Pollution Distribution of Potentially Toxic Elements in a Karstic River Affected by Manganese Mining in Changyang, Western Hubei, Central China
Abstract
:1. Introduction
2. Materials and Methods
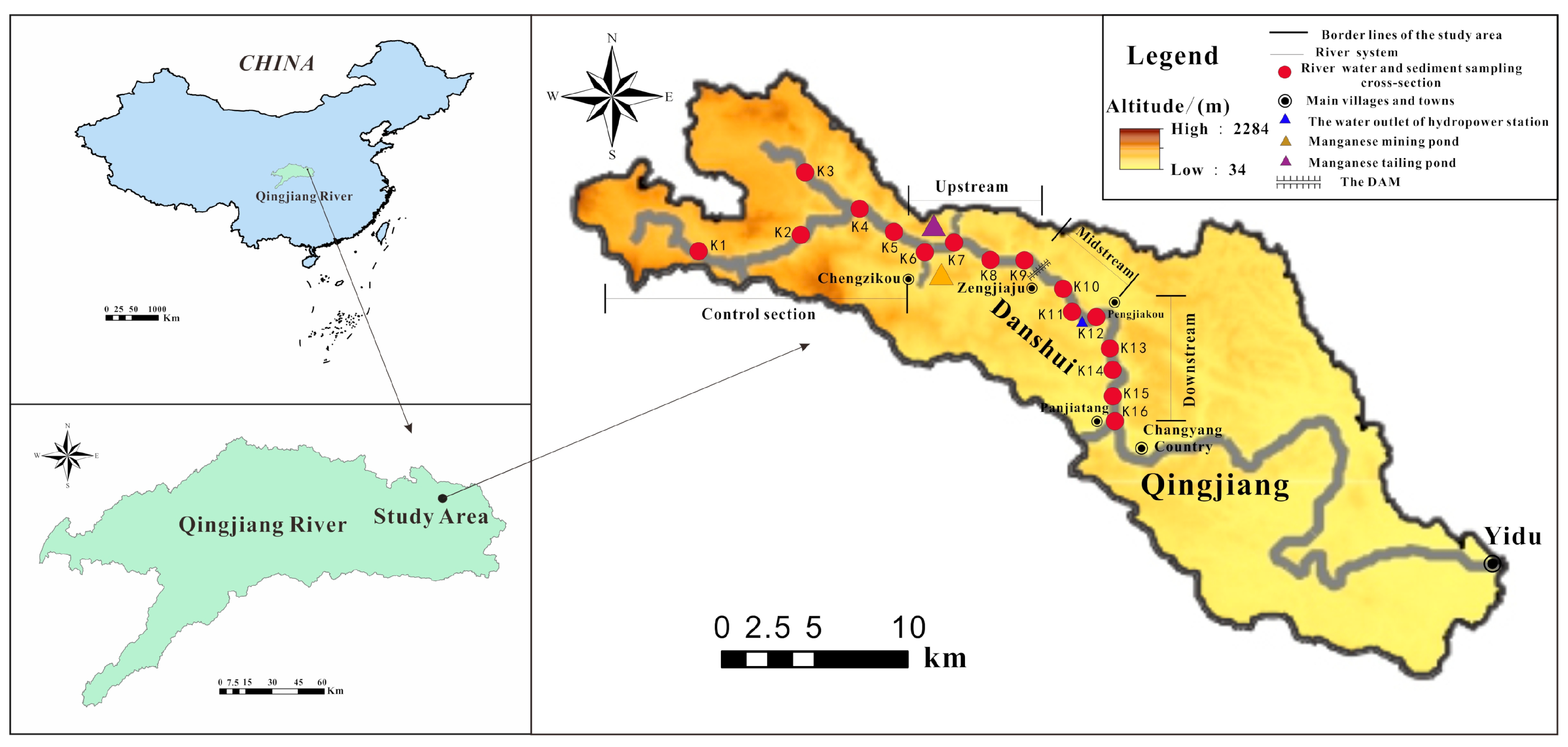
2.1. Study Area
2.2. Sample Collection and Chemical Analysis
2.3. Assessment of Pollution in River Water
2.3.1. Contamination Factor (CF)
2.3.2. Pollution Load Index (IPL)
2.4. Assessment of Pollution in River Sediments
2.4.1. Geo-Accumulation Index (Igeo)
2.4.2. Potential Ecological Risk Index (IPER)
3. Results and Discussion
