Association Between PM2.5 Exposure Level and Primary Open-Angle Glaucoma in Taiwanese Adults: A Nested Case–control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Collection of PM2.5 Concentration Data
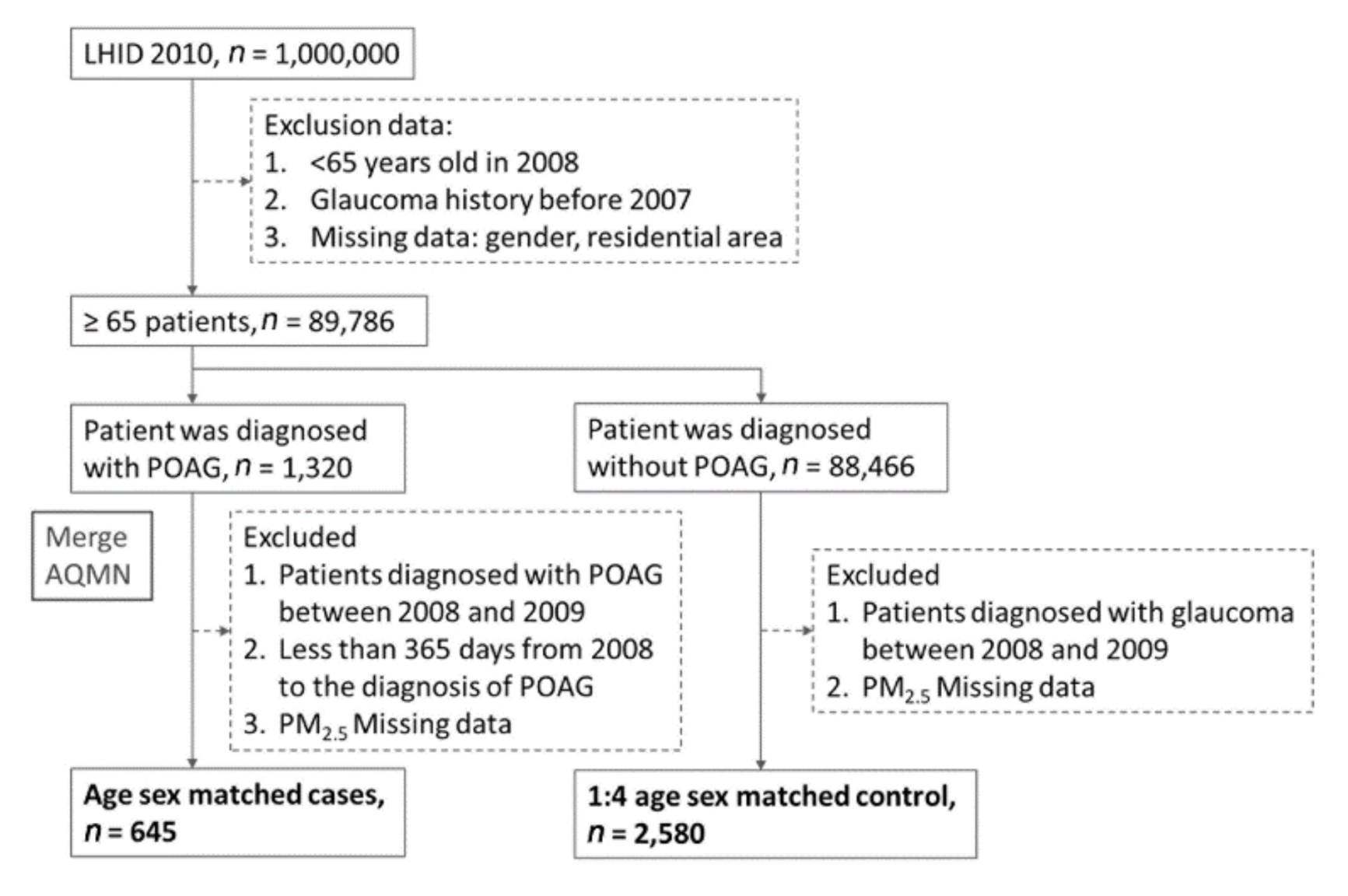
2.3. Study Population
2.4. Comorbidities
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. OR of PM2.5 Exposure as Risk Factor for POAG by Logistic Regression
3.3. ORs of PM2.5 Level As a Risk Factor for POAG in Subgroups.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Air quality guidelines: Global update 2005. Particulate matter, ozone, nitrogen dioxide and sulfur dioxide. Indian J. Med Res. 2007, 4, 492–493.
