Cord Blood Adipocytokines and Body Composition in Early Childhood: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
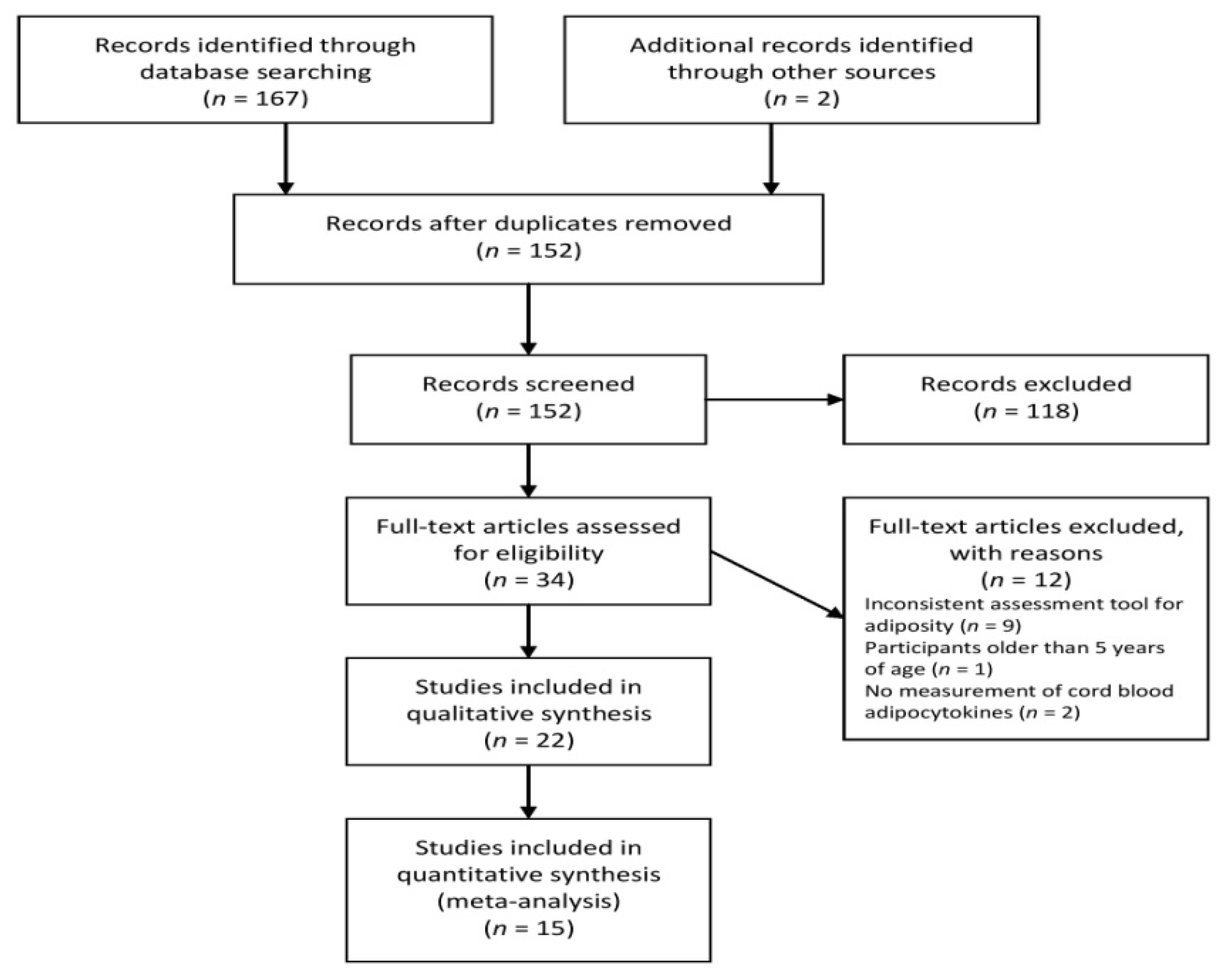
2.1. Search Strategy and Study Selection
2.2. Data Extraction
2.3. Statistical Analysis
2.4. Assessment of Risk Bias
3. Results
3.1. Characteristics
3.2. Studies Reporting Leptin
3.3. Studies Reporting Adiponectin
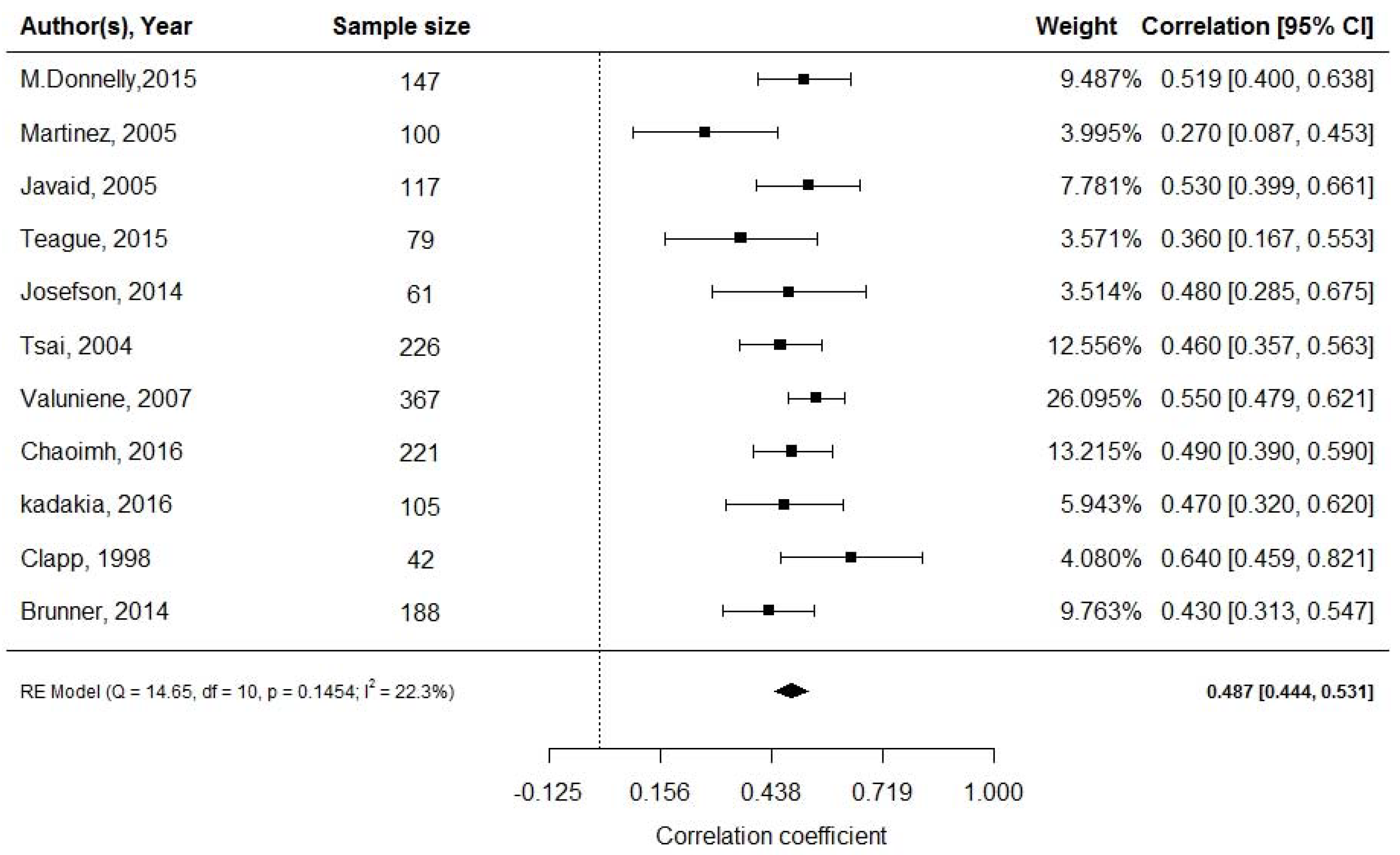
3.4. Leptin and Neonatal Adiposity at Birth
3.5. Leptin and Adiposity in Early Childhood
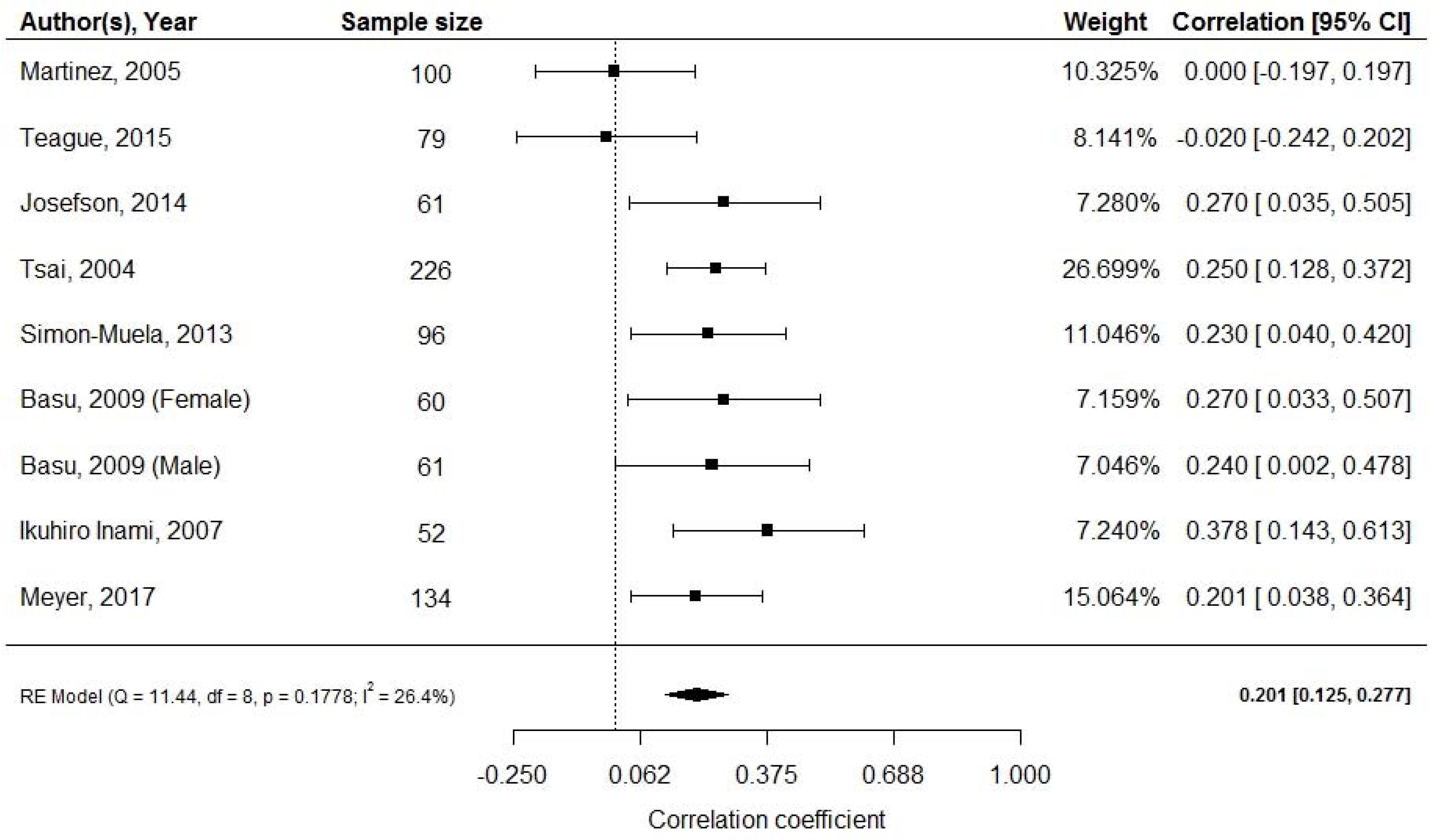
3.6. Adiponectin and Neonatal Adiposity at Birth
3.7. Adiponectin and Adiposity in Early Childhood
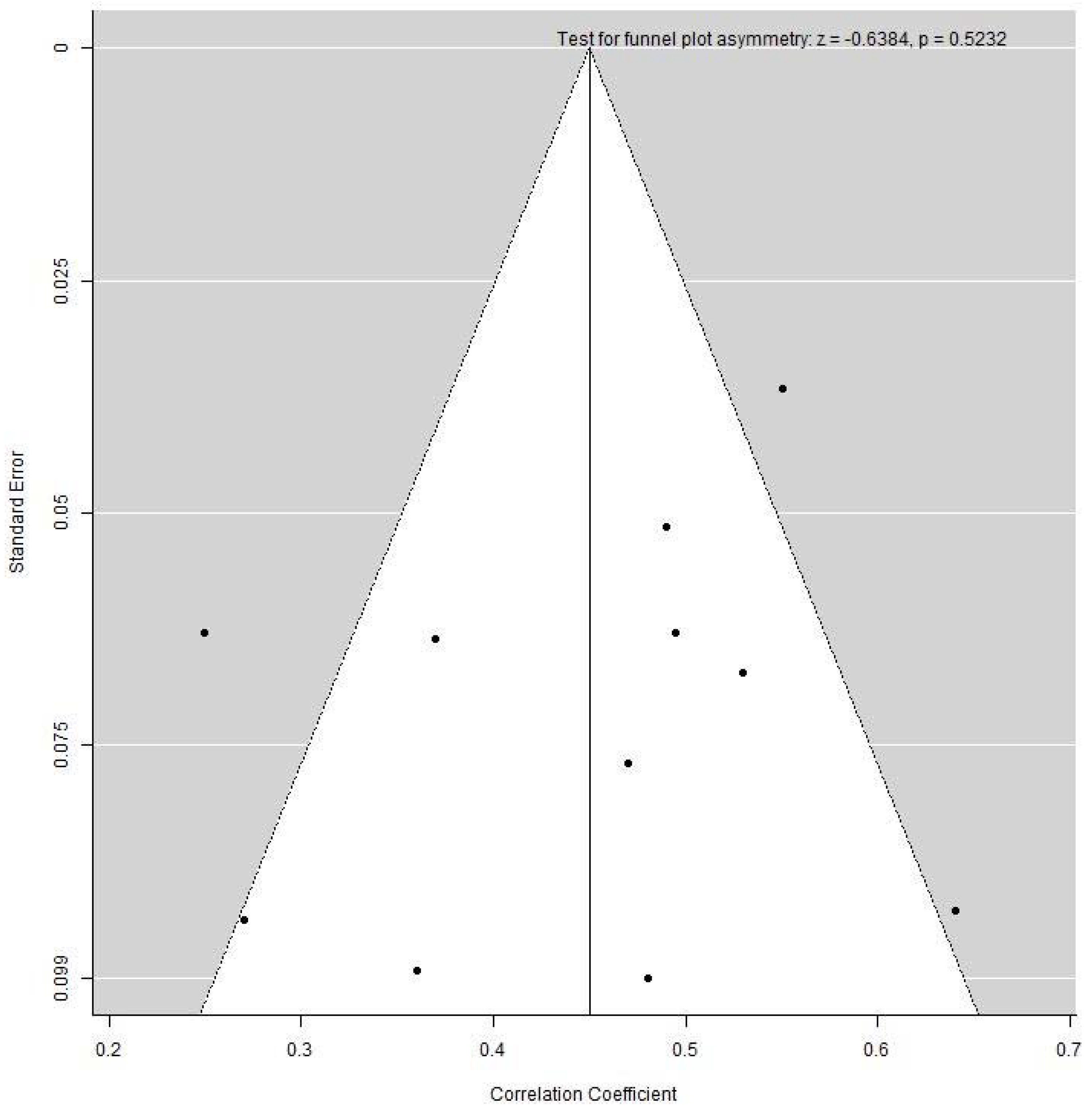
3.8. Publication Bias
4. Discussion
4.1. Leptin and Adiposity
4.2. Adiponectin and Adiposity
4.3. Strengths and Weakness
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Report of the commission on ending childhood obesity; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Simmonds, M.; Llewellyn, A.; Owen, C.G.; Woolacott, N. Predicting adult obesity from childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 95–107. [Google Scholar] [CrossRef]
- Tirosh, A.; Shai, I.; Afek, A.; Dubnov-Raz, G.; Ayalon, N.; Gordon, B.; Derazne, E.; Tzur, D.; Shamis, A.; Vinker, S.; et al. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N. Engl. J. Med. 2011, 364, 1315–1325. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.W.; Hanson, R.L.; Knowler, W.C.; Sievers, M.L.; Bennett, P.H.; Looker, H.C. Childhood obesity, other cardiovascular risk factors, and premature death. N. Engl. J. Med. 2010, 362, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body fatness and cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, K.; Sahoo, B.; Choudhury, A.K.; Sofi, N.Y.; Kumar, R.; Bhadoria, A.S. Childhood obesity: Causes and consequences. In. J. Family Med. Prim. Care 2015, 4, 187–192. [Google Scholar]
- Barker, D.J.P.; Godfrey, K.M.; Gluckman, P.D.; Harding, J.E.; Owens, J.A.; Robinson, J.S. Fetal nutrition and cardiovascular disease in adult life. Lancet 1993, 341, 938–941. [Google Scholar] [CrossRef]
- Kuzawa, C.W. Adipose tissue in human infancy and childhood: An evolutionary perspective. Am. J. Phys. Anthropol. 1998, 107, 177–209. [Google Scholar] [CrossRef]
- Pelletier, D.L.; Frongillo Jr, E.A.; Habicht, J.-P. Epidemiologic evidence for a potentiating effect of malnutrition on child mortality. Am. J. Public Health 1993, 83, 1130–1133. [Google Scholar] [CrossRef]
- Victora, C.G.; Barros, F.C.; Kirkwood, B.R.; Vaughan, J.P. Pneumonia, diarrhea, and growth in the first 4 y of life: A longitudinal study of 5914 urban Brazilian children. Am. J. Clin. Nutr. 1990, 52, 391–396. [Google Scholar] [CrossRef]