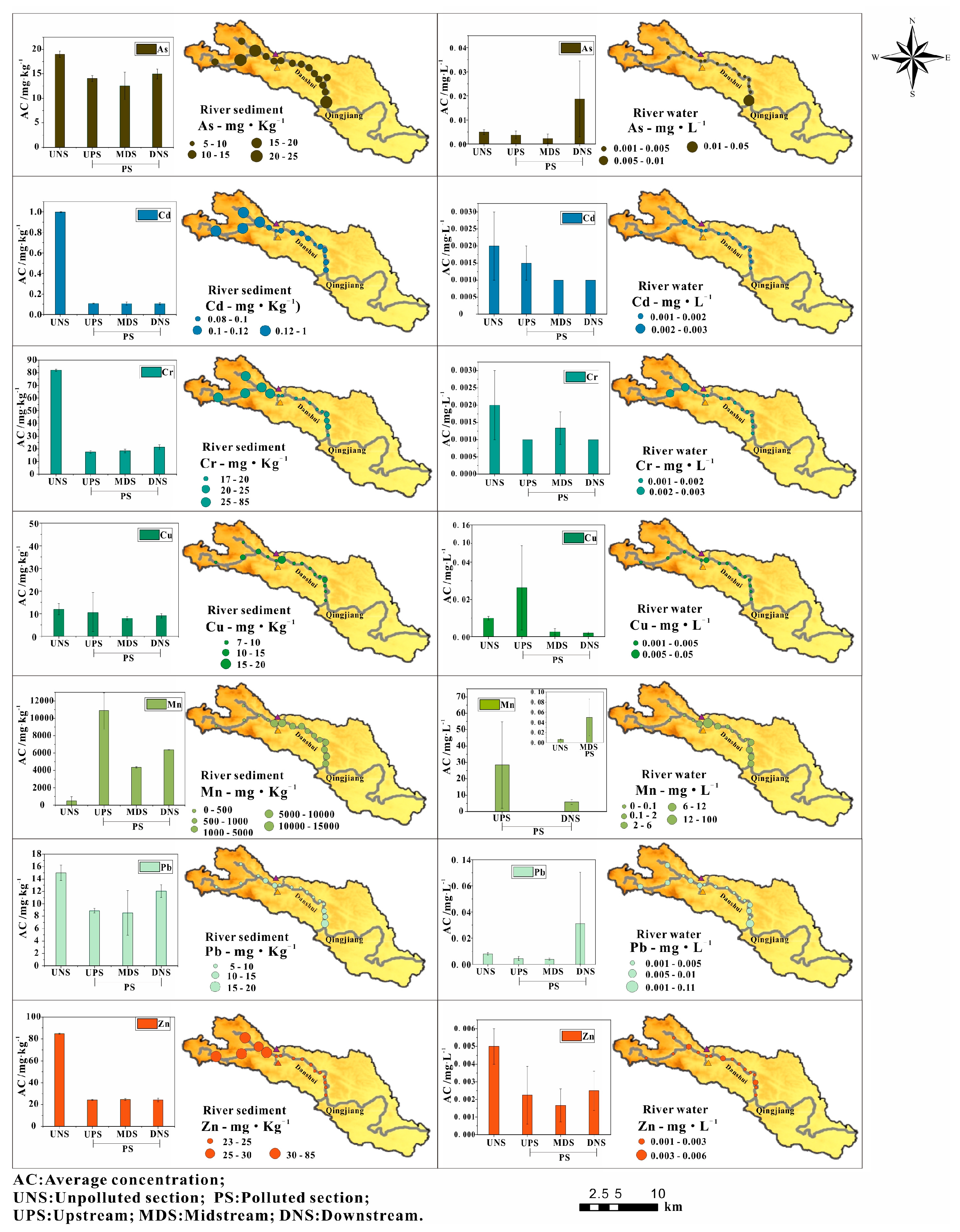
3.1. Concentration Distribution of PTEs
3.2. Identification of Sources of PTEs
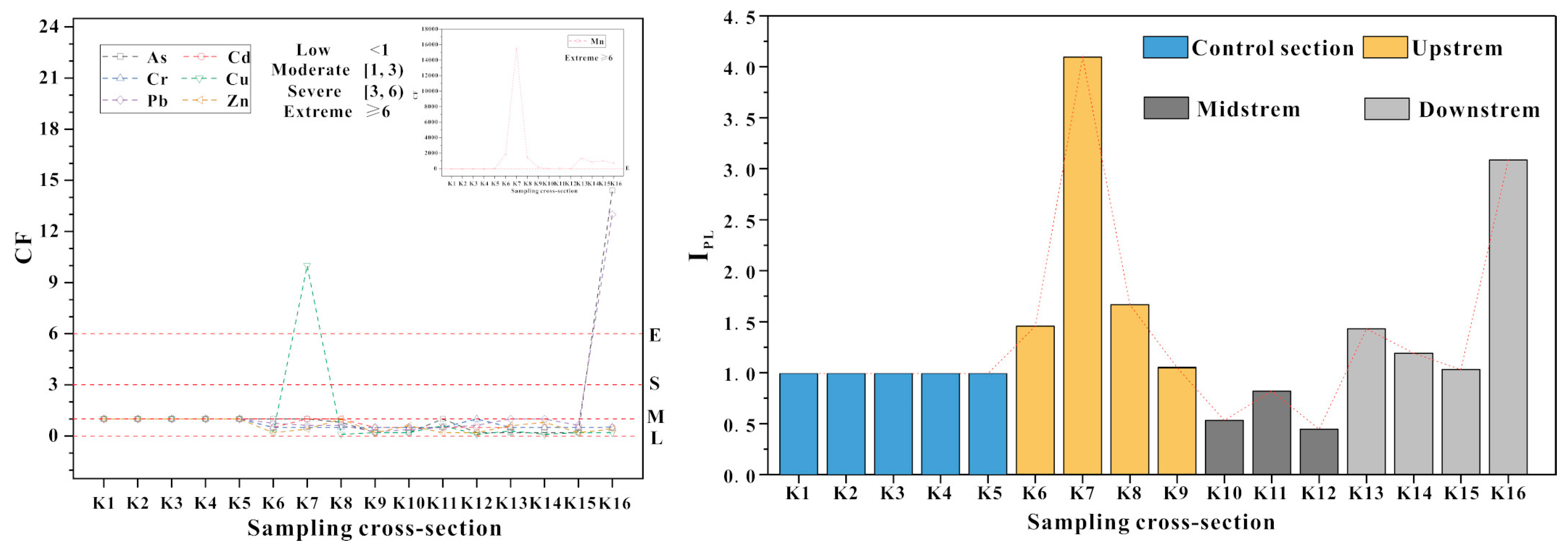
3.3. Pollution Assessment of PTEs
4. Conclusions
- (1)
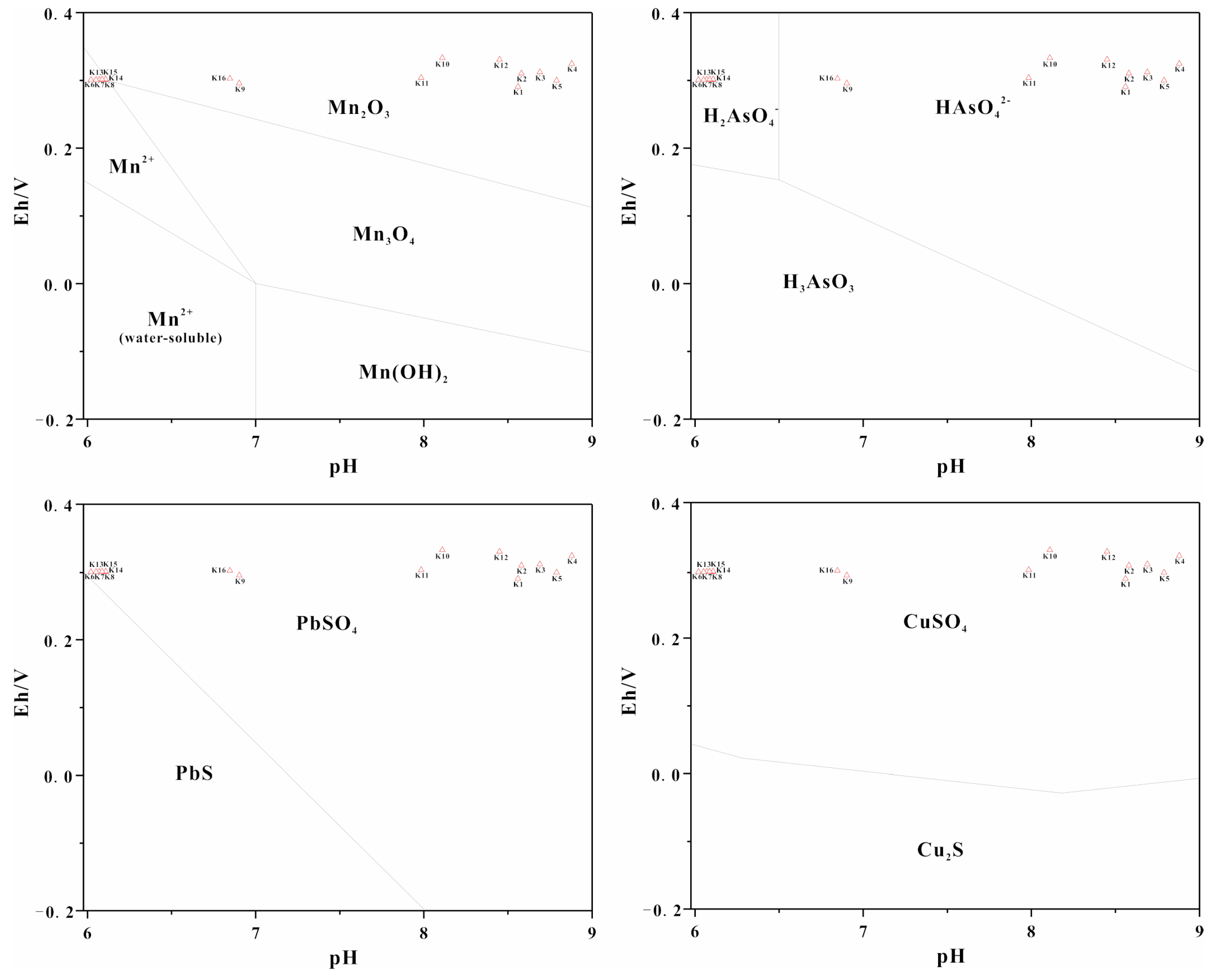
- PET pollution from manganese mining in the Danshui River was analyzed and it was determined that the main pollutant was Mn, which exhibited concentrations higher than the GBIII and CBG levels. The river water environment was mainly acidic and oxidizing and only the spatial distribution of Mn was greatly affected by the water environment. The pollution distribution of Mn was dominated by natural processes and anthropogenic activities. Upstream and downstream of the river were the main polluted areas, while midstream was lower. The water environment in the study area had a significant influence on the state of Mn as well as its pollution distribution. In addition, the concentration of As and Pb in the river water and sediments were high at K16, and Cu was high at K7. All of these locations were located upstream and downstream of the river.
