The GGLEAM Study: Understanding Glaucoma in the Ohio Amish
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
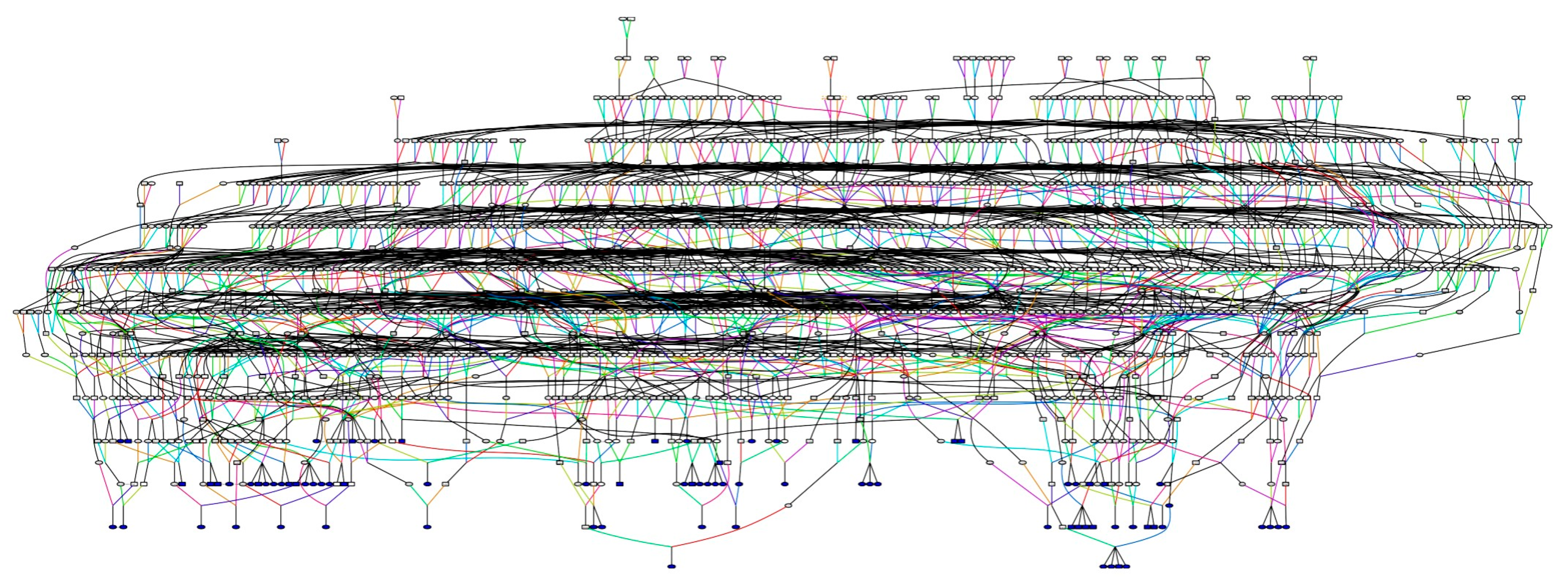
2.2. Amish Pedigree
3. Results
3.1. GGLEAM Study Participants
3.2. Family History of Glaucoma
3.3. Quantitative Ocular Measurements
3.4. Genealogy of GGLEAM Study Participants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Report on Vision; World Health Organization: Geneva, Switzarland, 2019. [Google Scholar]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Wiggs, J.L.; Pasquale, L.R. Genetics of glaucoma. Hum. Mol. Genet. 2017, 26, R21–R27. [Google Scholar] [CrossRef] [PubMed]
- Doucette, L.P.; Rasnitsyn, A.; Seifi, M.; Walter, M.A. The interactions of genes, age, and environment in glaucoma pathogenesis. Surv. Ophthalmol. 2015, 60, 310–326. [Google Scholar] [CrossRef] [PubMed]
- Trivli, A.; Zervou, M.I.; Goulielmos, G.N.; Spandidos, D.A.; Detorakis, E.T. Primary open angle glaucoma genetics: The common variants and their clinical associations. Mol. Med. Rep. 2020, 22, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.F.; Gorgels, T.G.; Ramdas, W.D.; Klaver, C.C.; van Duijn, C.M.; Jansonius, N.M.; Bergen, A.A. The vast complexity of primary open angle glaucoma: Disease genes, risks, molecular mechanisms and pathobiology. Prog. Retin. Eye Res. 2013, 37, 31–67. [Google Scholar] [CrossRef] [PubMed]
- Choquet, H.; Wiggs, J.L.; Khawaja, A.P. Clinical implications of recent advances in primary open-angle glaucoma genetics. Eye (London) 2020, 34, 29–39. [Google Scholar] [CrossRef]
- MacGregor, S.; Ong, J.S.; An, J.; Han, X.; Zhou, T.; Siggs, O.M.; Law, M.H.; Souzeau, E.; Sharma, S.; Lynn, D.J.; et al. Genome-wide association study of intraocular pressure uncovers new pathways to glaucoma. Nat. Genet. 2018, 50, 1067–1071. [Google Scholar] [CrossRef]
- Choquet, H.; Thai, K.K.; Yin, J.; Hoffmann, T.J.; Kvale, M.N.; Banda, Y.; Schaefer, C.; Risch, N.; Nair, K.S.; Melles, R.; et al. A large multi-ethnic genome-wide association study identifies novel genetic loci for intraocular pressure. Nat. Commun. 2017, 8, 2108. [Google Scholar] [CrossRef]
- Hysi, P.G.; Cheng, C.Y.; Springelkamp, H.; Macgregor, S.; Bailey, J.N.C.; Wojciechowski, R.; Vitart, V.; Nag, A.; Hewitt, A.W.; Hohn, R.; et al. Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nat. Genet. 2014, 46, 1126–1130. [Google Scholar] [CrossRef]
- Springelkamp, H.; Hohn, R.; Mishra, A.; Hysi, P.G.; Khor, C.C.; Loomis, S.J.; Bailey, J.N.; Gibson, J.; Thorleifsson, G.; Janssen, S.F.; et al. Meta-analysis of genome-wide association studies identifies novel loci that influence cupping and the glaucomatous process. Nat. Commun. 2014, 5, 4883. [Google Scholar] [CrossRef]
- Springelkamp, H.; Iglesias, A.I.; Mishra, A.; Hohn, R.; Wojciechowski, R.; Khawaja, A.P.; Nag, A.; Wang, Y.X.; Wang, J.J.; Cuellar-Partida, G.; et al. New insights into the genetics of primary open-angle glaucoma based on meta-analyses of intraocular pressure and optic disc characteristics. Hum. Mol. Genet. 2017, 26, 438–453. [Google Scholar] [CrossRef]
- Khawaja, A.P.; Bailey, J.N.C.; Wareham, N.J.; Scott, R.A.; Simcoe, M.; Igo, R.P.; Song, Y.E.; Wojciechowski, R.; Cheng, C.Y.; Khaw, P.T.; et al. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat. Genet. 2018, 50, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, J.; Kramer, P.L.; Dyer, T.; Diego, V.; Samples, J.R.; Craig, J.E.; Mackey, D.A.; Hewitt, A.W.; Blangero, J.; Wirtz, M.K. The path to open-angle glaucoma gene discovery: Endophenotypic status of intraocular pressure, cup-to-disc ratio, and central corneal thickness. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3509–3514. [Google Scholar] [CrossRef] [PubMed]