- World Health Organization. 7 Million Premature Deaths Annually Linked to Air Pollution. 2014. Available online: http://www.who.int/mediacentre/news/releases/2014/air-pollution/en (accessed on 15 December 2020).
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Block, M.L.; Calder’on-Garcidue˜nas, L. Air pollution: Mechanisms of neuro inflammation and CNS disease. Trends Neurosci. 2009, 32, 506–516. [Google Scholar] [CrossRef]
- Min, J.Y.; Min, K.B. Exposure to ambient PM10 and NO2 and the incidence of attention-deficit hyperactivity disorder in childhood. Environ. Int. 2017, 99, 221–227. [Google Scholar] [CrossRef]
- Cheng, H.; Saffari, A.; Sioutas, C.; Forman, H.J.; Morgan, T.E.; Finch, C.E. NanoScale Particulate Matter from Urban Traffic Rapidly Induces Oxidative Stress and Inflammation in Olfactory Epithelium with Concomitant Effects on Brain. Environ. Health Perspect. 2016, 37, 644–667. [Google Scholar] [CrossRef] [PubMed]
- Thomson, E.M.; Breznan, D.; Karthikeyan, S.; MackinnonRoy, C.; Vuong, N.Q.; Dabek-Zlotorzynska, E.; Celo, V.; Charland, J.P.; Kumarathasan, P.; Brook, J.R.; et al. Contrasting biological potency of particulate matter collected at sites impacted by distinct industrial sources. Part. Fibre Toxicol. 2016, 13, 65. [Google Scholar] [CrossRef]
- Santiago-Lopez, D.; Bautista-Martinez, J.A.; ReyesHernandez, C.I.; Aguilar-Martinez, M.; Rivas-Arancibia, S. Oxidative stress, progressive damage in the substantia nigra and plasma dopamine oxidation, in rats chronically exposed to ozone. Toxicol. Lett. 2010, 197, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Levesque, S.; Surace, M.J.; Mcdonald, J.; Block, M.L. Air pollution and the brain: Subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J. Neuroinflamm. 2011, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.A.; Novaes, P.; Matsuda, M.; Alves, M.R.; Monteiro, M.L. Ocular surface adverse effects of ambient levels of air pollution. Arq. Bras. Oftalmol. 2011, 74, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Moen, B.E.; Norbäck, D.; Wieslander, G.; Bakke, J.V.; Magerøy, N.; Irgens, Å.; Bråtveit, M.; Hollund, B.E.; Aasen, T. Can air pollution affect tear film stability? A cross-sectional study in the aftermath of an explosion accident. BMC Public Health 2011, 11, 1–6. [Google Scholar] [CrossRef]
- Gupta, S.K.; Gupta, V.; Joshi, S.; Tandon, R. Sub clinically dry eyes in urban Delhi: An impact of air pollution? Ophthalmologica 2002, 216, 368–371. [Google Scholar] [CrossRef]
- Fu, Q.; Mo, Z.; Lyu, D.; Zhang, L.; Qin, Z.; Tang, Q.; Yin, H.; Xu, P.; Wu, L.; Lou, X.; et al. Air pollution and outpatient visits for conjunctivitis: A case-crossover study in Hangzhou, China. Environ. Pollut. 2017, 231, 1344–1350. [Google Scholar] [CrossRef]
- Torricelli, A.A.M.; Novaes, P.; Matsuda, M.; Braga, A.; Saldiva, P.H.N.; Alves, M.R.; Monteiro, M.L.R. Correlation between signs and symptoms of ocular surface dysfunction and tear osmolarity with ambient levels of air pollution in a large metropolitan area. Cornea 2013, 32, 11–15. [Google Scholar] [CrossRef]