- WHO. Physical status: The use and interpretation of anthropometry. World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Wells, J.C.K. Toward Body Composition Reference Data for Infants, Children, and Adolescents. Adv. Nutr. 2014, 5, 320S–329S. [Google Scholar] [CrossRef]
- Friedman, J. 20 years of leptin: Leptin at 20: An overview. J. Endocrinol. 2014, 223, T1–T8. [Google Scholar] [CrossRef]
- Hassink, S.G.; de Lancey, E.; Sheslow, D.V.; Smith-Kirwin, S.M.; O’Connor, D.M.; Considine, R.V.; Opentanova, I.; Dostal, K.; Spear, M.L.; Leef, K.; et al. Placental leptin: An important new growth factor in intrauterine and neonatal development? Pediatrics 1997, 100, E1. [Google Scholar] [CrossRef]
- Wang, J.; Liu, R.; Hawkins, M.; Barzilai, N.; Rossetti, L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 1998, 393, 684. [Google Scholar] [CrossRef]
- Laharrague, P.; Larrouy, D.; Fontanilles, A.M.; Truel, N.; Campfield, A.; Tenenbaum, R.; Galitzky, J.; Corberand, J.X.; Penicaud, L.; Casteilla, L.; et al. High expression of leptin by human bone marrow adipocytes in primary culture. FASEB J. 1998, 12, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Bado, A.; Levasseur, S.; Attoub, S.; Kermorgant, S.; Laigneau, J.P.; Bortoluzzi, M.N.; Moizo, L.; Lehy, T.; Guerre-Milllo, M.; Le Marchand-Brustel, Y.; et al. The stomach is a source of leptin. Nature 1998, 394, 790. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.L.; Heist, K.; DePaoli, A.M.; Veldhuis, J.D.; Mantzoros, C.S. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J. Clin. Investig. 2003, 111, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, L.A.; Dembski, M.; Weng, X.; Deng, N.; Culpepper, J.; Devos, R.; Richards, G.J.; Campfield, L.A.; Clark, F.T.; Deeds, J.; et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995, 83, 1263–1271. [Google Scholar] [CrossRef]
- Alexe, D.-M.; Syridou, G.; Petridou, E.T. Determinants of early life leptin levels and later life degenerative outcomes. Clin. Med. Res. 2006, 4, 326–335. [Google Scholar] [CrossRef]
- Brochu-Gaudreau, K.; Rehfeldt, C.; Blouin, R.; Bordignon, V.; Murphy, B.D.; Palin, M.-F. Adiponectin action from head to toe. Endocrine 2010, 37, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Liang, P.; Spiegelman, B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996, 271, 10697–10703. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef]
- Chan, T.-F.; Yuan, S.-S.F.; Chen, H.-S.; Guu, C.-F.; Wu, L.-C.; Yeh, Y.-T.; Chung, Y.-F.; Jong, S.-B.; Su, J.-H. Correlations between umbilical and maternal serum adiponectin levels and neonatal birthweights. Acta Obstet. Gynecol. Scand. 2004, 83, 165–169. [Google Scholar] [CrossRef]