- Gharahkhani, P.; Jorgenson, E.; Hysi, P.; Khawaja, A.P.; Pendergrass, S.; Han, X.; Ong, J.S.; Hewitt, A.W.; Segre, A.; Igo, R.P.; et al. A large cross-ancestry meta-analysis of genome-wide association studies identifies 127 risk loci for primary open-angle glaucoma, with most loci showing consistent effects across ancestries. Nat. Commun. 2020, in press. [Google Scholar]
- Manolio, T.A.; Collins, F.S.; Cox, N.J.; Goldstein, D.B.; Hindorff, L.A.; Hunter, D.J.; McCarthy, M.I.; Ramos, E.M.; Cardon, L.R.; Chakravarti, A.; et al. Finding the missing heritability of complex diseases. Nature 2009, 461, 747–753. [Google Scholar] [CrossRef]
- Matovinovic, E.; Kho, P.F.; Lea, R.A.; Benton, M.C.; Eccles, D.A.; Haupt, L.M.; Hewitt, A.W.; Sherwin, J.C.; Mackey, D.A.; Griffiths, L.R. Genome-wide linkage and association analysis of primary open-angle glaucoma endophenotypes in the Norfolk Island isolate. Mol. Vis. 2017, 23, 660–665. [Google Scholar]
- van Koolwijk, L.M.; Despriet, D.D.; van Duijn, C.M.; Cortes, L.M.P.; Vingerling, J.R.; Aulchenko, Y.S.; Oostra, B.A.; Klaver, C.C.; Lemij, H.G. Genetic contributions to glaucoma: Heritability of intraocular pressure, retinal nerve fiber layer thickness, and optic disc morphology. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3669–3676. [Google Scholar] [CrossRef]
- Lee, M.K.; Woo, S.J.; Kim, J.I.; Cho, S.I.; Kim, H.; Sung, J.; Seo, J.S.; Kim, D.M. Replication of a glaucoma candidate gene on 5q22.1 for intraocular pressure in mongolian populations: The GENDISCAN Project. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1335–1340. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kitsos, G.; Petrou, Z.; Grigoriadou, M.; Samples, J.R.; Hewitt, A.W.; Kokotas, H.; Giannoulia-Karantana, A.; Mackey, D.A.; Wirtz, M.K.; Moschou, M.; et al. Primary open angle glaucoma due to T377M MYOC: Population mapping of a Greek founder mutation in Northwestern Greece. Clin. Ophthalmol. 2010, 4, 171–178. [Google Scholar] [CrossRef][Green Version]
- Faucher, M.; Anctil, J.L.; Rodrigue, M.A.; Duchesne, A.; Bergeron, D.; Blondeau, P.; Cote, G.; Dubois, S.; Bergeron, J.; Arseneault, R.; et al. Founder TIGR/myocilin mutations for glaucoma in the Quebec population. Hum. Mol. Genet. 2002, 11, 2077–2090. [Google Scholar] [CrossRef]
- Karunaratne, V.K.K. Quantitative Traits Related to Primary Open Angle Glaucoma in the Scottish Population Isolate of Orkney; University of Edinburgh: Edinburgh, UK, 2012. [Google Scholar]
- Axenovich, T.; Zorkoltseva, I.; Belonogova, N.; van Koolwijk, L.M.; Borodin, P.; Kirichenko, A.; Babenko, V.; Ramdas, W.D.; Amin, N.; Despriet, D.D.; et al. Linkage and association analyses of glaucoma related traits in a large pedigree from a Dutch genetically isolated population. J. Med. Genet. 2011, 48, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Tanigawa, Y.; Wainberg, M.; Karjalainen, J.; Kiiskinen, T.; Venkataraman, G.; Lemmela, S.; Turunen, J.A.; Graham, R.R.; Havulinna, A.S.; Perola, M.; et al. Rare protein-altering variants in ANGPTL7 lower intraocular pressure and protect against glaucoma. PLoS Genet. 2020, 16, e1008682. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.D.; Cooke Bailey, J.N.; D’Aoust, L.; Cade, W.; Ayala-Haedo, J.; Fuzzell, D.; Laux, R.; Adams, L.D.; Reinhart-Mercer, L.; Caywood, L.; et al. Rare complement factor H variant associated with age-related macular degeneration in the Amish. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4455–4460. [Google Scholar] [CrossRef]