- Adar, S.D.; Klein, R.; Klein, B.E.; Szpiro, A.A.; Cotch, M.F.; Wong, T.Y.; O’Neill, M.S.; Shrager, S.; Barr, R.G.; Siscovick, D.S.; et al. Air Pollution and the microvasculature: A cross-sectional assessment of in vivo retinal images in the population-based multi-ethnic study of atherosclerosis (MESA). PLoS Med. 2010, 7, e1000372. [Google Scholar] [CrossRef]
- Cheng, H.C.; Pan, R.H.; Yeh, H.J.; Lai, K.R.; Yen, M.Y.; Chan, C.L.; Wang, A.G. Ambient Air Pollution and the Risk of Central Retinal Artery Occlusion. Ophthalmology 2016, 123, 2603–2609. [Google Scholar] [CrossRef]
- Pascolini, D.; Mariotti, S.P. Global estimates of visual impairment: 2010. Br. J. Ophthalmol. 2012, 96, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Le, A.; Mukesh, B.N.; McCarty, C.A.; Taylor, H.R. Risk factors associated with the incidence of open-angle glaucoma: The visual impairment project. Invest. Ophthalmol. Vis. Sci. 2003, 44, 3783–3789. [Google Scholar] [CrossRef]
- Suzuki, Y.; Iwase, A.; Araie, M.; Yamamoto, T.; Abe, H.; Shirato, S.; Kuwayama, Y.; Mishima, H.; Shimizu, H.; Tomita, G.; et al. Risk factors for open-angle glaucoma in a Japanese population: The Tajimi Study. Ophthalmology 2006, 113, 1613–1617. [Google Scholar] [CrossRef]
- Lin, C.C.; Hu, C.C.; Ho, J.D.; Chiu, H.W.; Lin, H.C. Obstructive sleep apnea and increased risk of glaucoma. Ophthalmology 2013, 120, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Vijaya, L.; George, R.; Arvind, H.; Baskaran, M.; Ve Ramesh, S.; Raju, P.; Kumaramanickavel, G.; McCarty, C. Prevalence of primary open-angle glaucoma in an urban south Indian population and comparison with a rural population: The Chennai Glaucoma Study. Ophthalmology 2008, 115, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Li, R.L.; Ho, Y.C.; Luo, C.W.; Lee, S.S.; Kuan, Y.H. Influence of PM 2.5 Exposure Level on the Association between Alzheimer’s Disease and Allergic Rhinitis: A National Population-Based Cohort Study. Int. J. Environ. Res. Public Health 2019, 16, 3357. [Google Scholar]
- Wang, Y.; Shi, L.; Lee, M.; Liu, P.; Di, Q.; Zanobetti, A.; Schwartz, J.D. Long-term exposure to PM2.5 and mortality among older adults in the Southeastern US. Epidemiology 2017, 28, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, G.A.; Lee, W.; Bae, H.W.; Seong, G.J.; Kim, C.Y. Vascular and metabolic comorbidities in open-angle glaucoma with low- and high-teen intraocular pressure: A cross-sectional study from South Korea. Acta Ophthalmol. 2017, 95, e564–e574. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.C.; Chien, C.W.; Hu, C.C.; Ho, J.D. Comparison of comorbid conditions between open-angle glaucoma patients and a control cohort: A case-control study. Ophthalmology 2010, 117, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Kwak, C.; Clayton-Matthews, A. Multinomial logistic regression. Nurs. Res. 2002, 51, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Stephen, T.J.; Mark, E.L. Pollen dispersal models in Quaternary plant ecology: Assumptions, parameters, and prescriptions. Bot. Rev. 1999, 65, 65–75. [Google Scholar]