- McDonald, E.A.; Wolfe, M.W. Adiponectin attenuation of endocrine function within human term trophoblast cells. Endocrinology 2009, 150, 4358–4365. [Google Scholar] [CrossRef] [PubMed]
- Mazaki-Tovi, S.; Kanety, H.; Pariente, C.; Hemi, R.; Wiser, A.; Schiff, E.; Sivan, E. Maternal serum adiponectin levels during human pregnancy. J. Perinatol. 2007, 27, 77. [Google Scholar] [CrossRef] [PubMed]
- Sivan, E.; Mazaki-Tovi, S.; Pariente, C.; Efraty, Y.; Schiff, E.; Hemi, R.; Kanety, H. Adiponectin in human cord blood: Relation to fetal birth weight and gender. J. Clin. Endocrinol. Metab. 2003, 88, 5656–5660. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, S.; Bulfamante, G.; Cortelazzi, D.; Barresi, V.; Cetin, I.; Mantovani, G.; Bondioni, S.; Beck-Peccoz, P.; Spada, A. Adiponectin expression in human fetal tissues during mid-and late gestation. J. Clin. Endocrinol. Metab. 2005, 90, 2397–2402. [Google Scholar] [CrossRef]
- Donnelly, J.M.; Lindsay, K.L.; Walsh, J.M.; Horan, M.; Molloy, E.J.; McAuliffe, F.M. Fetal metabolic influences of neonatal anthropometry and adiposity. BMC Pediatrics 2015, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cordero, C.; Amador-Licona, N.; Guizar-Mendoza, J.M.; Hernandez-Mendez, J.; Ruelas-Orozco, G. Body fat at birth and cord blood levels of insulin, adiponectin, leptin, and insulin-like growth factor-I in small-for-gestational-age infants. Arch. Med. Res. 2006, 37, 490–494. [Google Scholar] [CrossRef]
- Javaid, M.K.; Godfrey, K.M.; Taylor, P.; Robinson, S.M.; Crozier, S.R.; Dennison, E.M.; Robinson, J.S.; Breier, B.R.; Arden, N.K.; Cooper, C. Umbilical cord leptin predicts neonatal bone mass. Calcif. Tissue Int. 2005, 76, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Teague, A.M.; Fields, D.A.; Aston, C.E.; Short, K.R.; Lyons, T.J.; Chernausek, S.D. Cord blood adipokines, neonatal anthropometrics and postnatal growth in offspring of Hispanic and Native American women with diabetes mellitus. Reprod. Biol. Endocrinol. 2015, 13, 68. [Google Scholar] [CrossRef]
- Josefson, J.L.; Zeiss, D.M.; Rademaker, A.W.; Metzger, B.E. Maternal leptin predicts adiposity of the neonate. Horm. Res. Paediatr. 2014, 81, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.J.; Yu, C.H.; Hsu, S.P.; Lee, H.Y.; Chiou, C.H.; Hsu, Y.W.; Ho, S.C.; Chu, C.H. Cord plasma concentrations of adiponectin and leptin in healthy term neonates: Positive correlation with birthweight and neonatal adiposity. Clin. Endocrinol. 