- Nittala, M.G.; Velaga, S.B.; Hariri, A.; Pfau, M.; Birch, D.G.; Haines, J.; Pericak-Vance, M.A.; Stambolian, D.; Sadda, S.R. Retinal Sensitivity Using Microperimetry in Age-Related Macular Degeneration in an Amish Population. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, e236–e241. [Google Scholar] [CrossRef]
- Sardell, R.J.; Nittala, M.G.; Adams, L.D.; Laux, R.A.; Bailey, J.N.C.; Fuzzell, D.; Fuzzell, S.; Reinhart-Mercer, L.; Caywood, L.J.; Horst, V.; et al. Heritability of Choroidal Thickness in the Amish. Ophthalmology 2016, 123, 2537–2544. [Google Scholar] [CrossRef]
- Waksmunski, A.R.; Igo, R.P., Jr.; Song, Y.E.; Cooke Bailey, J.N.; Laux, R.; Fuzzell, D.; Fuzzell, S.; Adams, L.D.; Caywood, L.; Prough, M.; et al. Rare variants and loci for age-related macular degeneration in the Ohio and Indiana Amish. Hum. Genet. 2019, 138, 1171–1182. [Google Scholar] [CrossRef]
- Kraybill, D.B.; Johnson-Weiner, K.M.; Nolt, S.M. The Amish; Johns Hopkins University Press: Baltimore, MD, USA, 2013. [Google Scholar]
- Mitchell, B.D.; McArdle, P.F.; Shen, H.; Rampersaud, E.; Pollin, T.I.; Bielak, L.F.; Jaquish, C.; Douglas, J.A.; Roy-Gagnon, M.H.; Sack, P.; et al. The genetic response to short-term interventions affecting cardiovascular function: Rationale and design of the Heredity and Phenotype Intervention (HAPI) Heart Study. Am. Heart J. 2008, 155, 823–828. [Google Scholar] [CrossRef]
- Cross, H.E.; Crosby, A.H. Amish Contributions to Medical Genetics. Mennon. Q. Rev. 2008, 82, 449–467. [Google Scholar]
- Strauss, K.A.; Puffenberger, E.G. Genetics, medicine, and the Plain people. Annu. Rev. Genom. Hum. Genet. 2009, 10, 513–536. [Google Scholar] [CrossRef]
- Hatzikotoulas, K.; Gilly, A.; Zeggini, E. Using population isolates in genetic association studies. Brief. Funct. Genom. 2014, 13, 371–377. [Google Scholar] [CrossRef] [PubMed]
- McKusick, V.A.; Hostetler, J.A.; Egeland, J.A. Genetic Studies of the Amish. In Background and Potentialities; Bulletin of the Johns Hopkins Hospital: Baltimore, MD, USA, 1964; Volume 115, pp. 203–222. [Google Scholar]
- Donnermeyer, J.F.; Anderson, C.; Cooksey, E.C. The Amish Population: County Estimates and Settlement Patterns. J. Amish Plain Anabapt. Stud. 2013, 1, 72–109. [Google Scholar] [CrossRef]
- Nolt, S.M. A History of the Amish, 3rd ed.; Good Books: New York, NY, USA, 2016. [Google Scholar]
- Pericak-Vance, M.A.; Johnson, C.C.; Rimmler, J.B.; Saunders, A.M.; Robinson, L.C.; D’Hondt, E.G.; Jackson, C.E.; Haines, J.L. Alzheimer’s disease and apolipoprotein E-4 allele in an Amish population. Ann. Neurol. 1996, 39, 700–704. [Google Scholar] [CrossRef] [PubMed]
- van der Walt, J.M.; Scott, W.K.; Slifer, S.; Gaskell, P.C.; Martin, E.R.; Welsh-Bohmer, K.; Creason, M.; Crunk, A.; Fuzzell, D.; McFarland, L.; et al. Maternal lineages and Alzheimer disease risk in the Old Order Amish. Hum. Genet. 2005, 118, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ashley-Koch, A.E.; Shao, Y.; Rimmler, J.B.; Gaskell, P.C.; Welsh-Bohmer, K.A.; Jackson, C.E.; Scott, W.K.; Haines, J.L.; Pericak-Vance, M.A. An autosomal genomic screen for dementia in an extended Amish family. Neurosci. Lett. 2005, 379, 199–204. [Google Scholar] [CrossRef]