- Lin, Y.J.; Tian, W.H.; Chen, C.C. Urbanization and the utilization of outpatient services under National Health Insurance in Taiwan. Health Policy 2011, 103, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, M.; Li, Z.; Huang, W. Epidemiological variations and trends in health burden of glaucoma worldwide. Acta Ophthalmol. 2019, 97, e349–e355. [Google Scholar] [CrossRef]
- Nwanaji-Enwerem, J.C.; Wang, W.; Nwanaji-Enwerem, O.; Vokonas, P.; Baccarelli, A.; Weisskopf, M.; Herndon, L.W.; Wiggs, J.L.; Park, S.K.; Schwartz, J. Association of Long-term Ambient Black Carbon Exposure and Oxidative Stress Allelic Variants With Intraocular Pressure in Older Men. JAMA Ophthalmol. 2019, 137, 129–137. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Cao, Q.; Rui, G.; Liang, Y. Study on PM2. 5 pollution and the mortality due to lung cancer in China based on geographic weighted regression model. BMC Public Health 2018, 18, 925. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Woodward, A.; Vardoulakis, S.; Kovats, S.; Wilkinson, P.; Kovats, S.; Wilkinson, P.; Li, L.; Xu, L.; Li, J.; et al. Haze, public health and mitigation measures in China: A review of the current evidence for further policy response. Sci. Total Environ. 2016, 578, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.J.; Zhang, Y.; Bozzetti, C.; Ho, K.F.; Cao, J.J.; Han, Y.; Daellenbach, K.R.; Slowik, J.G.; Platt, S.M.; Canonaco, F.; et al. High secondary aerosol contribution to particulate pollution during haze events in China. Nature 2014, 514, 218–222. [Google Scholar] [CrossRef]
- Zheng, M.; Salmon, L.G.; Schauer, J.J.; Zeng, L.; Kiang, C.S.; Zhang, Y.; Cassa, G.R. Seasonal trends in PM2.5 source contributions in Beijing, China. Atmos. Environ. 2005, 39, 3967–3976. [Google Scholar] [CrossRef]
- Tao, J.; Zhang, L.; Engling, G.; Zhang, R.; Yang, Y.; Cao, J.; Zhu, C.; Wang, Q.; Luo, L. Chemical composition of PM2.5, in an urban environment in Chengdu, China: Importance of springtime dust storms and biomass burning. Atmos. Res. 2013, 122, 270–283. [Google Scholar] [CrossRef]
- Abu-Allaban, M.; Gillies, J.A.; Gertler, A.W.; Clayton, R.; Proffitt, D. Motor vehicle contributions to ambient PM 10 and PM 2.5 at selected urban areas in the USA. Environ. Monit. Assess. 2007, 132, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Hansen, A.B.; Ravnskjaer, L.; Loft, S.; Andersen, K.K.; Brauner, E.V.; Baastrup, R.; Yao, C.; Ketzel, M.; Becker, T.; Brandt, J.; et al. Long-term exposure to fine particulate matter and incidence of diabetes in the Danish nurse cohort. Environ. Int. 2016, 91, 243–250. [Google Scholar] [CrossRef]
- Zhang, Z.; Shao, C.; Guan, Y.; Xue, C. Socioeconomic factors and regional differences of PM2. 5 health risks in China. J. Environ. Manag. 2019, 251, 109564. [Google Scholar] [CrossRef]
- Costa, L.G.; Cole, T.B.; Coburn, J.; Chang, Y.C.; Dao, K.; Roque, P.J. Neurotoxicity of traffic-related air pollution. Neurotoxicology 2017, 59, 133–139. [Google Scholar] [CrossRef]