2004, 61, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Valuniene, M.; Verkauskiene, R.; Boguszewski, M.; Dahlgren, J.; Lasiene, D.; Lasas, L.; Albertssson Wikland, K. Leptin levels at birth and in early postnatal life in small- and appropriate-for-gestational-age infants. Medicina 2007, 43, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Chaoimh, C.N.; Murray, D.M.; Kenny, L.C.; Irvine, A.D.; Hourihane, J.O.; Kiely, M. Cord blood leptin and gains in body weight and fat mass during infancy. Eur. J. Endocrinol. 2016, 175, 403–410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kadakia, R.; Zheng, Y.; Zhang, Z.; Zhang, W.; Hou, L.; Josefson, J.L. Maternal pre-pregnancy BMI downregulates neonatal cord blood LEP methylation. Pediatric Obes. 2017, 12, 57–64. [Google Scholar] [CrossRef]
- Clapp Iii, J.F.; Kiess, W. Cord blood leptin reflects fetal fat mass. J. Soc. Gynecol. Investig. 1998, 5, 300–303. [Google Scholar] [CrossRef]
- Brunner, S.; Schmid, D.; Huttinger, K.; Much, D.; Bruderl, M.; Seldmeier, E.-M.; Kratzsch, J.; Amann-Gassnerl, U.; Bader, B.; Hauner, H. Effect of reducing the n-6/n-3 fatty acid ratio on the maternal and fetal leptin axis in relation to infant body composition. Obesity 2014, 22, 217–224. [Google Scholar] [CrossRef]
- Simon-Muela, I.; Naf, S.; Ballesteros, M.; Vendrell, J.; Ceperuelo-Mallafre, V.; De la Flor, M.; Megia, A. Gender determines the actions of adiponectin multimers on fetal growth and adiposity. Am. J. Obstet. Gynecol. 2013, 208, e481–e487. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Laffineuse, L.; Presley, L.; Minium, J.; Catalano, P.M.; Hauguel-De Mouzon, S. In utero gender dimorphism of adiponectin reflects insulin sensitivity and adiposity of the fetus. Obesity 2009, 17, 1144–1149. [Google Scholar] [CrossRef]
- Inami, I.; Okada, T.; Fujita, H.; Makimoto, M.; Hosono, S.; Minato, M.; Takahashi, S.; Harada, K.; Yamamoto, T. Impact of serum adiponectin concentration on birth size and early postnatal growth. Pediatric Res. 2007, 61, 604. [Google Scholar] [CrossRef]
- Karakosta, P.; Roumeliotaki, T.; Chalkiadaki, G.; Sarri, K.; Vassilaki, M.; Venihaki, M.; Malliaraki, N.; Kampa, M.; Castanas, E.; Kogevinas, M. Cord blood leptin levels in relation to child growth trajectories. Metabolism 2016, 65, 874–882. [Google Scholar] [CrossRef]
- Mantzoros, C.S.; Rifas-Shiman, S.L.; Williams, C.J.; Fargnoli, J.L.; Kelesidis, T.; Gillman, M.W. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: A prospective cohort study. Pediatrics 2009, 123, 682–689. [Google Scholar] [CrossRef]
