Investigation of Acinetobacter baumannii Activity in Vascular Surgery Units through Epidemiological Management Based on the Analysis of Antimicrobial Resistance, Biofilm Formation and Genotyping
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates
2.2. Antibiotic Resistance
2.3. Screening for Metallo-β-Lactamase Phenotype
2.4. Screening for Carbapenemase Type Beta-Lactamases Phenotype
2.5. Polymerase Chain Reaction
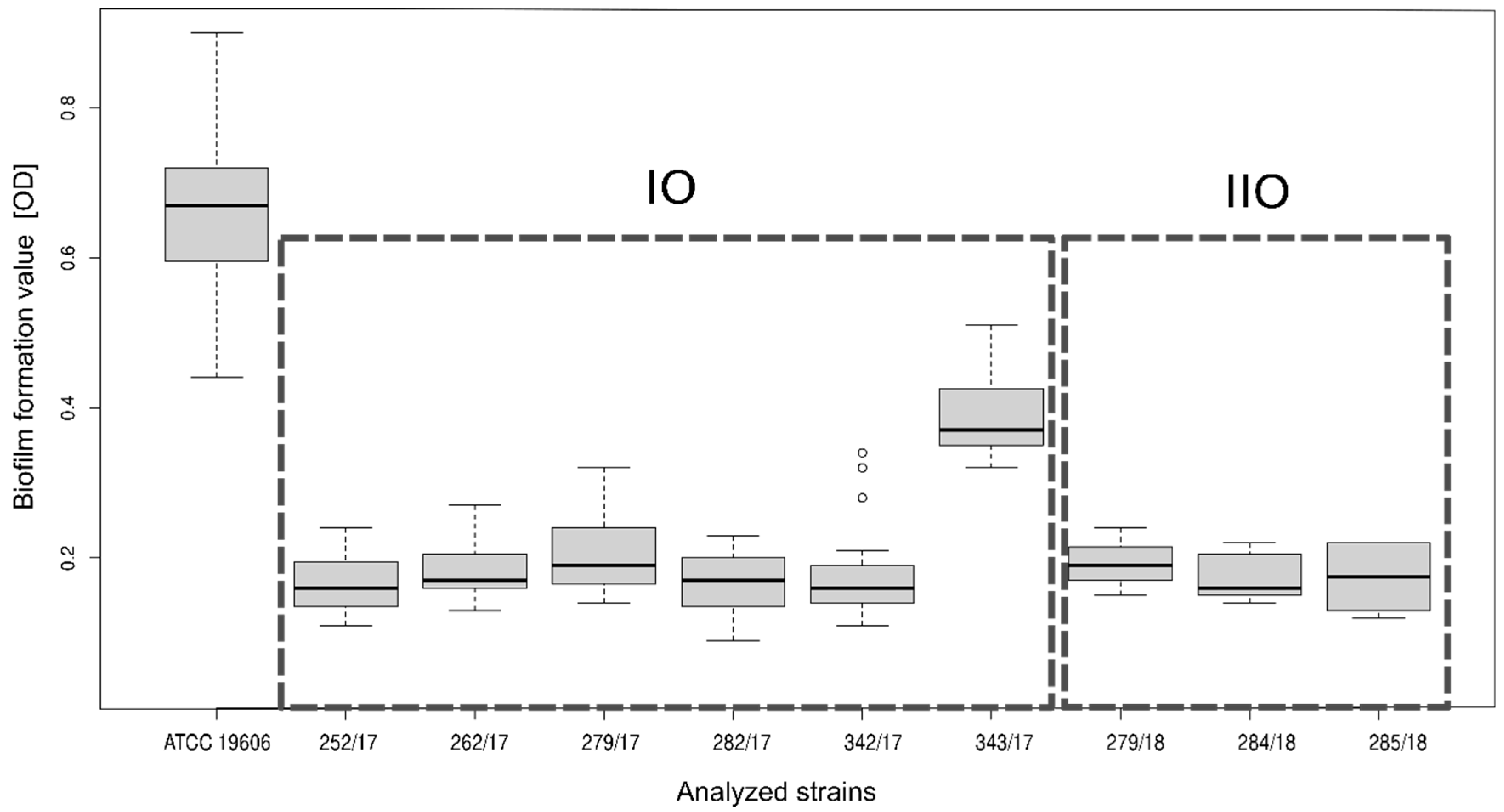
2.6. Biofilm Formation
2.7. Multi-Locus Sequence Typing
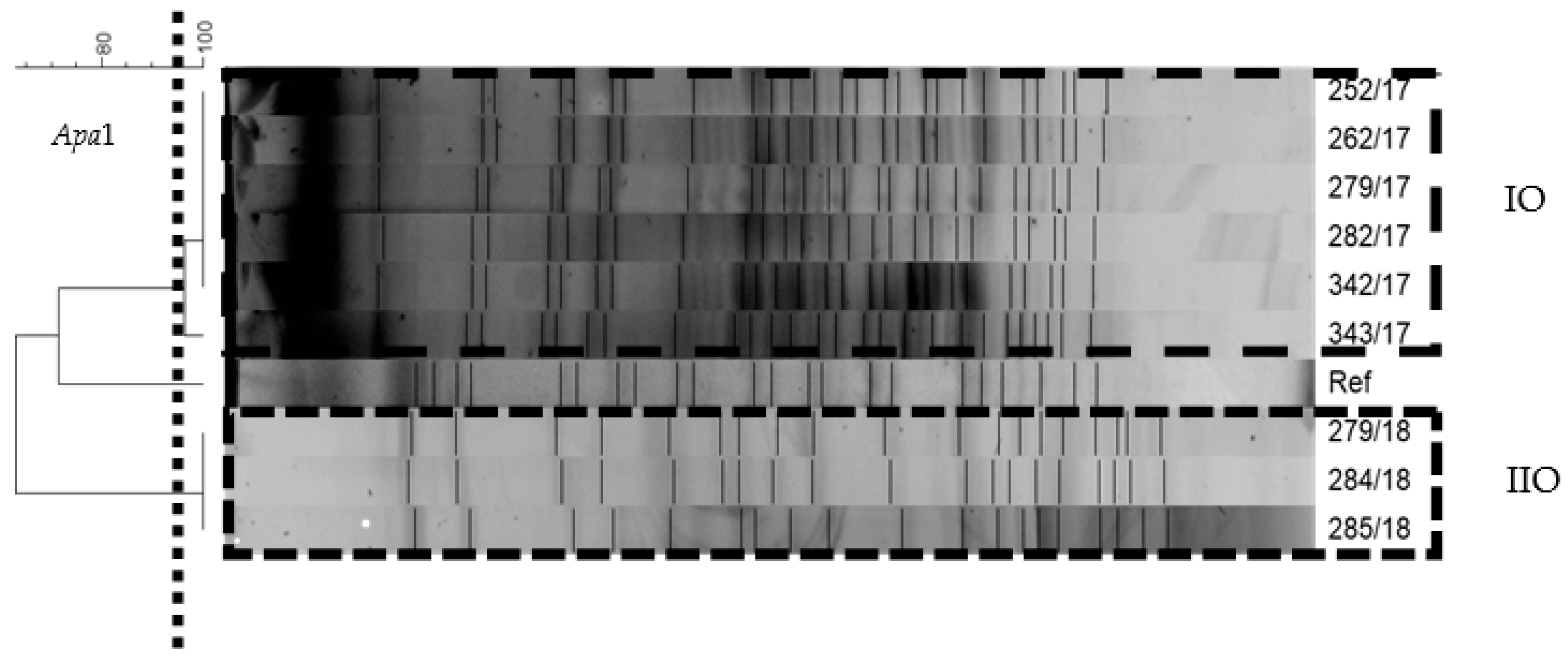
2.8. Pulsed-Field Gel Electrophoresis
2.9. Characteristics of Hospital
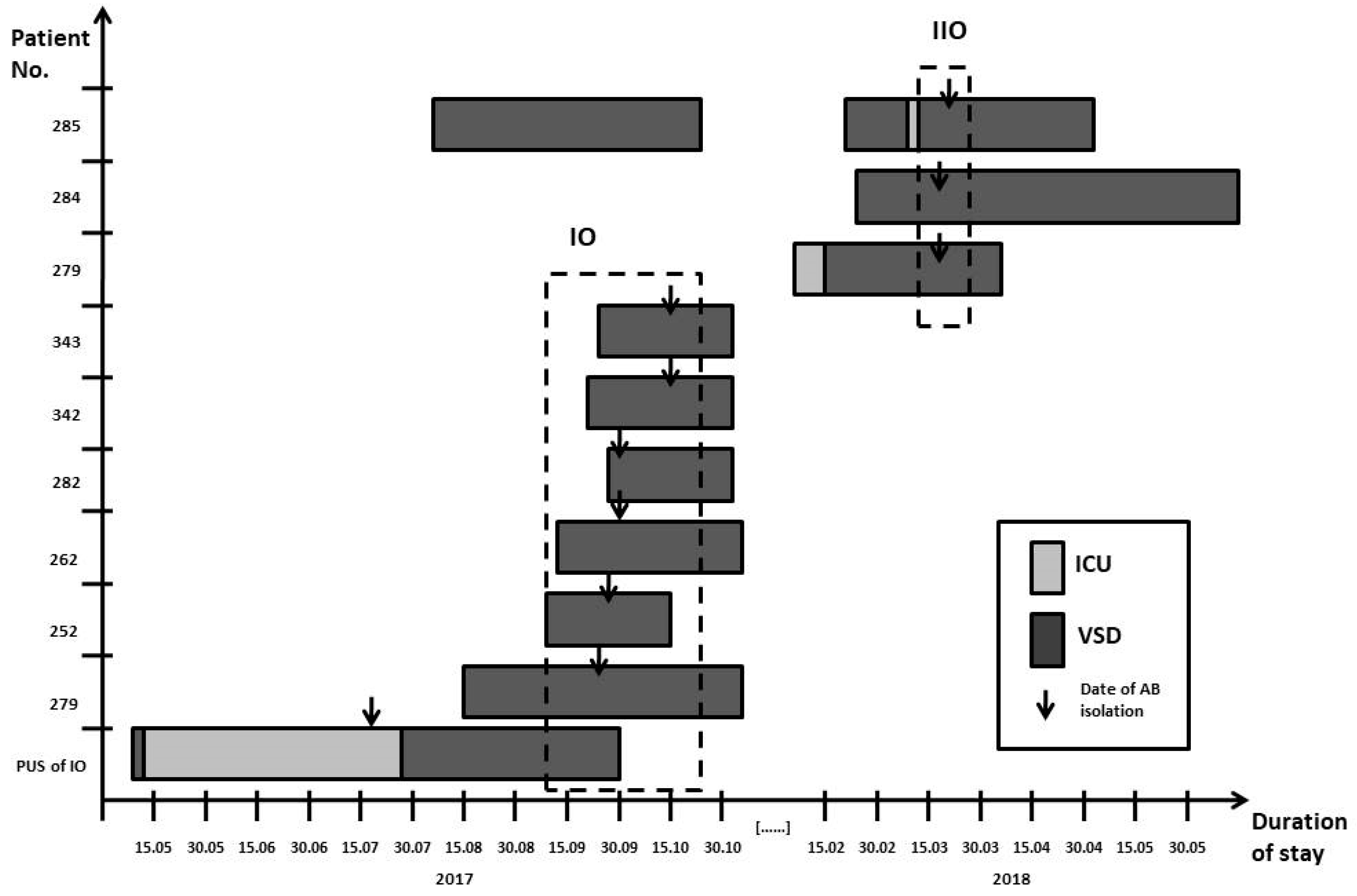
2.10. Epidemiological Investigation
2.11. Statistical Methods
3. Results
3.1. Characteristics of Patients