- Cesaroni, G.; Badaloni, C.; Gariazzo, C.; Stafoggia, M.; Sozzi, R.; Davoli, M.; Forastiere, F. Long-term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ. Health Perspect. 2013, 121, 324–331. [Google Scholar] [CrossRef]
- Wong, C.M.; Tsang, H.; Lai, H.K.; Thomas, G.N.; Lam, K.B.; Chan, K.P.; Zheng, Q.; Ayres, J.G.; Lee, S.Y.; Lam, T.H. Cancer mortality risks from long-term exposure to ambient fine particle. Cancer Epidemiol. Biomark. Prev. 2016, 25, 839–845. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H.J.; Schoetzau, A.; Flammer, J. Blood flow velocity in the extraocular vessels in chronic smokers. Br. J. Ophthalmol. 1997, 81, 133–135. [Google Scholar] [CrossRef]
- Wang, S.; Bao, X. Hyperlipidemia, blood lipid level, and the risk of glaucoma: A meta-analysis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1028–1043. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Lin, C.L. Comparison of medical comorbidity between patients with primary angle-closure glaucoma and a control cohort: A population-based study from Taiwan. BMJ Open 2019, 9, e024209. [Google Scholar] [CrossRef] [PubMed]
- Zaleska-Żmijewska, A.; Janiszewski, M.; Wawrzyniak, Z.M.; Kuch, M.; Szaflik, J.; Szaflik, J.P. Is atrial fibrillation a risk factor for normal-tension glaucoma? Medicine 2017, 96, 43. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.C.; Kappelle, L.J.; Eliasziw, M.; Babikian, V.L.; Pearce, L.A.; Barnett, H.J.M. Occurrence of hemispheric and retinal ischemia in atrial fibrillation compared with carotid stenosis. Stroke 2002, 33, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, C.B.; Lip, G.Y.; Lamberts, M.; Gislason, G.; Torp-Pedersen, C.; Olesen, J.B. Retinal vein and artery occlusions: A risk factor for stroke in atrial fibrillation. J. Thromb. Haemost. 2013, 11, 1485–1492. [Google Scholar] [CrossRef]
- Callizo, J.; Feltgen, N.; Pantenburg, S.; Wolf, A.; Neubauer, A.S.; Jurklies, B.; Wachter, R.; Schmoor, C.; Schumacher, M.; Junker, B.; et al. European Assessment Group for Lysis in the EyeCardiovascular risk factors in central retinal artery occlusion: Results of a prospective and standardized medical examination. Ophthalmology 2015, 122, 1881–1888. [Google Scholar] [CrossRef]
- DeMaman, A.S.; Melo, P.; Homem, J.M.; Tavares, M.A.; Lachat, J.J. Effectiveness of iron repletion in the diet for the optic nerve development of anaemic rats. Eye 2010, 24, 901–908. [Google Scholar] [CrossRef]
- Chua, S.Y.; Khawaja, A.P.; Morgan, J.; Strouthidis, N.; Reisman, C.; Dick, A.D.; Khaw, P.T.; Patel, P.J.; Foster, P.J. UK Biobank Eye and Vision Consortium. The relationship between ambient atmospheric fine particulate matter (PM2. 5) and glaucoma in a large community cohort. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4915–4923. [Google Scholar] [CrossRef]
- MIN, K.B.; MIN, J.Y. Association of ambient particulate matter exposure with the incidence of glaucoma in childhood. Am. J. Ophthalmol. 2020, 211, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Sakai, H.; Murata, H.; Fujino, Y.; Matsuura, M.; Garway-Heath, D.; Weinreb, R.; Asaoka, R. Rates of Visual Field Loss in Primary Open-Angle Glaucoma and Primary Angle-Closure Glaucoma: Asymmetric Patterns. Invest. Ophthalmol. Vis. Sci. 2018, 59, 5717–5725. [Google Scholar] [CrossRef]