- (2)
- Manganese mining activities were the main source of pollution. A wasteyard located along the river was also identified as a source of pollution. According to Pearson’s correlation and PCA, it showed that three groups of PTEs, Cd, Cr and Zn, mainly originated from nature and Pb and As were mainly related to the wasteyard. Mn mainly originated from manganese mining activities, and Cu was from both manganese mining activities and the wasteyard.
- (3)
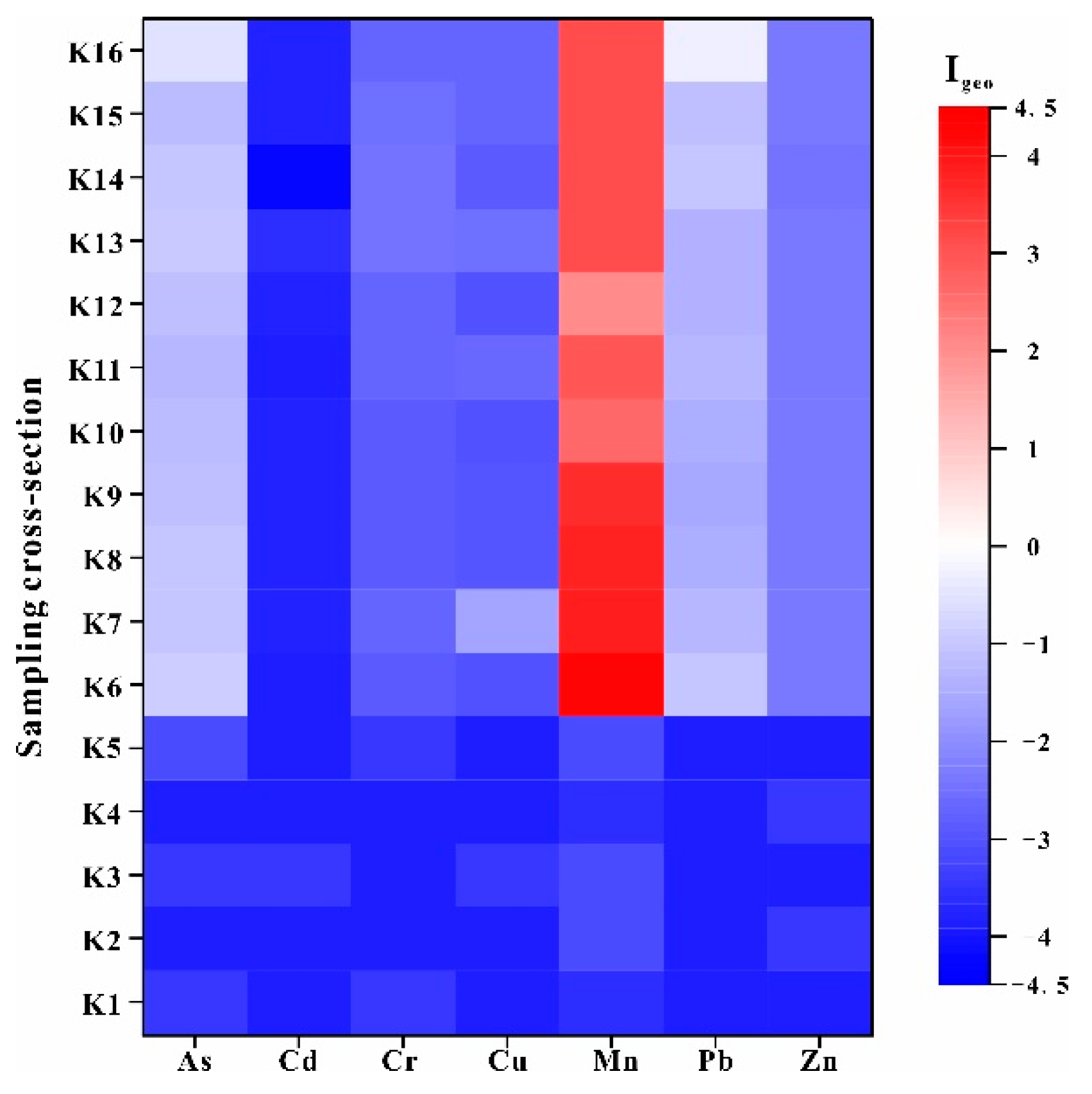
- According to the CF and IPL, As and Pb were low in river water from downstream, and Cu in river water was higher upstream. Mn was considered as the main pollutant of the river water in the whole river. Upstream and downstream were the main polluted sections of river water. Based on the results of Igeo and IPER, river sediments were heavily polluted by Mn and the other PTEs were at normal levels in this area. The IPER of PTEs in the river sediments were mostly at a low-risk level, only Mn in the upstream showed a moderate ecological risk, downstream showed low ecological risk and higher risk than midstream. Therefore, Mn was the main pollutant in the upstream and downstream polluted sections of the river.