- McCauley, J.L.; Hahs, D.W.; Jiang, L.; Scott, W.K.; Welsh-Bohmer, K.A.; Jackson, C.E.; Vance, J.M.; Pericak-Vance, M.A.; Haines, J.L. Combinatorial Mismatch Scan (CMS) for loci associated with dementia in the Amish. BMC Med. Genet. 2006, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Hahs, D.W.; McCauley, J.L.; Crunk, A.E.; McFarland, L.L.; Gaskell, P.C.; Jiang, L.; Slifer, S.H.; Vance, J.M.; Scott, W.K.; Welsh-Bohmer, K.A.; et al. A genome-wide linkage analysis of dementia in the Amish. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006, 141B, 160–166. [Google Scholar] [CrossRef]
- Lee, S.L.; Murdock, D.G.; McCauley, J.L.; Bradford, Y.; Crunk, A.; McFarland, L.; Jiang, L.; Wang, T.; Schnetz-Boutaud, N.; Haines, J.L. A genome-wide scan in an Amish pedigree with parkinsonism. Ann. Hum. Genet. 2008, 72, 621–629. [Google Scholar] [CrossRef]
- Edwards, D.R.V.; Gilbert, J.R.; Jiang, L.; Gallins, P.J.; Caywood, L.; Creason, M.; Fuzzell, D.; Knebusch, C.; Jackson, C.E.; Pericak-Vance, M.A.; et al. Successful aging shows linkage to chromosomes 6, 7, and 14 in the Amish. Ann. Hum. Genet. 2011, 75, 516–528. [Google Scholar] [CrossRef]
- D’Aoust, L.N.; Cummings, A.C.; Laux, R.; Fuzzell, D.; Caywood, L.; Reinhart-Mercer, L.; Scott, W.K.; Pericak-Vance, M.A.; Haines, J.L. Examination of candidate exonic variants for association to Alzheimer disease in the Amish. PLoS ONE 2015, 10, e0118043. [Google Scholar] [CrossRef]
- Nittala, M.G.; Song, Y.E.; Sardell, R.; Adams, L.D.; Pan, S.; Velaga, S.B.; Horst, V.; Dana, D.; Caywood, L.; Laux, R.; et al. AMISH EYE STUDY: Baseline Spectral Domain Optical Coherence Tomography Characteristics of Age-Related Macular Degeneration. Retina 2019, 39, 1540–1550. [Google Scholar] [CrossRef]
- Prum, B.E., Jr.; Rosenberg, L.F.; Gedde, S.J.; Mansberger, S.L.; Stein, J.D.; Moroi, S.E.; Herndon, L.W., Jr.; Lim, M.C.; Williams, R.D. Primary Open-Angle Glaucoma Preferred Practice Pattern((R)) Guidelines. Ophthalmology 2016, 123, P41–P111. [Google Scholar] [CrossRef] [PubMed]
- Prum, B.E., Jr.; Lim, M.C.; Mansberger, S.L.; Stein, J.D.; Moroi, S.E.; Gedde, S.J.; Herndon, L.W., Jr.; Rosenberg, L.F.; Williams, R.D. Primary Open-Angle Glaucoma Suspect Preferred Practice Pattern((R)) Guidelines. Ophthalmology 2016, 123, P112–P151. [Google Scholar] [CrossRef] [PubMed]
- McKusick, V.A. The Amish. Endeavour 1980, 4, 52–57. [Google Scholar] [CrossRef]
- Colyer, C.; Anderson, C.; Stein, R.; Donnermeyer, J.F.; Wasao, S. Reviving the Demographic Study of the Amish. J. Amish Plain Anabapt. Stud. 2017, 5, 96–119. [Google Scholar] [CrossRef]
- Agarwala, R.; Biesecker, L.G.; Schaffer, A.A. Anabaptist genealogy database. Am. J. Med. Genet. C Semin. Med. Genet. 2003, 121C, 32–37. [Google Scholar] [CrossRef]
- Garbe, J.R.; Da, Y. Pedigraph: A Software Tool for the Graphing and Analysis of Large Complex Pedigree; Department of Animal Science, University of Minnesota: Minneapolis, MN, USA, 2008. [Google Scholar]
- Bourgain, C. KinInbcoef: Calculation of Kinship and Inbreeding Coefficients. 2003. Available online: http://www.stat.uchicago.edu/~mcpeek/software/KinInbcoef/index.html (accessed on 22 September 2020).