- Guo, T.; Guo, L.; Fan, Y.; Fang, F.; Wei, J.; Tan, Y.; Chen, Y.; Fan, X. Aqueous humor levels of TGFβ2 and SFRP1 in different types of glaucoma. BMC Ophthalmol. 2019, 19, 170. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Zhang, Y.; Yang, K.Q.; Yang, Y.K.; Liang, X.; Zhou, X.L. Potential Harmful Effects of PM2.5 on Occurrence and Progression of Acute Coronary Syndrome: Epidemiology, Mechanisms, and Prevention Measures. Int. J. Environ. Res. Public Health 2016, 13, 748. [Google Scholar] [CrossRef]
- Perez, C.I.; Singh, K.; Lin, S. Relationship of lifestyle, exercise, and nutrition with glaucoma. Curr. Opin. Ophthalmol. 2019, 30, 82–88. [Google Scholar] [CrossRef] [PubMed]
| Comparison | POAG | P-value | ||||
|---|---|---|---|---|---|---|
| (n = 2580) | (n = 645) | |||||
| Gender | ||||||
| Female | 1228 | (47.6%) | 307 | (47.6%) | 1.0000 | |
| Male | 1352 | (52.4%) | 338 | (52.4%) | ||
| Age | ||||||
| Mean ± SD | 72.64 ± 5.58 | 72.57 ± 5.66 | 0.7915 | |||
| Low income | ||||||
| Yes | 1620 | (62.79%) | 405 | (62.79%) | 1.0000 | |
| No | 960 | (37.21%) | 240 | (37.21%) | ||
| Urbanization level | ||||||
| Highly urbanized | 563 | (21.82%) | 198 | (30.7%) | <.0001 | |
| Moderate urbanization | 667 | (25.85%) | 180 | (27.91%) | ||
| Emerging town | 402 | (15.58%) | 79 | (12.25%) | ||
| General town | 489 | (18.95%) | 105 | (16.28%) | ||
| Aged Township | 101 | (3.91%) | 21 | (3.26%) | ||
| Agricultural town | 195 | (7.56%) | 37 | (5.74%) | ||
| Remote township | 163 | (6.32%) | 25 | (3.88%) | ||
| Comorbidity | ||||||
| Hypertension | 1616 | (62.64%) | 449 | (69.61%) | 0.0010 | |
| Ischemic heart disease | 452 | (17.52%) | 146 | (22.64%) | 0.0048 | |
| Hyperlipidemia | 879 | (34.07%) | 313 | (48.53%) | <.0001 | |
| Conge stive heart failure | 256 | (9.92%) | 77 | (11.94%) | 0.1523 | |
| Peripheral vascular disease | 157 | (6.09%) | 56 | (8.68%) | 0.0002 | |
| Atrial fibrillation | 64 | (2.48%) | 34 | (5.27%) | 0.0028 | |
| Ischemic stroke | 85 | (3.29%) | 19 | (2.95%) | 0.6539 | |
| Headaches | 438 | (16.98%) | 137 | (21.24%) | 0.0047 | |
| Migraines | 75 | (2.91%) | 29 | (4.5%) | 0.0716 | |
| Epilepsy and recurrent | 41 | (1.59%) | 16 | (2.48%) | 0.1775 | |
| Dementia | 173 | (6.71%) | 44 | (6.82%) | 0.9161 | |
| Rheumatoid arthritis | 82 | (3.18%) | 24 | (3.72%) | 0.5093 | |
| Systemic lupus erythematosus | 3 | (0.12%) | 2 | (0.31%) | 0.3979 | |
| Diabetes | 689 | (26.71%) | 226 | (35.04%) | <.0001 | |
| Asthma | 622 | (24.11%) | 161 | (24.96%) | 0.6516 | |
| Chronic kidney disease | 131 | (5.08%) | 39 | (6.05%) | 0.3489 | |
| Hepatitis B | 39 | (1.51%) | 12 | (1.86%) | 0.5505 | |
| Fluid, Electrolyte, Acid–Base Disorders | 55 | (2.13%) | 23 | (3.57%) | 0.0678 | |
| Tuberculosis | 63 | (2.44%) | 16 | (2.48%) | 0.9546 | |
| Anemia | 274 | (10.62%) | 102 | (15.81%) | 0.0009 | |
| Peptic ulcer | 170 | (6.59%) | 66 | (10.23%) | 0.0049 | |
| Depression | 52 | (2.02%) | 16 | (2.48%) | 0.4893 | |