- Boeke, C.E.; Mantzoros, C.S.; Hughes, M.D.; Rifas-Shiman, S.L.; Villamor, E.; Zera, C.; Gillman, M. Differential associations of leptin with adiposity across early childhood. Obesity 2013, 21, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Persson, B.; Westgren, M.; Celsi, G.; Nord, E.; Ortqvist, E. Leptin concentrations in cord blood in normal newborn infants and offspring of diabetic mothers. Horm. Metab. Res. 1999, 31, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.A.; Solenberger, T.; Philpott, T.; Holcomb, W.L.; Landt, M. Plasma leptin concentrations in newborns of diabetic and nondiabetic mothers. Am. J. Perinatol. 1998, 11, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.R.; Catalano, P.M.; Biggio, J.R.; Gower, B.A.; Chandler-Laney, P.C. Associations of neonatal adiponectin and leptin with growth and body composition in African American infants. Pediatric Obes. 2018, 13, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.M.; Brei, C.; Stecher, L.; Much, D.; Brunner, S.; Hauner, H. Leptin in Maternal Plasma and Cord Blood as a Predictor of Offspring Adiposity at 5 Years: A Follow-up Study. Obesity 2018, 26, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.M.; Brei, C.; Stecher, L.; Much, D.; Brunner, S.; Hauner, H. Cord blood and child plasma adiponectin levels in relation to childhood obesity risk and fat distribution up to 5 y. Pediatric Res. 2017, 81, 745. [Google Scholar] [CrossRef]
- Lindsay, R.S.; Walker, J.D.; Havel, P.J.; Hamilton, B.A.; Calder, A.A.; Johnstone, F.D. Adiponectin is present in cord blood but is unrelated to birth weight. Diabetes Care 2003, 26, 2244–2249. [Google Scholar] [CrossRef]
- Geary, M.; Pringle, P.J.; Persaud, M.; Wilshin, J.; Hindmarsh, P.C.; Rodeck, C.H.; Brook, C.G. Leptin concentrations in maternal serum and cord blood: Relationship to maternal anthropometry and fetal growth. BJOG Int. J. Obstet. Gynaecol. 1999, 106, 1054–1060. [Google Scholar] [CrossRef]
- Linnemann, K.; Malek, A.; Sager, R.; Blum, W.F.; Schneider, H.; Fusch, C. Leptin production and release in the dually in vitro perfused human placenta. J. Clin. Endocrinol. Metab. 2000, 85, 4298–4301. [Google Scholar]
- Schubring, C.; Kiess, W.; Englaro, P.; Rascher, W.; Dotsch, J.; Hanitsch, S.; Attanasio, A.; Blum, W.F. Levels of leptin in maternal serum, amniotic fluid, and arterial and venous cord blood: Relation to neonatal and placental weight. J. Clin. Endocrinol. Metab. 1997, 82, 1480–1483. [Google Scholar] [CrossRef] [PubMed]
- Jaquet, D.; Leger, J.; Levy-Marchal, C.; Oury, J.F.; Czernichow, P. Ontogeny of leptin in human fetuses and newborns: Effect of intrauterine growth retardation on serum leptin concentrations. J. Clin. Endocrinol. Metab. 1998, 83, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Karakosta, P.; Georgiou, V.; Fthenou, E.; Margioris, A.; Castanas, E.; Kogevinas, M.; Kampa, M.; Chatzi, L. Gender-specific reference intervals for cord blood leptin in Crete, Greece. Eur. J. Pediatrics 2012, 171, 1563–1566. [Google Scholar] [CrossRef]