- Wiggs, J.L. Glaucoma Genes and Mechanisms. Prog. Mol. Biol. Transl. Sci. 2015, 134, 315–342. [Google Scholar] [CrossRef]
- Wiggs, J.L. Genetic etiologies of glaucoma. Arch. Ophthalmol. 2007, 125, 30–37. [Google Scholar] [CrossRef]
- Tielsch, J.M.; Katz, J.; Sommer, A.; Quigley, H.A.; Javitt, J.C. Family history and risk of primary open angle glaucoma. The Baltimore Eye Survey. Arch. Ophthalmol. 1994, 112, 69–73. [Google Scholar] [CrossRef]
- Wolfs, R.C.; Klaver, C.C.; Ramrattan, R.S.; van Duijn, C.M.; Hofman, A.; de Jong, P.T. Genetic risk of primary open-angle glaucoma. Population-based familial aggregation study. Arch. Ophthalmol. 1998, 116, 1640–1645. [Google Scholar] [CrossRef]
- Francomano, C.A.; McKusick, V.A.; Biesecker, L.G. Medical genetic studies in the Amish: Historical perspective. Am. J. Med. Genet. C Semin. Med. Genet. 2003, 121C, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.D.; Lee, W.J.; Tolea, M.I.; Shields, K.; Ashktorab, Z.; Magder, L.S.; Ryan, K.A.; Pollin, T.I.; McArdle, P.F.; Shuldiner, A.R.; et al. Living the good life? Mortality and hospital utilization patterns in the Old Order Amish. PLoS ONE 2012, 7, e51560. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.N.; Sutherland, J.; Levin, A.V.; Klose, R.; Priston, M.; Heon, E. Molecular characterisation of congenital glaucoma in a consanguineous Canadian community: A step towards preventing glaucoma related blindness. J. Med. Genet. 2000, 37, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Xin, B.; Jones, S.; Puffenberger, E.G.; Hinze, C.; Bright, A.; Tan, H.; Zhou, A.; Wu, G.; Vargus-Adams, J.; Agamanolis, D.; et al. Homozygous mutation in SAMHD1 gene causes cerebral vasculopathy and early onset stroke. Proc. Natl. Acad. Sci. USA 2011, 108, 5372–5377. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.E.; Klein, R.; Lee, K.E. Heritability of risk factors for primary open-angle glaucoma: The Beaver Dam Eye Study. Investig. Ophthalmol. Vis. Sci. 2004, 45, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Auer, P.L.; Lettre, G. Rare variant association studies: Considerations, challenges and opportunities. Genome Med. 2015, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Arcos-Burgos, M.; Muenke, M. Genetics of population isolates. Clin. Genet. 2002, 61, 233–247. [Google Scholar] [CrossRef]
- Agarwala, R.; Schaffer, A.A.; Tomlin, J.F. Towards a complete North American Anabaptist Genealogy II: Analysis of inbreeding. Hum. Biol. 2001, 73, 533–545. [Google Scholar] [CrossRef]
- Wang, L.; Choi, S.; Lee, S.; Park, T.; Won, S. Comparing family-based rare variant association tests for dichotomous phenotypes. BMC Proc. 2016, 10, 181–186. [Google Scholar] [CrossRef]
- Choi, S.; Lee, S.; Cichon, S.; Nothen, M.M.; Lange, C.; Park, T.; Won, S. FARVAT: A family-based rare variant association test. Bioinformatics 2014, 30, 3197–3205. [Google Scholar] [CrossRef]
- Laird, N.M.; Lange, C. Family-based designs in the age of large-scale gene-association studies. Nat. Rev. Genet. 2006, 7, 385–394. [Google Scholar] [CrossRef]
- Pasquale, L.R.; Kang, J.H. Lifestyle, nutrition, and glaucoma. J. Glaucoma 2009, 18, 423–428. [Google Scholar] [CrossRef]
- Wiggs, J.L. The cell and molecular biology of complex forms of glaucoma: Updates on genetic, environmental, and epigenetic risk factors. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2467–2469. [Google Scholar] [CrossRef]
- Ong, S.R.; Crowston, J.G.; Loprinzi, P.D.; Ramulu, P.Y. Physical activity, visual impairment, and eye disease. Eye (London) 2018, 32, 1296–1303. [Google Scholar] [CrossRef]