| Malignant disease | 310 | (12.02%) | 105 | (16.28%) | 0.0074 | |
| n (%) | Distribution of PM2.5 (μg/m3) | Odd ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Comparison | POAG | Median | Mean (SD) | Follow up Months Mean (SD) | Univariate | Multivariate | |
| Total participants | |||||||
| Per WHO standard level increase | 2580 | 645 | 1159.84 | 1262.18 (629.57) | 48.05 (15.07) | 1.248 (1.106–1.408) | 1.193 (1.050–1.356) |
| PM2.5 WHO standard level (reference: normal standard) | |||||||
| Normal level | 210 (8.14%) | 56 (8.56%) | 691.30 | 688.4 (283.57) | 43.30 (16.05) | Reference | Reference |
| WHO 1.0 level | 1457 (56.47%) | 318 (48.62%) | 1046.42 | 1080.31 (469.86) | 42.90 (15.08) | 0.818 (0.595–1.126) | 0.825 (0.588–1.158) |
| WHO 1.5 level | 797 (30.89%) | 209 (31.96%) | 1478.70 | 1569.97 (680.75) | 52.00 (13.93) | 0.983 (0.706–1.370) | 0.982 (0.690–1.396) |
| WHO 2.0 level | 116 (4.5%) | 62 (9.48%) | 2221.87 | 2193.60 (133.81) | 53.56 (11.83) | 2.004 (1.308–3.071) | 1.668 (1.045–2.663) |
| Odd ratio (95% CI) | |||
|---|---|---|---|
| Univariate | Multivariate | ||
| PM2.5 WHO standard level (reference: normal level) | |||
| WHO 1.0 level | 0.818 (0.595–1.126) | 0.825 (0.588–1.158) | |
| WHO 1.5 level | 0.983 (0.706–1.370) | 0.982 (0.690–1.396) | |
| WHO 2.0 level | 2.004 (1.308–3.071) | 1.668 (1.045–2.663) | |
| Gender (reference: female) | |||
| Male | 1.000 (0.841–1.189) | 1.093 (0.911–1.311) | |
| Age (reference:general population) | |||
| Per year | 0.998 (0.983–1.013) | 0.999 (0.982–1.015) | |
| Low income (reference: no) | |||
| yes | 1.000 (0.837–1.195) | 0.744 (0.603–0.919) | |
| Urbanization level (reference: highly urbanized) | |||
| Moderate urbanization | 0.767 (0.609–0.967) | 0.822 (0.642–1.053) | |
| Emerging town | 0.559 (0.418–0.747) | 0.640 (0.469–0.872) | |
| General town | 0.611 (0.468–0.796) | 0.628 (0.464–0.852) | |
| Aged Township | 0.591 (0.360–0.972) | 0.545 (0.320–0.927) | |
| Agricultural town | 0.540 (0.366–0.794) | 0.471 (0.307–0.723) | |
| Remote township | 0.436 (0.278–0.685) | 0.413 (0.254–0.669) | |
| Comorbidity (reference: no) | |||
| Hypertension | 1.366 (1.135–1.645) | 1.074 (0.873–1.320) | |
| Ischemic heart disease | 1.378 (1.116–1.700) | 1.090 (0.865–1.374) | |
| Hyperlipidemia | 1.824 (1.532–2.172) | 1.588 (1.305–1.932) | |
| Congestive heart failure | 1.231 (0.939–1.613) | 1.015 (0.752–1.371) | |
| Peripheral vascular disease | 1.708 (1.304–2.236) | 1.393 (0.998–1.943) | |
| Atrial fibrillation | 2.188 (1.430–3.347) | 2.088 (1.324–3.292) | |
| Ischemic stroke | 0.891 (0.538–1.476) | 0.796 (0.471–1.347) | |
| Headaches | 1.319 (1.064–1.635) | 1.204 (0.954–1.519) | |
| Migraines | 1.573 (1.016–2.437) | 1.417 (0.891–2.253) | |
| Epilepsy and recurrent | 1.576 (0.879–2.827) | 1.635 (0.891–3.002) | |
| Dementia | 1.019 (0.723–1.435) | 0.902 (0.625–1.301) | |
| Rheumatoid arthritis | 1.177 (0.741–1.871) | 1.031 (0.635–1.675) | |
| Systemic lupus erythematosus | 2.672 (0.446–16.023) | 3.126 (0.468–20.892) | |
| Diabetes | 1.481 (1.232–1.779) | 1.175 (0.958–1.440) | |