- (4)
- According to the pollution distribution of PTEs, element speciation, the discharge of pollutants and water conservancy facilities were the key impacts observed and should be considered for the pollution treatment and environmental protection in this study area.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azhari, A.E.; Rhoujjati, A.; Hachimi, M.L.E.; Ambrosi, J.P. Pollution and ecological risk assessment of heavy metals in the soil-plant system and the sediment-water column around a former Pb/Zn-mining area in NE Morocco. Ecotoxicol. Environ. Saf. 2017, 144, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Q.; Sheng, Y.Q.; Jiang, M. Heavy metal pollution in a reforested mangrove ecosystem (Can Gio Biosphere Reserve, Southern Vietnam): Effects of natural and anthropogenic stressors over a thirty-year history. Chemosphere 2020, 245, 125596. [Google Scholar] [CrossRef]
- Pourret, O.; Hursthouse, A. It’s Time to Replace the Term “Heavy Metals” with “Potentially Toxic Elements” When Reporting Environmental Research. Int. J. Environ. Res. Public Health 2019, 16, 4446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, R.; Guo, D.; Ali, A. Accumulation, ecological-health risks assessment, and source apportionment of heavy metals in paddy soils: A case study in Hanzhong, Shaanxi, China. Environ. Pollut. 2019, 248, 349–357. [Google Scholar] [CrossRef]
- Wu, W.; Wu, P.; Yang, F. Assessment of heavy metal pollution and human health risks in urban soils around an electronics manufacturing facility. Sci. Total Environ. 2018, 630, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, H.; Naik, P.K.; Rishi, M.S. A new indexing approach for evaluating heavy metal contamination in groundwater. Chemosphere 2020, 245, 125598. [Google Scholar] [CrossRef]
- Adimalla, N.; Li, P.Y.; Qian, H. Evaluation of groundwater contamination for fluoride and nitrate in semi-arid region of Nirmal Province, South India: A special emphasis on human health risk assessment (HHRA). Hum. Ecol. Risk Assess. 2019, 25, 1107–1124. [Google Scholar] [CrossRef]
- Chibuike, G.U.; Obiora, S.C. Heavy metal polluted soils: Effect on plants and bioremediation methods. Appl. Environ. Soil Sci. 2014, 25, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.H.; Xie, X.D.; Wang, P. Heavy metal pollution caused by small-scale metal ore mining activities: A case study from a polymetallic mine in South China. Sci. Total Environ. 2018, 639, 217–227. [Google Scholar] [CrossRef]
- Lin, Y.; Ma, J.; Zhang, Z. Linkage between human population and trace elements in soils of the Pearl River Delta: Implications for source identification and risk assessment. Sci. Total Environ. 2018, 610–611, 944–950. [Google Scholar] [CrossRef]
- Kumar, A.R.; Riyazuddin, P. Seasonal variation of redox species and redox potentials in shallow groundwater: A comparison of measured and calculated redox potentials. J. Hydrol. 2012, 444, 187–198. [Google Scholar] [CrossRef]
- Mcgrath, S.P.; Lane, P.W. An explanation for the apparent losses of metals in a long-term field experiment with sewage sludge. Environ. Pollut. 1989, 60, 235–256. [Google Scholar] [CrossRef]
- Paraguassú, L.; Leite, M.G.P.; Moreira, F.W.A.; Mendonça, F.P.C.; Eskinazi-Sant’Anna, E.M. Impacts of mining in artificial lake of Iron Quadrangle-MG: Past marks and changes of the present. Environ. Earth Sci. 2019, 167, 1–10. [Google Scholar] [CrossRef]
- Barbieri, M.; Sappa, G.; Nigro, A. Soil pollution: Anthropogenic versus geogenic contributions over large areas of the Lazio region. J. Geochem. Explor. 2018, 195, 78–86. [Google Scholar] [CrossRef]
- Li, R.; Tang, X.Q.; Guo, W.J. Spatiotemporal distribution dynamics of heavy metals in water, sediment, and zoobenthos in mainstream sections of the middle and lower Changjiang River. Sci. Total Environ. 2020, 714, 136779. [Google Scholar] [CrossRef]
- Zeng, Y.S.; Bi, C.J.; Jia, J.P. Impact of intensive land use on heavy metal concentrations and ecological risks in an urbanized river network of Shanghai. Ecol. Indic. 2020, 16, 106501. [Google Scholar] [CrossRef]
- Sun, X.S.; Fan, D.J.; Liu, M. Source identification, geochemical normalization and influence factors of heavy metals in Yangtze River Estuary sediment. Environ. Pollut. 2018, 241, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, H.; Chen, L. Spatial distribution characteristics and pollution assessment of heavy metals in river surface sediments in a manganese mining area, Western Hubei. Saf. Environ. Eng. 2020, 27, 110–117. [Google Scholar]
- Liu, Z.; Zhou, H.; Cao, W.J. Seasonal Distribution Characteristics and Health Risk Assessment of Heavy Metals in Surface Water of Qingjiang River. Environ. Sci. 2021, 42, 175–183. [Google Scholar]
- Kimleang, K.; Asumi, S.; Shingo, T. Long-term acid generation and heavy metal leaching from the tailings of Shimokawa mine, Hokkaido, Japan: Column study under natural condition. J. Geochem. Explor. 2019, 201, 1–12. [Google Scholar]
- Bi, B.; Liu, X.H.; Guo, X.C. Occurrence and risk assessment of heavy metals in water, sediment, and fish from Dongting Lake, China. Environ. Sci. Pollut. Res. 2018, 34, 34076–34090. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Ren, B.Z.; Hursthouse, A.S. Soil from an Abandoned Manganese Mining Area (Hunan, China): Significance of Health Risk from Potentially Toxic Element Pollution and Its Spatial Context. Int. J. Environ. Res. Public Health 2020, 18, 6554. [Google Scholar] [CrossRef]
- Jiang, F.; Ren, B.Z.; Hursthouse, A.S. Trace Metal Pollution in Topsoil Surrounding the Xiangtan Manganese Mine Area (South-Central China): Source Identification, Spatial Distribution and Assessment of Potential Ecological Risks. Int. J. Environ. Res. Public Health 2018, 15, 2412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acosta, J.A.; Gabarrón, M.; Faz, A. Influence of population density on the concentration and speciation of metals in the soil and street dust from urban areas. Chemosphere 2015, 134, 328–337. [Google Scholar] [CrossRef]
- Mico, C.; Recatalá, L.; Peris, M. Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere 2006, 65, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Chinese State Environmental Protection Administration. Environmental Quality Standards for Surface Water (GB3838-2002); Chinese State Environmental Protection Administration: Beijing, China, 2002.