- Katz, M.L.; Ferketich, A.K.; Broder-Oldach, B.; Harley, A.; Reiter, P.L.; Paskett, E.D.; Bloomfield, C.D. Physical activity among Amish and non-Amish adults living in Ohio Appalachia. J. Community Health 2012, 37, 434–440. [Google Scholar] [CrossRef]
- Carter, G.B.C.; Katz, M.L.; Ferketich, A.K.; Clinton, S.K.; Grainger, E.M.; Paskett, E.D.; Bloomfield, C.D. Dietary intake, food processing, and cooking methods among Amish and non-Amish adults living in Ohio Appalachia: Relevance to nutritional risk factors for cancer. Nutr. Cancer 2011, 63, 1208–1217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferketich, A.K.; Katz, M.L.; Kauffman, R.M.; Paskett, E.D.; Lemeshow, S.; Westman, J.A.; Clinton, S.K.; Bloomfield, C.D.; Wewers, M.E. Tobacco use among the Amish in Holmes County, Ohio. J. Rural Health 2008, 24, 84–90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johnson, C.C.; Rybicki, B.A.; Brown, G.; D’Hondt, E.; Herpolsheimer, B.; Roth, D.; Jackson, C.E. Cognitive impairment in the Amish: A four county survey. Int. J. Epidemiol. 1997, 26, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, B.D.; Hsueh, W.C.; King, T.M.; Pollin, T.I.; Sorkin, J.; Agarwala, R.; Schaffer, A.A.; Shuldiner, A.R. Heritability of life span in the Old Order Amish. Am. J. Med. Genet. 2001, 102, 346–352. [Google Scholar] [CrossRef]
- Racette, B.A.; Rundle, M.; Wang, J.C.; Goate, A.; Saccone, N.L.; Farrer, M.; Lincoln, S.; Hussey, J.; Smemo, S.; Lin, J.; et al. A multi-incident, Old-Order Amish family with PD. Neurology 2002, 58, 568–574. [Google Scholar] [CrossRef]
- Sorkin, J.; Post, W.; Pollin, T.I.; O’Connell, J.R.; Mitchell, B.D.; Shuldiner, A.R. Exploring the genetics of longevity in the Old Order Amish. Mech. Ageing Dev. 2005, 126, 347–350. [Google Scholar] [CrossRef]
- Stambolian, D.; Ciner, E.B.; Reider, L.C.; Moy, C.; Dana, D.; Owens, R.; Schlifka, M.; Holmes, T.; Ibay, G.; Bailey-Wilson, J.E. Genome-wide scan for myopia in the Old Order Amish. Am. J. Ophthalmol. 2005, 140, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Peet, J.A.; Cotch, M.F.; Wojciechowski, R.; Bailey-Wilson, J.E.; Stambolian, D. Heritability and familial aggregation of refractive error in the Old Order Amish. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4002–4006. [Google Scholar] [CrossRef][Green Version]
- Post, W.; Bielak, L.F.; Ryan, K.A.; Cheng, Y.C.; Shen, H.; Rumberger, J.A.; Sheedy, P.F., 2nd; Shuldiner, A.R.; Peyser, P.A.; Mitchell, B.D. Determinants of coronary artery and aortic calcification in the Old Order Amish. Circulation 2007, 115, 717–724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pollin, T.I.; Damcott, C.M.; Shen, H.; Ott, S.H.; Shelton, J.; Horenstein, R.B.; Post, W.; McLenithan, J.C.; Bielak, L.F.; Peyser, P.A.; et al. A null mutation in human APOC3 confers a favorable plasma lipid profile and apparent cardioprotection. Science 2008, 322, 1702–1705. [Google Scholar] [CrossRef] [PubMed]
- Racette, B.A.; Good, L.M.; Kissel, A.M.; Criswell, S.R.; Perlmutter, J.S. A population-based study of parkinsonism in an Amish community. Neuroepidemiology 2009, 33, 225–230. [Google Scholar] [CrossRef]
- Wojciechowski, R.; Stambolian, D.; Ciner, E.; Ibay, G.; Holmes, T.N.; Bailey-Wilson, J.E. Genomewide linkage scans for ocular refraction and meta-analysis of four populations in the Myopia Family Study. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2024–2032. [Google Scholar] [CrossRef]
- Wojciechowski, R.; Bailey-Wilson, J.E.; Stambolian, D. Fine-mapping of candidate region in Amish and Ashkenazi families confirms linkage of refractive error to a QTL on 1p34-p36. Mol. Vis. 2009, 15, 1398–1406. [Google Scholar]