| Asthma | 1.047 (0.857–1.279) | 0.934 (0.755–1.155) | |
| Chronic kidney disease | 1.203 (0.832–1.739) | 0.943 (0.638–1.394) | |
| Hepatitis B | 1.235 (0.643–2.373) | 0.993 (0.502–1.963) | |
| Fluid, Electrolyte, Acid–Base Disorders | 1.698 (1.035–2.784) | 1.455 (0.866–2.446) | |
| Tuberculosis | 1.016 (0.583–1.771) | 1.004 (0.562–1.793) | |
| Anemia | 1.581 (1.236–2.021) | 1.410 (1.085–1.833) | |
| Peptic ulcer | 1.616 (1.199–2.178) | 1.577 (1.157–2.149) | |
| Depression | 1.237 (0.701–2.180) | 1.025 (0.568–1.850) | |
| Malignant disease | 1.424 (1.120–1.811) | 1.280 (0.995–1.646) | |
| Odd Ratio (95% CI), Reference: Normal Level | Ptrend | |||
|---|---|---|---|---|
| WHO 1.0 level | WHO 1.5 level | WHO 2.0 level | ||
| Total | 0.825 (0.588–1.158) | 0.982 (0.690–1.396) | 1.668 (1.045–2.663) | 0.0068 |
| Hyperlipidemia | ||||
| Yes | 0.678 (0.419–1.097) | 0.876 (0.532–1.441) | 1.291 (0.661–2.521) | 0.1567 |
| No | 0.999 (0.609–1.639) | 1.122 (0.670–1.880) | 2.148 (1.100–4.194) | 0.0231 |
| Atrial fibrillation | ||||
| Yes | 0.474 (0.025–8.961) | 2.163 (0.108–43.256) | 0.196 (0.004–9.417) | 0.9929 |
| No | 0.818 (0.58–1.155) | 0.957 (0.67–1.369) | 1.673 (1.037–2.699) | 0.0120 |
| Anemia | ||||
| Yes | 0.640 (0.262–1.566) | 0.559 (0.210–1.489) | 0.843 (0.223–3.193) | 0.5377 |
| No | 0.828 (0.571–1.202) | 1.021 (0.695–1.500) | 1.814 (1.092–3.012) | 0.0062 |
| Peptic ulcer | ||||
| Yes | 0.877 (0.243–3.166) | 0.777 (0.214–2.814) | 5.602 (0.597–52.587) | 0.6761 |
| No | 0.832 (0.581–1.191) | 1.014 (0.698–1.471) | 1.651 (1.012–2.692) | 0.0028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.-Y.; Luo, C.-W.; Chiang, Y.-W.; Li, K.-L.Y.Y.-C.; Ho, Y.-C.; Lee, S.-S.; Chen, W.-Y.; Chen, C.-J.; Kuan, Y.-H. Association Between PM2.5 Exposure Level and Primary Open-Angle Glaucoma in Taiwanese Adults: A Nested Case–control Study. Int. J. Environ. Res. Public Health 2021, 18, 1714. https://doi.org/10.3390/ijerph18041714
Sun H-Y, Luo C-W, Chiang Y-W, Li K-LYY-C, Ho Y-C, Lee S-S, Chen W-Y, Chen C-J, Kuan Y-H. Association Between PM2.5 Exposure Level and Primary Open-Angle Glaucoma in Taiwanese Adults: A Nested Case–control Study. International Journal of Environmental Research and Public Health. 2021; 18(4):1714. https://doi.org/10.3390/ijerph18041714
Chicago/Turabian StyleSun, Han-Yin, Ci-Wen Luo, Yun-Wei Chiang, Kun-Lin Yeh Yi-Ching Li, Yung-Chung Ho, Shiuan-Shinn Lee, Wen-Ying Chen, Chun-Jung Chen, and Yu-Hsiang Kuan. 2021. "Association Between PM2.5 Exposure Level and Primary Open-Angle Glaucoma in Taiwanese Adults: A Nested Case–control Study" International Journal of Environmental Research and Public Health 18, no. 4: 1714. https://doi.org/10.3390/ijerph18041714
APA StyleSun, H.-Y., Luo, C.-W., Chiang, Y.-W., Li, K.-L. Y. Y.-C., Ho, Y.-C., Lee, S.-S., Chen, W.-Y., Chen, C.-J., & Kuan, Y.-H. (2021). Association Between PM2.5 Exposure Level and Primary Open-Angle Glaucoma in Taiwanese Adults: A Nested Case–control Study. International Journal of Environmental Research and Public Health, 18(4), 1714. https://doi.org/10.3390/ijerph18041714