- Chinese State Environmental Protection Administration. Technical Specifications Requirements for Monitoring of Surface Water and Waste Water (HJ/T 91-2002); Chinese State Environmental Protection Administration: Beijing, China, 2002.
- Chinese Ministry of Land and Resources. Specification of Geochemical Reconnaissance Survey (1:50000) (DZ/T0011-2015); Chinese Ministry of Land and Resources: Beijing, China, 2015. [Google Scholar]
- Shen, F.; Mao, L.; Sun, R. Contamination evaluation and source identification of heavy metals in the sediments from the Lishui River Watershed, Southern China. Int. J. Environ. Res. Public Health 2019, 16, 336. [Google Scholar] [CrossRef] [Green Version]
- Usman, Q.A.; Muhammad, S.; Ali, W. Spatial distribution and provenance of heavy metal contamination in the sediments of the Indus River and its tributaries, North Pakistan: Evaluation of pollution and potential risks. Environ. Technol. Innov. 2020, 28, 101184. [Google Scholar] [CrossRef]
- Liu, X.; Li, D.; Song, G. Assessment of heavy metal levels in surface sediments of estuaries and adjacent coastal areas in China. Front. Earth Sci. 2017, 11, 85–94. [Google Scholar] [CrossRef]
- Jorfi, S.; Maleki, R.; Jaafarzadeh, N. Pollution load index for heavy metals in Mian-Ab plain soil, Khuzestan, Iran. Data Brief. 2017, 15, 584–590. [Google Scholar] [CrossRef]
- Hasan, A.B.; Kabir, S.A.; Reza, H.M.S. Enrichment factor and geo-accumulation index of trace metals in sediments of the ship breaking area of Sitakund Upazilla (Bhatiary–Kumira), Chittagong, Bangladesh. J. Geochem. Explor. 2013, 125, 130–137. [Google Scholar]
- Usese, A.; Chukwu, O.L.; Rahamn, M.M. Enrichment, contamination and geo-accumulation factors for assessing arsenic contamination in sediment of a Tropical Open Lagoon, Southwest Nigeria. Environ. Technol. Innov. 2017, 8, 126–131. [Google Scholar] [CrossRef]
- Han, X.F.; Lu, X.W.; Qinggeletu; Wu, Y.F. Health risks and contamination levels of heavy metals in dusts from parks and squares of an industrial city in semi-arid area of China. Int. J. Environ. Res. Public Health 2017, 8, 886. [Google Scholar] [CrossRef] [Green Version]
- Maanan, M.; Saddik, M.; Maanan, M.; Chaibi, M.; Assobhei, O.; Zourarah, B. Environmental and ecological risk assessment of heavy metals in sediments of Nador lagoon, Morocco. Ecol. Indic. 2015, 48, 616–626. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, L.; Kong, L.; Liu, E.; Wang, L.; Zhu, J. Spatial distribution, ecological risk assessment and source identification for heavy metals in surface sediments from Dongping lake, Shandong, East China. Catena 2015, 125, 200–205. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M. A review of Listeria monocytogenes: An update on outbreaks, virulence, dose-response, ecology, and risk assessments. Food Control 2015, 75, 1–13. [Google Scholar] [CrossRef]
- Semenzin, E.; Critto, A.; Rutgers, M. Integration of bioavailability, ecology and ecotoxicology by three lines of evidence into ecological risk indexes for contaminated soil assessment. Sci. Total Environ. 2008, 389, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Muhammad, S.; Jadoon, I.A.K. Potentially harmful elements contamination in water and sediment: Evaluation for risk assessment and provenance in the northern Sulaiman fold belt, Baluchistan, Pakistan. Microchem. J. 2019, 147, 1155–1162. [Google Scholar] [CrossRef]
- Chen, Y.X.; Jiang, X.S.; Wang, Y. Spatial characteristics of heavy metal pollution and the potential ecological risk of a typical mining area: A case study in China. Process Saf. Environ. Prot. 2018, 113, 204–219. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, X.; Xu, J.; Selim, H.M. Heavy metal contaminations in a soil-rice system: Identification of spatial dependence in relation to soil properties of paddy fields. J. Hazard. Mater. 2010, 181, 778–787. [Google Scholar]