- Wojciechowski, R.; Bailey-Wilson, J.E.; Stambolian, D. Association of matrix metalloproteinase gene polymorphisms with refractive error in Amish and Ashkenazi families. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4989–4995. [Google Scholar] [CrossRef][Green Version]
- Cummings, A.C.; Lee, S.L.; McCauley, J.L.; Jiang, L.; Crunk, A.; McFarland, L.L.; Gallins, P.J.; Fuzzell, D.; Knebusch, C.; Jackson, C.E.; et al. A genome-wide linkage screen in the Amish with Parkinson disease points to chromosome 6. Ann. Hum. Genet. 2011, 75, 351–358. [Google Scholar] [CrossRef]
- Cummings, A.C.; Jiang, L.; Velez Edwards, D.R.; McCauley, J.L.; Laux, R.; McFarland, L.L.; Fuzzell, D.; Knebusch, C.; Caywood, L.; Reinhart-Mercer, L.; et al. Genome-wide association and linkage study in the Amish detects a novel candidate late-onset Alzheimer disease gene. Ann. Hum. Genet. 2012, 76, 342–351. [Google Scholar] [CrossRef]
- Courtenay, M.D.; Gilbert, J.R.; Jiang, L.; Cummings, A.C.; Gallins, P.J.; Caywood, L.; Reinhart-Mercer, L.; Fuzzell, D.; Knebusch, C.; Laux, R.; et al. Mitochondrial haplogroup X is associated with successful aging in the Amish. Hum. Genet. 2012, 131, 201–208. [Google Scholar] [CrossRef]
- Davis, M.F.; Cummings, A.C.; D’Aoust, L.N.; Jiang, L.; Velez Edwards, D.R.; Laux, R.; Reinhart-Mercer, L.; Fuzzell, D.; Scott, W.K.; Pericak-Vance, M.A.; et al. Parkinson disease loci in the mid-western Amish. Hum. Genet. 2013, 132, 1213–1221. [Google Scholar] [CrossRef]
- Edwards, D.R.V.; Gilbert, J.R.; Hicks, J.E.; Myers, J.L.; Jiang, L.; Cummings, A.C.; Guo, S.; Gallins, P.J.; Konidari, I.; Caywood, L.; et al. Linkage and association of successful aging to the 6q25 region in large Amish kindreds. Age (Dordr) 2013, 35, 1467–1477. [Google Scholar] [CrossRef]
- Hou, L.; Faraci, G.; Chen, D.T.; Kassem, L.; Schulze, T.G.; Shugart, Y.Y.; McMahon, F.J. Amish revisited: Next-generation sequencing studies of psychiatric disorders among the Plain people. Trends Genet. 2013, 29, 412–418. [Google Scholar] [CrossRef]
- Crawford, D.C.; Dumitrescu, L.; Goodloe, R.; Brown-Gentry, K.; Boston, J.; McClellan, B., Jr.; Sutcliffe, C.; Wiseman, R.; Baker, P.; Pericak-Vance, M.A.; et al. Rare variant APOC3 R19X is associated with cardio-protective profiles in a diverse population-based survey as part of the Epidemiologic Architecture for Genes Linked to Environment Study. Circ. Cardiovasc. Genet. 2014, 7, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Chavali, V.R.; Diniz, B.; Huang, J.; Ying, G.S.; Sadda, S.R.; Stambolian, D. Association of OCT derived drusen measurements with AMD associated-genotypic SNPs in Amish population. J. Clin. Med. 2015, 4, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Musolf, A.M.; Simpson, C.L.; Alexander, T.A.; Portas, L.; Murgia, F.; Ciner, E.B.; Stambolian, D.; Bailey-Wilson, J.E. Genome-wide scans of myopia in Pennsylvania Amish families reveal significant linkage to 12q15, 8q21.3 and 5p15.33. Hum. Genet. 2019, 138, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, W.C.; Mitchell, B.D.; Aburomia, R.; Pollin, T.; Sakul, H.; Gelder Ehm, M.; Michelsen, B.K.; Wagner, M.J.; St Jean, P.L.; Knowler, W.C.; et al. Diabetes in the Old Order Amish: Characterization and heritability analysis of the Amish Family Diabetes Study. Diabetes Care 2000, 23, 595–601. [Google Scholar] [CrossRef]
- Medical Alumni Association of the University of Maryland. Expediting genetic research with help from the Amish. Bulletin Fall 2004. Available online: https://www.medicalalumni.org/bulletin/fall_2004/lead1.html (accessed on 8 October 2020).
- University of Maryland School of Medicine. Amish Research Program. Available online: https://www.medschool.umaryland.edu/endocrinology/Amish-Research-Program/ (accessed on 8 October 2020).
