4 |
| Non-Hispanic Black | 28.6 | 28.5 |
| American Indian/Alaska Native | 15.9 | 14.2 |
| Asian/Pacific Islander | 12.5 | 11.4 |
| Hispanic | 20.7 | 20.3 |
| Cervical Cancer | ||
| Non-Hispanic White | 2.5 * | 2.0 |
| Non-Hispanic Black | 4.6 * | 3.4 |
| American Indian/Alaska Native | 3.4 * | 1.9 |
| Asian/Pacific Islander | 1.8 | 1.8 |
| Hispanic | 2.7 * | 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahnd, W.E.; Murphy, C.; Knoll, M.; Benavidez, G.A.; Day, K.R.; Ranganathan, R.; Luke, P.; Zgodic, A.; Shi, K.; Merrell, M.A.; et al. The Intersection of Rural Residence and Minority Race/Ethnicity in Cancer Disparities in the United States. Int. J. Environ. Res. Public Health 2021, 18, 1384. https://doi.org/10.3390/ijerph18041384
Zahnd WE, Murphy C, Knoll M, Benavidez GA, Day KR, Ranganathan R, Luke P, Zgodic A, Shi K, Merrell MA, et al. The Intersection of Rural Residence and Minority Race/Ethnicity in Cancer Disparities in the United States. International Journal of Environmental Research and Public Health. 2021; 18(4):1384. https://doi.org/10.3390/ijerph18041384
Chicago/Turabian StyleZahnd, Whitney E., Cathryn Murphy, Marie Knoll, Gabriel A. Benavidez, Kelsey R. Day, Radhika Ranganathan, Parthenia Luke, Anja Zgodic, Kewei Shi, Melinda A. Merrell, and et al. 2021. "The Intersection of Rural Residence and Minority Race/Ethnicity in Cancer Disparities in the United States" International Journal of Environmental Research and Public Health 18, no. 4: 1384. https://doi.org/10.3390/ijerph18041384
APA StyleZahnd, W. E., Murphy, C., Knoll, M., Benavidez, G. A., Day, K. R., Ranganathan, R., Luke, P., Zgodic, A., Shi, K., Merrell, M. A., Crouch, E. L., Brandt, H. M., & Eberth, J. M. (2021). The Intersection of Rural Residence and Minority Race/Ethnicity in Cancer Disparities in the United States. International Journal of Environmental Research and Public Health, 18(4), 1384. https://doi.org/10.3390/ijerph18041384