- Li, X.; Yang, H.; Zhang, C.; Zeng, G.; Liu, Y.; Xu, W.; Wu, Y.; Lan, S. Spatial distribution and transport characteristics of heavy metals around an antimony mine area in central China. Chemosphere 2017, 170, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.H.; Qu, S.Y.; Nel, W. The impact of natural weathering and mining on heavy metal accumulation in the karst areas of the Pearl River Basin, China. Sci. Total Environ. 2020, 734, 139480. [Google Scholar] [CrossRef]
- Sappa, G.; Barbieri, M.; Andrei, M.; Ferranti, F. Assessment of arsenic mobility in a shallow aquifer from Bevera Valley Basin (Northern Italy). Arab. J. Geosci. 2019, 12, 678. [Google Scholar] [CrossRef]
- Barbieri, M.; Sappa, G.; Vitale, S.; Parisse, B.; Battistel, M. Soil control of trace metals concetrations in landfill: A case study of the largest landfill in Europe, Malagrotta, Rome. J. Geochem. Explor. 2014, 143, 146–154. [Google Scholar] [CrossRef]
- Barbieri, M.; Nigro, A.; Sappa, G. Soil contamination evaluation by Enrichment Factor (EF) and Geoaccumulation Index (Igeo). Senses Sci. 2015, 2, 94–97. [Google Scholar]
- Husson, O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: A transdisciplinary overview pointing to integrative. Plant Soil 2013, 362, 389–417. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Zhao, Y.; Zhao, X.; Wang, Y.; Deng, W. Sources of heavy metal pollution in agricultural soils of a rapidly industrializing area in the Yangtze Delta of China. Ecotoxicol. Environ. Saf. 2014, 108, 161–167. [Google Scholar] [CrossRef]
- Delplace, G.; Schreck, E.; Pokrovsky, O.S. Accumulation of heavy metals in phytoliths from reeds growing on mining environments in Southern Europe. Sci. Total Environ. 2020, 712, 135595. [Google Scholar] [CrossRef]
- Petrovic, J.V.; Alagic, S.C.; Milic, S.M. Chemometric characterization of heavy metals in soils and shoots of the two pioneer species sampled near the polluted water bodies in the close vicinity of the copper mining and metallurgical complex in Bor (Serbia): Phytoextraction and biomonitoring contexts. Chemosphere 2021, 262, 127808. [Google Scholar]
- Qu, S.Y.; Wu, W.H.; Nel, W.; Ji, J.F. The behavior of metals/metalloids during natural weathering: A systematic study of the mono-lithological watersheds in the upper Pearl River Basin, China. Sci. Total Environ. 2020, 708, 134572. [Google Scholar] [CrossRef] [PubMed]


| Classification | RI | |||
|---|---|---|---|---|
| 1 | <16 | Low Risk | <60 | Low Risk |
| 2 | (16,32] | Moderate Risk | (60,120] | Moderate Risk |
| 3 | (32,64] | Considerable Risk | (120,240] | Considerable Risk |
| 4 | (64,128] | High Risk | (240,480] | High Risk |
| 5 | >128 | Very High Risk | >480 | Very High Risk |
| Sampling Size | Element | As | Cd | Cr | Cu | Mn | Pb | Zn |
|---|---|---|---|---|---|---|---|---|
| 48 | CV in river sediments (Mean ± SD%) | 35 ± 2 | 11 ± 1 | 13 ± 2 | 55 ± 4 | 206 ± 12 | 37 ± 2 | 15 ± 2 |
| 48 | CV in river water (Mean ± SD%) | 36 ± 3 | 9 ± 1 | 10 ± 1 | 36 ± 3 | 103 ± 2 | 35 ± 2 | 13 ± 1 |
| Sampling Cross-Section | Sampling Size | pH (Mean ± SD) | Eh/mV (Mean ± SD) |
|---|---|---|---|
| K1 | 3 | 8.56 ± 0.1 | 290.6 ± 2.1 |
| K2 | 3 | 8.58 ± 0.2 | 310.5 ± 2.2 |
| K3 | 3 | 8.69 ± 0.2 | 312.2 ± 2.6 |
| K4 | 3 | 8.88 ± 0.1 | 324.5 ± 2.3 |
| K5 | 3 | 8.79 ± 0.2 | 299.8 ± 2.5 |
| K6 | 3 | 6.01 ± 0.1 | 300.8 ± 1.9 |
| K7 | 3 | 6.08 ± 0.2 | 300.4 ± 1.8 |
| K8 | 3 | 6.18 ± 0.2 | 300.8 ± 1.5 |
| K9 | 3 | 6.98 ± 0.2 | 301.9 ± 2.1 |
| K10 | 3 | 8.11 ± 0.1 | 335.6 ± 2.2 |