3.2. Characteristics of A. baumannii Strains
3.3. Epidemiological Investigation
3.4. Outbreak Response
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garnacho-Montero, J.; Amaya-Villar, R. Multiresistant Acinetobacter baumannii infections: Epidemiology and management. Curr. Opin. Infect. Dis. 2010, 23, 332–339. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godziszewska, J.; Guzek, D.; Głąbski, K.; Wierzbicka, A. Mobile antibiotic resistance—The spread of genes determining the resistance of bacteria through food products. Postepy Hig. Med. Dosw. 2016, 70, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Lambiase, A.; Piazza, O.; Rossano, F.; Del Pezzo, M.; Tufano, R.; Catania, M.R. Persistence of carbapenem-resistant Acinetobacter baumannii strains in an Italian intensive care unit during a forty-six month study period. New Microbiol. 2012, 35, 199–206. [Google Scholar] [PubMed]
- Antunes, L.C.S.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef] [Green Version]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzyściak, P.; Chmielarczyk, A.; Pobiega, M.; Romaniszyn, D.; Wójkowska-Mach, J. Acinetobacter baumannii isolated from hospital-acquired infection: Biofilm production and drug susceptibility. APMIS 2017, 125, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- European Antimicrobial Resistance Surveillance, (EARS-Net). ECDC Surveillance Atlas—Antimicrobial Resistance. 2018. Available online: https://www.ecdc.europa.eu/en/antimicrobial-resistance/surveillance-and-disease-data/data-ecdc (accessed on 2 July 2020).