- Mistry, A.M.; Swick, A.; Romsos, D.R. Leptin alters metabolic rates before acquisition of its anorectic effect in developing neonatal mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999, 277, R742–R747. [Google Scholar] [CrossRef]
- Koutcherov, Y.; Mai, J.K.; Paxinos, G. Hypothalamus of the human fetus. J. Chem. Neuroanat. 2003, 26, 253–270. [Google Scholar] [CrossRef]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J. Neurosci. 2004, 24, 2797–2805. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004, 304, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Kajantie, E.; Hytinantti, T.; Hovi, P.; Andersson, S. Cord plasma adiponectin: A 20-fold rise between 24 weeks gestation and term. J. Clin. Endocrinol. Metab. 2004, 89, 4031–4036. [Google Scholar] [CrossRef]
- Weyermann, M.; Beermann, C.; Brenner, H.; Rothenbacher, D. Adiponectin and leptin in maternal serum, cord blood, and breast milk. Clin. Chem. 2006, 52, 2095–2102. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, G.; Wong, W.P.S.; Vliegenthart, J.F.G.; Gerwig, G.J.; Lam, K.S.L.; Cooper, G.J.S.; Xu, A. Proteomic and functional characterization of endogenous adiponectin purified from fetal bovine serum. Proteomics 2004, 4, 3933–3942. [Google Scholar] [CrossRef] [PubMed]
- Asayama, K.; Hayashibe, H.; Dobashi, K.; Uchida, N.; Nakane, T.; Kodera, K.; Shirahata, A.; Taniyama, M. Decrease in serum adiponectin level due to obesity and visceral fat accumulation in children. Obes. Res. 2003, 11, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Viengchareun, S.; Zennaro, M.-C.; Pascual-Le Tallec, L.; Lombes, M. Brown adipocytes are novel sites of expression and regulation of adiponectin and resistin. FEBS Lett. 2002, 532, 345–350. [Google Scholar] [CrossRef]
- Milan, G.; Granzotto, M.; Scarda, A.; Calcagno, A.; Pagano, C.; Federspil, G.; Vettor, R. Resistin and adiponectin expression in visceral fat of obese rats: Effect of weight loss. Obes. Res. 2002, 10, 1095–1103. [Google Scholar] [CrossRef]
- Kamon, J.; Yamauchi, T.; Waki, H.; Uchika, S.; Ito, Y.; Suzuki, R.; Aoyama, M.; Takasawa, T.; Kubota, N.; Terauchi, Y.; et al. Mechanism for the regulation of adiponectin expression. Diabetes 2002, 51, A87. [Google Scholar]
- Simpson, J.; Smith, A.D.; Fraser, A.; Sattar, N.; Lindsay, R.S.; Ring, S.M.; Tilling, K.; Smith, G.D.; Lawlor, D.; Nelson, S.M. Programming of Adiposity in Childhood and Adolescence: Associations with Birth Weight and Cord Blood Adipokines. J. Clin. Endocrinol. Metab. 2017, 102, 499–506. [Google Scholar] [CrossRef]
- National Heart, Lung and Blood Institute Home Page. Available online: http://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 12 February 2021).