| K11 | 3 | 7.98 ± 0.2 | 301.9 ± 1.2 |
| K12 | 3 | 8.45 ± 0.2 | 330.8 ± 1.4 |
| K13 | 3 | 6.14 ± 0.1 | 300.9 ± 1.6 |
| K14 | 3 | 6.26 ± 0.2 | 302.2 ± 1.9 |
| K15 | 3 | 6.26 ± 0.1 | 301.9 ± 1.1 |
| K16 | 3 | 6.78 ± 0.1 | 310.5 ± 2.0 |
| Element in Water | As | Cd | Cr | Cu | Mn | Pb | Zn |
|---|---|---|---|---|---|---|---|
| As | 1 | 0.65 * | 0.69 * | 0.59 * | 0.16 | 0.99 ** | 0.69 * |
| Cd | 1 | 0.98 ** | 0.64 * | 0.26 | 0.69 * | 0.99 ** | |
| Cr | 1 | 0.67 * | 0.30 | 0.66 * | 0.99 ** | ||
| Cu | 1 | 0.66 * | 0.61 * | 0.69 * | |||
| Mn | 1 | 0.26 | 0.33 | ||||
| Pb | 1 | 0.56 * | |||||
| Zn | 1 | ||||||
| Element in Sediment | As | Cd | Cr | Cu | Mn | Pb | Zn |
| As | 1 | 0.63 * | 0.67 ** | 0.58 * | 0.15 | 0.99 ** | 0.67 * |
| Cd | 1 | 0.98 ** | 0.62 * | 0.24 | 0.66 * | 0.99 ** | |
| Cr | 1 | 0.66 * | 0.31 | 0.66 * | 0.99 ** | ||
| Cu | 1 | 0.63 * | 0.62 * | 0.68 * | |||
| Mn | 1 | 0.21 | 0.34 | ||||
| Pb | 1 | 0.56 * | |||||
| Zn | 1 |
| Element in Water | PC1 | PC2 | PC3 | Element in Sediment | PC1 | PC2 | PC3 |
|---|---|---|---|---|---|---|---|
| As | 0.47 | 0.01 | 0.79 | As | 0.48 | 0.13 | 0.71 |
| Cd | 0.94 | −0.16 | −0.13 | Cd | 0.97 | −0.02 | −0.13 |
| Cr | 0.99 | −0.02 | −0.08 | Cr | 0.99 | −0.01 | −0.08 |
| Cu | 0.57 | 0.75 | 0.04 | Cu | 0.53 | 0.71 | 0.04 |
| Mn | −0.04 | 0.98 | 0.06 | Mn | 0.10 | 0.95 | 0.02 |
| Pb | 0.48 | −0.09 | 0.83 | Pb | 0.50 | −0.04 | 0.79 |
| Zn | 0.98 | 0.04 | 0.06 | Zn | 0.95 | 0.02 | 0.02 |
| eigenvalues | 3.13 | 1.12 | 1.11 | eigenvalues | 3.16 | 1.27 | 1.11 |
| variance/% | 46.30 | 16.78 | 15.71 | variance/% | 48.89 | 17.67 | 16.67 |
| cumulative variance/% | 46.30 | 63.08 | 78.79 | cumulative variance/% | 48.89 | 66.56 | 83.23 |
| River Section | Sampling Size | Ei (Mean ± SD) | RI (Mean ± SD) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| As | Cd | Cr | Cu | Mn | Pb | Zn | |||
| Upstream | 12 | 7.4 ± 0.4 | 3.2 ± 0.1 | 0.4 ± 0.0 | 1.3 ± 0.6 | 22.4 ± 4.1 | 3.0 ± 0.4 | 0.3 ± 0.0 | 38.1 ± 4.8 |
| Midstream | 9 | 6.6 ± 0.3 | 3.2 ± 0.1 | 0.4 ± 0.0 | 1.0 ± 0.1 | 9.0 ± 2.2 | 2.8 ± 0.1 | 0.3 ± 0.0 | 23.4 ± 1.9 |
| Downstream | 12 | 7.9 ± 1.5 | 3.2 ± 0.4 | 0.5 ± 0.0 | 1.2 ± 0.1 | 13.1 ± 0.2 | 4.0 ± 1.2 | 0.3 ± 0.0 | 30.1 ± 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Kuang, Y.; Lan, S.; Cao, W.; Yan, Z.; Chen, L.; Chen, Q.; Feng, Q.; Zhou, H. Pollution Distribution of Potentially Toxic Elements in a Karstic River Affected by Manganese Mining in Changyang, Western Hubei, Central China. Int. J. Environ. Res. Public Health 2021, 18, 1870. https://doi.org/10.3390/ijerph18041870
Liu Z, Kuang Y, Lan S, Cao W, Yan Z, Chen L, Chen Q, Feng Q, Zhou H. Pollution Distribution of Potentially Toxic Elements in a Karstic River Affected by Manganese Mining in Changyang, Western Hubei, Central China. International Journal of Environmental Research and Public Health. 2021; 18(4):1870. https://doi.org/10.3390/ijerph18041870
Chicago/Turabian StyleLiu, Zhao, Ye Kuang, Shengtao Lan, Wenjia Cao, Ziqi Yan, Li Chen, Qianlong Chen, Qi Feng, and Hong Zhou. 2021. "Pollution Distribution of Potentially Toxic Elements in a Karstic River Affected by Manganese Mining in Changyang, Western Hubei, Central China" International Journal of Environmental Research and Public Health 18, no. 4: 1870. https://doi.org/10.3390/ijerph18041870
APA StyleLiu, Z., Kuang, Y., Lan, S., Cao, W., Yan, Z., Chen, L., Chen, Q., Feng, Q., & Zhou, H. (2021). Pollution Distribution of Potentially Toxic Elements in a Karstic River Affected by Manganese Mining in Changyang, Western Hubei, Central China. International Journal of Environmental Research and Public Health, 18(4), 1870. https://doi.org/10.3390/ijerph18041870