- Chmielarczyk, A.; Pobiega, M.; Ziółkowski, G.; Pomorska-Wesołowska, M.; Romaniszyn, D.; Krawczyk, L.; Wójkowska-Mach, J. Severe infections caused by multidrug-resistant non-fermentative bacilli in southern Poland. Adv. Clin. Exp. Med. 2018, 27, 401–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, P.G.; Janßen, K.; Fresen, M.M.; Wisplinghoff, H.; Seifert, H. Molecular Epidemiology of Acinetobacter baumannii Bloodstream Isolates Obtained in the United States from 1995 to 2004 Using rep-PCR and Multilocus Sequence Typing. J. Clin. Microbiol. 2012, 50, 3493–3500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.H.; Li, J.F.; Huang, L.Y.; Lin, F.M.; Yang, Y.S.; Siu, L.K.; Chang, F.Y.; Lin, J.C. Outbreak of imipenem-resistant Acinetobacter baumannii in different wards at a regional hospital related to untrained bedside caregivers. Am. J. Infect. Control. 2017, 45, 1086–1090. [Google Scholar] [CrossRef]
- Zollner-Schwetz, I.; Zechner, E.; Ullrich, E.; Luxner, J.; Pux, C.; Pichler, G.; Schippinger, W.; Krause, R.; Leitner, E. Colonization of long term care facility patients with MDR-Gram-negatives during an Acinetobacter baumannii outbreak. Antimicrob. Resist. Infect. Control. 2017, 16, 49. [Google Scholar] [CrossRef] [Green Version]
- Tanguy, M.; Kouatchet, A.; Tanguy, B.; Pichard Fanello, S.; Joly-Guillou, M.L. Prise en charge d’une épidémie à Acinetobacter baumannii en service de réanimation médicale. Med. Mal. Infect. 2017, 47, 409–414. [Google Scholar] [CrossRef]
- Duszynska, W.; Litwin, A.; Rojek, S.; Szczesny, A.; Ciasullo, A.; Gozdzik, W. Analysis of Acinetobacter baumannii hospital infections in patients treated at the intensive care unit of the University Hospital, Wroclaw, Poland: A 6-year, single-center, retrospective study. Infect. Drug Resist. 2018, 11, 629–635. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.; Daikos, G.L.; Durante-Mangoni, E.; Yahav, D.; Carmeli, Y.; Benattar, Y.D.; Skiada, A.; Andini, R.; Eliakim-Raz, N.; Nutman, A.; et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: An open-label, randomised controlled trial. Lancet Infect. Dis. 2018, 18, 391–400. [Google Scholar] [CrossRef]
- Lorenzin, G.; Scaltriti, E.; Gargiulo, F.; Caccuri, F.; Piccinelli, G.; Gurrieri, F.; Caruso, A.; De Francesco, M.A. Extensively drug-resistant Acinetobacter baumannii isolated from intensive care units in northern Italy: A genomic approach to characterize new sequence types. Future Microbiol. 2019, 14, 1281–1292. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Sharma, A.; Sen, M.K.; Rani, V.; Gaind, R.; Suri, J.C. Phenotypic and molecular characterization of Acinetobacter baumannii isolates causing lower respiratory infections among ICU patients. Microb. Pathog. 2019, 128, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Boral, B.; Unaldi, Ö.; Ergin, A.; Durmaz, R.; Köseoğlu Eser, Ö. A prospective multicenter study on the evaluation of antimicrobial resistance and molecular epidemiology of multidrug-resistant Acinetobacter baumannii infections in intensive care units with clinical and environmental features. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 19. [Google Scholar] [CrossRef]
- Feretzakis, G.; Loupelis, E.; Sakagianni, A.; Skarmoutsou, N.; Michelidou, S.; Velentza, A.; Martsoukou, M.; Valakis, K.; Petropoulou, S.; Koutalas, E. A 2-Year Single-Centre Audit on Antibiotic Resistance of Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae Strains from an Intensive Care Unit and Other Wards in a General Public Hospital in Greece. Antibiotics 2019, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Kołpa, M.; Wałaszek, M.; Gniadek, A.; Wolak, Z.; Dobroś, W. Incidence, microbiological profile and risk factors of healthcare-associated infections in intensive care units: A 10 year observation in a provincial hospital in southern Poland. Int. J. Environ. Res. Public Health 2018, 15, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duszyńska, W.; Rosenthal, V.D.; Szczęsny, A.; Woźnica, E.; Ulfik, K.; Ostrowska, E.; Litwin, A.; Kübler, A. Urinary tract infections in intensive care unit patients-a single-centre, 3-year observational study according to the INICC project. Anaesthesiol. Intensive Ther. 2016, 48, 1–6. [Google Scholar] [CrossRef]
- Sieniawski, K.; Kaczka, K.; Rucinska, M.; Gagis, L.; Pomorski, L. Acinetobacter baumannii nosocomial infections. Pol. J. Surg. 2013, 85, 483–490. [Google Scholar] [CrossRef]
- Chmielarczyk, A.; Pilarczyk-Żurek, M.; Kamińska, W.; Pobiega, M.; Romaniszyn, D.; Ziółkowski, G.; Wójkowska-Mach, J.; Bulanda, M. Molecular Epidemiology and Drug Resistance of Acinetobacter baumannii Isolated from Hospitals in Southern Poland: ICU as a Risk Factor for XDR Strains. Microb. Drug Resist. 2016, 22, 328–335. [Google Scholar] [CrossRef]
- The European Committe on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint Tables for Interpretation of MICs and Zone Diameters European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 6.0. 2016, pp. 16–20. Available online: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.1_Breakpoint_Tables.pdf (accessed on 2 July 2020).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.T.; Carmeli, Y.; Falagas, M.T.; Giske, C.T.; Harbarth, S.; Hindler, J.T.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Lim, Y.S.; Yong, D.; Yum, J.H.; Chong, Y. Evaluation of the Hodge Test and the Imipenem-EDTA Double-Disk Synergy Test for Differentiating Metallo-β-Lactamase-Producing Isolates of Pseudomonas spp. and Acinetobacter spp. J. Clin. Microbiol. 2003, 41, 4623–4629. [Google Scholar] [CrossRef] [Green Version]
- Żabicka, D.; Baraniak, A.; Literacka, E.; Gniadkowski, M.; Hryniewicz, W. Wykrywanie Karbapenemaz—Zalecenia 2015. Warsaw. 2015. Available online: http://www.korld.edu.pl/pdf/Wykrywanie karbapenemaz-zalecenia2015-logoKORLD.pdf (accessed on 3 July 2020).