| Study | Country (Sample Size) | Year of Data Collection | Follow-Up Duration | Assay Method | Adiposity Method | Limitations | Author’s Conclusion | |
|---|---|---|---|---|---|---|---|---|
| Leptin | Adiponectin | |||||||
| Meyer, 2018 [50] | Germany (n = 89) | NR | 5 years | ELISA | - | Skinfold, MRI | Drop out, maternal BMI | L does not predict adiposity |
| Meyer, 2017 [51] | Germany (n = 90) | NR | 5 years | - | ELISA | Skinfold, MRI | Drop out, maternal BMI | Ad does not predict adiposity |
| Schneider, 2017 [49] | USA (n = 36) | NR | 3 months | RIA | RIA | ADP | Sample size | Ad but not L predicts adiposity |
| Kadakia, 2016 [37] | USA (n = 105) | 2011–2014 | At birth | RIA | - | ADP | Sample size | Early life L resistance |
| Karakosta, 2016 [43] | Greece (n = 578) | NR | 4 years | ELISA | - | Skinfold | NR | L does not predict adiposity |
| Chaoimh, 2016 [36] | Ireland (n = 221) | 2008–2011 | 2 months | ELISA | - | ADP | Sample size, maternal BMI, infant feeding not quantified | L predicts adiposity, longitudinal data required |
| Donnelly, 2015 [29] | Ireland (n = 147) | 2007–2011 | At birth | RIA | - | Skinfold | Selection bias | L predicts adiposity |
| Teague, 2015 [32] | USA (n = 124) | 2010–2013 | 1 month | ELISA | ELISA | Skinfold, DXA | Drop out | L and Ad predict adiposity |
| Josefson, 2014 [33] | USA (n = 61) | NR | At birth | RIA | RIA | ADP | Sample size | L and Ad correlate with FM |
| Brunner, 2014 [39] | Germany (n = 188) | NR | 2 years | ELISA | - | Skinfold | Sample size | L predicts adiposity |
| Boeke, 2013 [45] | USA (n = 508) | 1999–2002 | 7 years | ELISA | - | Skinfold | Circadian L variation | L predicts adiposity |
| Simon-Muela, 2013 [40] | Spain (n = 96) | NR | At birth | ELISA | ELISA | Skinfold | NR | Ad action shows sex dimorphism |
| Basu, 2009 [41] | USA (n = 121) | NR | At birth | - | ELISA | Skinfold | Ad assay | Ad correlates with FM, sex dimorphism |
| Mantzoros, 2009 [44] | USA (n = 588) | 1999–2002 | 3 years | RIA | RIA | Skinfold | Socioeconomic status | Ad but not L predicts adiposity |
| Inami, 2007 [42] | Japan (n = 52) | 2004–2005 | 1 month | - | ELISA | Skinfold | NR | Ad correlates with FM |
| Valuniene, 2007 [35] | Lithuania (n = 367) | 1998–2000 | At birth | RIA | - | Skinfold | NR | L correlates with FM |
| Martinez, 2005 [30] | Mexico (n = 100) | NR | At birth | RIA | RIA | Skinfold | Sample size | L but not Ad correlates with FM |
| Javaid, 2005 [31] | England (n = 117) | NR | At birth | RIA | - | DXA | Sample size | L correlates with bone and FM |
| Tsai, 2004 [34] | Taiwan (n = 226) | 2001–2002 | At birth | RIA | RIA | Skinfold | NR | L and Ad correlate with FM |
| Lindsay, 2003 [52] | Scotland (n = 73) | 1999–2001 | At birth | - | RIA | Skinfold | NR | Ad not associated with skinfold |
| Geary, 1999 [53] | England (n = 39) | 1996–1997 | At birth | RIA | - | Skinfold | NR | L correlates with FM |
| Clapp, 1998 [38] | USA (n = 42) | NR | At birth | RIA | - | Skinfold | NR | L correlates with FM |
| Study (Sample size) | Adiposity Measure Studied | Adjustments | Results | p-Value |
|---|---|---|---|---|
| Schneider, 2017 [49] (n = 36) | FM (g) at 2 weeks and conditional change from 2 weeks to 3 months | 2 weeks: gestational age, age at measurement, FFM 3 months: above + 2 weeks measurement and time between measurements | 2 weeks: r = 0.27 3 months: r = −0.19 | p > 0.05 |
| Chaoimh, 2016 [36] (n = 221) | Conditional change to FMI (kg/m2) from birth to 2 months | Maternal age-education-smoking, maternal BMI at 15 weeks gestation, family income, sex, gestational age, breast feeding | Β-Coef (95% CI): −0.021 (−0.034, −0.007) | p = 0.003 |