- Kaplan, B.; Caddle-Steele, C.; Chisholm, G.; Esmond, W.A.; Ferryman, K.; Gertner, M.; Goytia, C.; Hauser, D.; Richardson, L.D.; Robinson, M.; et al. A Culture of Understanding: Reflections and Suggestions from a Genomics Research Community Board. Prog. Community Health Partnersh. 2017, 11, 161–165. [Google Scholar] [CrossRef]
- Skinner, J.S.; Williams, N.A.; Richmond, A.; Brown, J.; Strelnick, A.H.; Calhoun, K.; de Loney, E.H.; Allen, S.; Pirie, A.; Wilkins, C.H. Community Experiences and Perceptions of Clinical and Translational Research and Researchers. Prog. Community Health Partnersh. 2018, 12, 263–271. [Google Scholar] [CrossRef]
| Affected | Unaffected | ||
|---|---|---|---|
| N | 42 | 20 | |
| Age | Mean ± SD | 67.60 ± 11.39 | 56.95 ± 10.33 |
| Sex | Male | 19 | 7 |
| Female | 23 | 13 | |
| Family History of Glaucoma | Affected (%) | Unaffected (%) | All (%) | |
|---|---|---|---|---|
| First-Degree Relatives | No | 8 (19.05) | 13 (65) | 21 (33.87) |
| Yes | 34 (80.95) | 7 (35) | 41 (66.13) | |
| Extended Family | Unknown | 1 (2.38) | 1 (5) | 2 (3.23) |
| No | 12 (28.57) | 14 (70) | 26 (41.94) | |
| Yes | 29 (69.05) | 5 (25) | 34 (54.84) | |
| Affected | Unaffected | |
|---|---|---|
| Average ± SD (Range) | Average ± SD (Range) | |
| OD IOP (mmHg) | 15.1 ± 4.0 (3–22) | 13.5 ± 2.8 (8–19) |
| OS IOP (mmHg) | 15.4 ± 3.7 (9–22) | 13.8 ± 3.1 (8–21) |
| OD VCDR | 0.62 ± 0.12 (0.30–0.90) | 0.38 ± 0.15 (0.10–0.64) |
| OS VCDR | 0.66 ± 0.12 (0.40–0.90) | 0.37 ± 0.16 (0.06–0.62) |
| OD Refractive error (D) | 0.59 ± 1.55 (−4.00, +4.50) | −0.17 ± 2.34 (−6.00, +3.25) |
| OS Refractive error (D) | 0.49 ± 1.54 (−3.00, +5.00) | −0.21 ± 2.32 (−6.50, +3.00) |
| OD CCT (Microns) | 549 ± 43 (440–658) | 559 ± 29 (516–606) |
| OS CCT (Microns) | 547 ± 42 (466–638) | 564 ± 34 (514–627) |
| OD Axial Length * (mm) | 23.70 ± 0.86 (22.02–25.47) | 23.71 ± 1.13 (21.58–26.30) |
| OS Axial Length * (mm) | 23.60 ± 0.85 (21.86–25.20) | 23.58 ± 1.10 (21.59–25.98) |
| POAG | |
|---|---|
| Average ± SD (Range) | |
| OD IOP (mmHg) | 15.0 ± 4.2 (3–22) |
| OS IOP (mmHg) | 15.1 ± 3.6 (9–22) |
| OD VCDR | 0.63 ± 0.11 (0.30–0.85) |
| OS VCDR | 0.66 ± 0.11 (0.40–0.90) |
| OD Refractive error (D) | 0.56 ± 1.59 (−4.00, +4.50) |
| OS Refractive error (D) | 0.53 ± 1.57 (−3.00, +5.00) |
| OD CCT (Microns) | 547 ± 43 (440–658) |
| OS CCT (Microns) | 545 ± 42 (466–638) |
| OD Axial Length * (mm) | 23.66 ± 0.88 (22.02–25.47) |
| OS Axial Length * (mm) | 23.55 ± 0.84 (21.86–25.09) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waksmunski, A.R.; Song, Y.E.; Kinzy, T.G.; Laux, R.A.; Sewell, J.; Fuzzell, D.; Fuzzell, S.; Miller, S.; Wiggs, J.L.; Pasquale, L.R.; et al. The GGLEAM Study: Understanding Glaucoma in the Ohio Amish. Int. J. Environ. Res. Public Health 2021, 18, 1551. https://doi.org/10.3390/ijerph18041551
Waksmunski AR, Song YE, Kinzy TG, Laux RA, Sewell J, Fuzzell D, Fuzzell S, Miller S, Wiggs JL, Pasquale LR, et al. The GGLEAM Study: Understanding Glaucoma in the Ohio Amish. International Journal of Environmental Research and Public Health. 2021; 18(4):1551. https://doi.org/10.3390/ijerph18041551
Chicago/Turabian StyleWaksmunski, Andrea R., Yeunjoo E. Song, Tyler G. Kinzy, Reneé A. Laux, Jane Sewell, Denise Fuzzell, Sarada Fuzzell, Sherri Miller, Janey L. Wiggs, Louis R. Pasquale, and et al. 2021. "The GGLEAM Study: Understanding Glaucoma in the Ohio Amish" International Journal of Environmental Research and Public Health 18, no. 4: 1551. https://doi.org/10.3390/ijerph18041551
APA StyleWaksmunski, A. R., Song, Y. E., Kinzy, T. G., Laux, R. A., Sewell, J., Fuzzell, D., Fuzzell, S., Miller, S., Wiggs, J. L., Pasquale, L. R., Skarie, J. M., Haines, J. L., & Cooke Bailey, J. N. (2021). The GGLEAM Study: Understanding Glaucoma in the Ohio Amish. International Journal of Environmental Research and Public Health, 18(4), 1551. https://doi.org/10.3390/ijerph18041551