- Ellington, M.J.; Kistler, J.; Livermore, D.M.; Woodford, N. Multiplex PCR for rapid detection of genes encoding acquired metallo-b-lactamases. J. Antimicrob. Chemother. 2007, 59, 321–322. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.-Z.; Cash, D.M.; Chahine, M.A.; Nikolich, M.P.; Craft, D.W. Development and validation of a multiplex TaqMan real-time PCR for rapid detection of genes encoding four types of class D carbapenemase in Acinetobacter baumannii. J. Med. Microbiol. 2012, 61, 1532–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasanen, T.; Koskela, S.; Mero, S.; Tarkka, E.; Tissari, P.; Vaara, M.; Kirveskari, J. Rapid Molecular Characterization of Acinetobacter baumannii Clones with rep-PCR and Evaluation of Carbapenemase Genes by New Multiplex PCR in Hospital District of Helsinki and Uusimaa. PLoS ONE 2014, 9, e85854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merritt, J.H.; Kadouri, D.E.; O’Toole, G.A. Growing and analyzing static biofilms. Curr. Protoc. Microbiol. 2005, 1. Unit 1B.1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diancourt, L.; Passet, V.; Nemec, A.; Dijkshoorn, L.; Brisse, S.; Ahmed, N. The Population Structure of Acinetobacter baumannii: Expanding Multiresistant Clones from an Ancestral Susceptible Genetic Pool. PLoS ONE 2010, 5, e10034. [Google Scholar] [CrossRef] [Green Version]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 24, 124. [Google Scholar] [CrossRef]
- Seifert, H.; Dolzani, L.; Bressan, R.; van der Reijden, T.; van Strijen, B.; Stefanik, D.; Heersma, H.; Dijkshoorn, L. Standardization and Interlaboratory Reproducibility Assessment of Pulsed-Field Gel Electrophoresis-Generated Fingerprints of Acinetobacter baumannii. J. Clin. Microbiol. 2005, 43, 4328–4335. [Google Scholar] [CrossRef] [Green Version]
- Różańska, A.; Romaniszyn, D.; Chmielarczyk, A.; Bulanda, M. Bacteria contamination of touch surfaces in Polish hospital wards. Med. Pr. 2017, 68, 459–467. [Google Scholar] [CrossRef] [Green Version]
- WHO. Guidelines on Hand Hygiene in Health Care First Global Patient Safety Challenge Clean Care Is Safer Care. 2009. Available online: https://apps.who.int/iris/bitstream/handle/10665/44102/9789241597906_eng.pdf;jsessionid=8A7EF6DC6DA102FE83BE98E59557262F?sequence=1 (accessed on 16 June 2020).
- Kaiser, K.; Wolski, A. Kontrola czystości mikrobiologicznej powietrza. Tech. Chłodnicza Klim. 2007, 4, 158–162. [Google Scholar]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.r-project.org/ (accessed on 10 March 2020).