| Brunner, 2014 [39] (n = 90) a | FM (g) at 2 years | Maternal BMI, gestational weight gain, pregnancy duration, sex, breast feeding | Β-Coef (95% CI): −14.86 (−29.49, −0.23) | p = 0.04 |
| Boeke, 2013 [45] (n = 508) b | SSF (mm) at 3 years SSF (mm) and DXA derived FM (kg) at 7 years | Maternal age, weight gain, income, education, smoking, sex, ethnicity, breast feeding | 3 years Β-Coef (95% CI0: −1.4 (−2.7, −0.1) 7 years Β-Coef (95% CI): 1.1 (−1.5, 2.1) for SSF 0.3 (−0.7, 1.3) for DXA | p < 0.05 p > 0.05 |
| Mantzoros, 2009 [44] (n = 588) b | SS + TR (mm) and SS/TR (mm) at 3 years of age (regression per 10 ng/mL of leptin) | Maternal education, pre-pregnancy BMI, weight gain, gestational age, paternal BMI, sex, ethnicity, breast feeding | Β-Coef (95% CI): −0.24 (−0.88, 0.41) for SS + TR −0.22 (−2.61, 2.17) for SS/TR | p = 0.48 p = 0.86 |
| Meyer, 2018 [50] (n = 89) a | % FM at 3 and 5 years VAT (cm3) and SAT (cm3) at 5 years | Maternal BMI, gestational weight gain, pregnancy duration, sex, breast feeding | Β-Coef (95% CI) % FM at 3: −0.06 (−0.13, 0.01) % FM at 5: −0.09 (0.17, 0.00) VAT: 0.26 (−1.71, 2.23) SAT: −0.13 (−9.20, 8.94) | p = 0.07 p = 0.04 p = 0.78 p = 0.97 |
| Karakosta, 2016 [43] (n = 578) | SSF (mm) at 4 years of age | Sex, birthweight, maternal age and education, parity, pre-pregnancy BMI, breast feeding duration | Β-Coef (95% CI): 0.2 (−1.4, 1.7) | p > 0.05 |
| Teague, 2015 [32] (n = 52) | % FM at 1month | Diabetic status, sex, age in days | r = 0.19 | p = 0.19 |
| Study (Sample Size) | Adiposity Measure Studied | Adjustments | Results | p-Value |
|---|---|---|---|---|
| Teague, 2015 [32] (n = 52) | % FM at 1 month | Diabetic status, sex, age in days | r = 0.32 | p = 0.02 |
| Schneider, 2017 [49] (n = 36) | FM (g) at 2 weeks and conditional change from 2 weeks to 3 months | 2 weeks: gestational age, age at measurement, FFM 3 months: above + 2 weeks measurement and time between measurements | 2 weeks: r = 0.45 3 months: r = −0.38 | p < 0.001 p < 0.05 |
| Mantzoros, 2009 [44] (n = 588) | SS + TR (mm) and SS/TR (mm) at 3 years of age (regression per 10 μg/mL of adiponectin) | Maternal education, pre-pregnancy BMI, weight gain, gestational age, paternal BMI, sex, ethnicity, breast feeding | Β-Coef (95% CI): 0.42 (−0.11, 0.95) for SS + TR 2.01 (0.09, 3.93) for SS/TR | p = 0.12 p = 0.04 |
| Meyer, 2017 [51] (n = 90) | % FM at 3 and 5 years VAT (cm3) and SAT(cm3) at 5 years | Maternal BMI, gestational weight gain, pregnancy duration, sex, breast feeding | Β-Coef (95% CI) % FM at 3: 0.21 (0.06, 0.35_ % FM at 5: 0.08 (−0.10, 0.27) VAT: 1.57 (−2.20, 5.34) SAT: 7.22 (−10.17, 24.62) | p < 0.05 p = 0.36 p = 0.39 p = 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagias, C.; Sukumar, N.; Weldeselassie, Y.; Oyebode, O.; Saravanan, P. Cord Blood Adipocytokines and Body Composition in Early Childhood: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 1897. https://doi.org/10.3390/ijerph18041897
Bagias C, Sukumar N, Weldeselassie Y, Oyebode O, Saravanan P. Cord Blood Adipocytokines and Body Composition in Early Childhood: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(4):1897. https://doi.org/10.3390/ijerph18041897
Chicago/Turabian StyleBagias, Christos, Nithya Sukumar, Yonas Weldeselassie, Oyinlola Oyebode, and Ponnusamy Saravanan. 2021. "Cord Blood Adipocytokines and Body Composition in Early Childhood: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 4: 1897. https://doi.org/10.3390/ijerph18041897
APA StyleBagias, C., Sukumar, N., Weldeselassie, Y., Oyebode, O., & Saravanan, P. (2021). Cord Blood Adipocytokines and Body Composition in Early Childhood: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 18(4), 1897. https://doi.org/10.3390/ijerph18041897