- Chmielarczyk, A.; Pobiega, M.; Romaniszyn, D.; Wójkowska-Mach, J. Multi-locus sequence typing (MLST) of non-fermentative Gram-negative bacilli isolated from bloodstream infections in southern Poland. Folia Microbiol. 2018, 63, 191–196. [Google Scholar] [CrossRef]
- Hamidian, M.; Nigro, S.J. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii. Microb. Genom. 2019, 5, e000306. [Google Scholar] [CrossRef]
- Agodi, A.; Voulgari, E.; Barchitta, M.; Quattrocchi, A.; Bellocchi, P.; Poulou, A.; Santangelo, C.; Castiglione, G.; Giaquinta, L.; Romeo, M.A.; et al. Spread of a carbapenem- and colistin-resistant Acinetobacter baumannii ST2 clonal strain causing outbreaks in two Sicilian hospitals. J. Hosp. Infect. 2014, 86, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Izdebski, R.; Fiett, J.; Hryniewicz, W.; Gniadkowski, M. Molecular analysis of Acinetobacter baumannii isolates from invasive infections in 2009 in Poland. J. Clin. Microbiol. 2012, 50, 3813–3815. [Google Scholar] [CrossRef] [Green Version]
- Towner, K.J.; Levi, K.; Vlassiadi, M. Genetic diversity of carbapenem-resistant isolates of Acinetobacter baumannii in Europe. Clin. Microbiol. Infect. 2008, 14, 161–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, P.G.; Pérez-Llarena, F.J.; Zander, E.; Fernández, A.; Bou, G.; Seifert, H. OXA-235, a Novel Class D-Lactamase Involved in Resistance to Carbapenems in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2013, 57, 2121–2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, B.A.; Amyes, S.G.B. OXA β-Lactamases. Clin. Microbiol. Rev. 2014, 27, 241–263. [Google Scholar] [CrossRef] [Green Version]
- Liakopoulos, A.; Miriagou, V.; Katsifas, E.A.; Karagouni, A.D.; Daikos, G.L.; Tzouvelekis, L.S.; Petinaki, E. Identification of OXA-23-producing Acinetobacter baumannii in Greece, 2010 to 2011. Eurosurveillance 2012, 17, 20117. [Google Scholar] [PubMed]
- Turton, J.F.; Woodford, N.; Glover, J.; Yarde, S.; Kaufmann, M.E.; Pitt, T.L. Identification of Acinetobacter baumannii by Detection of the bla OXA-51-like Carbapenemase Gene Intrinsic to This Species. J. Clin. Microbiol. 2006, 44, 2974–2976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, P.; Paluchowska, P.; Budak, A. Distribution of bla OXA genes among carbapenem-resistant Acinetobacter baumannii nosocomial strains in Poland. New Microbiol. 2012, 35, 317–325. [Google Scholar] [PubMed]
- Qureshi, Z.A.; Hittle, L.E.; O’Hara, J.A.; Rivera, J.I.; Syed, A.; Shields, R.K.; Pasculle, A.W.; Ernst, R.K.; Doi, Y. Colistin-resistant Acinetobacter baumannii: Beyond carbapenem resistance. Clin. Infect. Dis. 2015, 60, 1295–1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawfal Dagher, T.; Al-Bayssari, C.; Chabou, S.; Antar, N.; Diene, S.M.; Azar, E.; Rolain, J.M. Investigation of multidrug-resistant ST2 Acinetobacter baumannii isolated from Saint George hospital in Lebanon. BMC Microbiol. 2019, 19, 29. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, K.; Zhang, J.; Guo, Y.; Fan, X.; Wang, Y.; Mensah, S.D.; Zhang, X. Outbreak of carbapenem-resistant Acinetobacter baumannii carrying the carbapenemase OXA-23 in ICU of the eastern Heilongjiang Province, China. BMC Infect. Dis. 2019, 19, 452. [Google Scholar]
- Espinal, P.; Martí, S.; Vila, J. Effect of biofilm formation on the survival of Acinetobacter baumannii on dry surfaces. J. Hosp. Infect. 2012, 80, 56–60. [Google Scholar] [CrossRef]
- Kaliterna, V.; Kaliterna, M.; Hrenović, J.; Barišić, Z.; Tonkić, M.; Goic-Barisic, I. Acinetobacter baumannii in Southern Croatia: Clonal lineages, biofilm formation, and resistance patterns. Infect. Dis. 2015, 47, 902–907. [Google Scholar] [CrossRef]
- King, L.B.; Pangburn, M.K.; McDaniel, L.S. Serine protease PKF of Acinetobacter baumannii results in serum resistance and suppression of biofilm formation. J. Infect. Dis. 2013, 207, 1128–1134. [Google Scholar] [CrossRef] [Green Version]
- Roberts, S.A.; Findlay, R.; Lang, S.D.R. Investigation of an outbreak of multi-drug resistant Acinetobacter baumannii in an intensive care burns unit. J. Hosp. Infect. 2001, 48, 228–232. [Google Scholar] [CrossRef]
- Markogiannakis, A.; Fildisis, G.; Tsiplakou, S.; Ikonomidis, A.; Koutsoukou, A.; Pournaras, S.; Manolis, E.N.; Baltopoulos, G.; Tsakris, A. Cross-Transmission of Multidrug-Resistant Acinetobacter baumannii Clonal Strains Causing Episodes of Sepsis in a Trauma Intensive Care Unit. Infect. Control. Hosp. Epidemiol. 2008, 29, 410–417. [Google Scholar] [CrossRef]
- Morgan, D.J.; Liang, S.Y.; Smith, C.L.; Johnson, J.K.; Harris, A.D.; Furuno, J.P.; Thom, K.A.; Snyder, G.M.; Day, H.R.; Perencevich, E.N. Frequent Multidrug-Resistant Acinetobacter baumannii Contamination of Gloves, Gowns, and Hands of Healthcare Workers. Infect. Control. Hosp. Epidemiol. 2010, 31, 716–721. [Google Scholar] [CrossRef] [Green Version]
- Kuziemski, A.; Czerniak, B.; Frankowska, K.; Gonia, E. Molecular epidemiology of Acinetobacter baumannii strains isolated in the years 2008-2010 in University Hospital no. 2 in Bydgoszcz. Prz. Epidemiol. 2012, 66, 403–407. [Google Scholar]
- Kevorkijan, B.K.; Petrovič, Ž.; Kocuvan, A.; Rupnik, M. MRSA diversity and the emergence of LA-MRSA in a large teaching hospital in Slovenia. Acta Microbiol. Immunol. Hung. 2018, 66, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangberg, A.; Larsen, A.L.; Kacelnik, O.; Sæther, H.S.; Bjørland, M.; Ringstad, J.; Jonassen, C.M. Molecular analysis and epidemiological typing of Vancomycin-resistant Enterococcus outbreak strains. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chmielarczyk, A.; Higgins, P.G.; Wojkowska-Mach, J.; Synowiec, E.; Zander, E.; Romaniszyn, D.; Gosiewski, T.; Seifert, H.; Heczko, P.; Bulanda, M. Control of an outbreak of Acinetobacter baumannii infections using vaporized hydrogen peroxide. J. Hosp. Infect. 2012, 81, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Perez, F.; Beltramini, A.M.; Jakubowycz, M.; Dimick, P.; Jacobs, M.R.; Roman, K.; Bonomo, R.A.; Salata, R.A. Use of Vaporized Hydrogen Peroxide Decontamination during an Outbreak of Multidrug-Resistant Acinetobacter baumannii Infection at a Long-Term Acute Care Hospital. Infect. Control. Hosp. Epidemiol. 2010, 31, 1236–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robustillo-Rodela, A.; Pérez-Blanco, V.; Espinel Ruiz, M.A.; Ruiz Carrascoso, G.; Figueira Iglesias, J.C.; Abad Martín, D. Successful control of 2 simultaneous outbreaks of OXA-48 carbapenemase-producing Enterobacteriaceae and multidrug-resistant Acinetobacter baumannii in an intensive care unit. Am. J. Infect. Control. 2017, 45, 1356–1362. [Google Scholar] [CrossRef]
- Chiguer, M.; Maleb, A.; Amrani, R.; Abda, N.; Alami, Z. Assessment of surface cleaning and disinfection in neonatal intensive care unit. Heliyon 2019, 5, e02966. [Google Scholar] [CrossRef] [Green Version]
- Stewardson, A.; Pittet, D. Anatomy of a successful multimodal hand hygiene campaign. BMJ Qual. Saf. 2012, 21, 973–975. [Google Scholar] [CrossRef] [Green Version]
- Cheng, V.C.; Tai, J.W.; Wong, L.M.; Ching, R.H.; Ng, M.M.; Ho, S.K.; Lee, D.W.; Li, W.S.; Lee, W.M.; Sridhar, S.; et al. Effect of proactive infection control measures on benchmarked rate of hospital outbreaks: An analysis of public hospitals in Hong Kong over 5 years. Am. J. Infect. Control. 2015, 43, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, N.; Gastmeier, P.; Vonberg, R.P. Effectiveness of healthcare worker screening in hospital outbreaks with gram-negative pathogens: A systematic review. Antimicrob. Resist. Infect. Control. 2018, 7, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Patient No. | Age (Years) | Median (Mean) of Age (Years) | Sex | Duration of Hospital Stay | Ward (Room) | Infection Type | Accompanying/Preceding Disease | Outbreak No. |
|---|---|---|---|---|---|---|---|---|
| PUS of IO | 72 | - | F | 11 May–12 May 2017 12 May–28 July 2017 28 July–29 September 2017 | VSD ICU VSD | BTC | VAP, UTI, CRBSI (none of them due to A. baumannii), previous surgeries | unclassified |
| 279 | 82 | 73.5 (73.5) | F | 15 August–06 November 2017 | VSD (217) | SSI | hypertension, COPD, CKD, DM, PAD, chronic ulceration | I |
| 252 | 64 | F | 11 September–20 October 2017 | VSD (218) | SSI | hypertension, DM, chronic ulceration | ||
| 262 | 81 | F | 15 September–03 November 2017 | VSD (218) | SSI | hypertension, heart failure, ulceration | ||
| 282 | 66 | M | 29 September–30 October 2017 | VSD (216) | SSI | hypertension, PAD, DM, previous surgeries | ||
| 342 | 66 | F | 19 September–30 October 2017 | VSD (216,218) | SSI | PAD, ulceration, previous hospitalization in another hospital, DM2 | ||
| 343 | 82 | F | 24 September–30 October 2017 | VSD (204,207) | SSI | hypertension, PAD, CKD, DM, previous hospitalization and surgeries | ||
| 279 | 82 | 70.0 (70.3) | M | 10 February–16 February 2018 16 February–13 April 2018 | ICU VSD (216) | SSI | hypertension, antibiotic therapy due to SSI, DM2, previous hospitalization and surgeries | II |
| 284 | 59 | M | 27 February–23 August 2018 | VSD (209) | SSI | PAD, antibiotic therapy due to SSI, previous hospitalization | ||
| 285 | 70 | F | 9 August–24 October 2017 23 February–6 March 2018 6 March–9 March 2018 9 March–9 May 2018 | VSD VSD (217) ICU VSD (217) | SSI | hypertension, PAD, previous hospitalization and surgeries |
| Strain No. | Outbreak No. | Date of Isolation | EUCAST Susceptibility Interpretation | MBL Phenotype | KPC phenotype | bla OXA Gene | MBL Gene | Biofilm Formation | Genotyping | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone Diameter Breakpoint (mm) (S/R) | MIC Breakpoint (mg/L) (S/R) | OXA type | ISAba 1 | PFGE | MLST | |||||||||||||||||
| CAZ | SXT | AK | GN | NET | TOB | CIP | LEV | IMP | MEM | TGC | CT | |||||||||||
| PUS | unclassified | 18.07.2017 | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 2.0/S | * | * | * | * | * | * | * | * |
| 252/17 | I | 26.09.2017 | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 2.0/S | - | - | 51/23/24 | - | - | + | A | ST2 |
| 262/17 | I | 28.09.2017 | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 1.0/S | - | - | 51/23/24 | - | - | + | A | ST2 |
| 279/17 | I | 02.10.2017 | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 4.0/R | - | - | 51/23/24 | - | - | + | A | ST2 |
| 282/17 | I | 02.10.2017 | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 2.0/S | - | - | 51/23/24 | - | - | + | A | ST2 |
| 342/17 | I | 16.10.2017 | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 2.0/S | - | - | 51/23/24 | - | - | + | A | ST2 |
| 343/17 | I | 16.10.2017 | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 2.0/S | - | - | 51/23/24 | - | - | + | A | ST2 |
| 279/18 | II | 19.03.2018 | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 0.5/S | - | - | 51/23/24 | - | - | + | B | ST2 |
| 284/18 | II | 19.03.2018 | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 0.5/S | - | - | 51/23/24 | - | - | + | B | ST2 |
| 285/18 | II | 23.03.2018 | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 6/R | 0.5/S | - | - | 51/23/24 | - | - | + | B | ST2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczypta, A.; Talaga-Ćwiertnia, K.; Kielar, M.; Krzyściak, P.; Gajewska, A.; Szura, M.; Bulanda, M.; Chmielarczyk, A. Investigation of Acinetobacter baumannii Activity in Vascular Surgery Units through Epidemiological Management Based on the Analysis of Antimicrobial Resistance, Biofilm Formation and Genotyping. Int. J. Environ. Res. Public Health 2021, 18, 1563. https://doi.org/10.3390/ijerph18041563
Szczypta A, Talaga-Ćwiertnia K, Kielar M, Krzyściak P, Gajewska A, Szura M, Bulanda M, Chmielarczyk A. Investigation of Acinetobacter baumannii Activity in Vascular Surgery Units through Epidemiological Management Based on the Analysis of Antimicrobial Resistance, Biofilm Formation and Genotyping. International Journal of Environmental Research and Public Health. 2021; 18(4):1563. https://doi.org/10.3390/ijerph18041563
Chicago/Turabian StyleSzczypta, Anna, Katarzyna Talaga-Ćwiertnia, Małgorzata Kielar, Paweł Krzyściak, Anna Gajewska, Mirosław Szura, Małgorzata Bulanda, and Agnieszka Chmielarczyk. 2021. "Investigation of Acinetobacter baumannii Activity in Vascular Surgery Units through Epidemiological Management Based on the Analysis of Antimicrobial Resistance, Biofilm Formation and Genotyping" International Journal of Environmental Research and Public Health 18, no. 4: 1563. https://doi.org/10.3390/ijerph18041563
APA StyleSzczypta, A., Talaga-Ćwiertnia, K., Kielar, M., Krzyściak, P., Gajewska, A., Szura, M., Bulanda, M., & Chmielarczyk, A. (2021). Investigation of Acinetobacter baumannii Activity in Vascular Surgery Units through Epidemiological Management Based on the Analysis of Antimicrobial Resistance, Biofilm Formation and Genotyping. International Journal of Environmental Research and Public Health, 18(4), 1563. https://doi.org/10.3390/ijerph18041563







