Metabolic Syndromes as Important Comorbidities in Patients of Inherited Retinal Degenerations: Experiences from the Nationwide Health Database and a Large Hospital-Based Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Samples
2.1.1. Data Source of the Nationwide Database
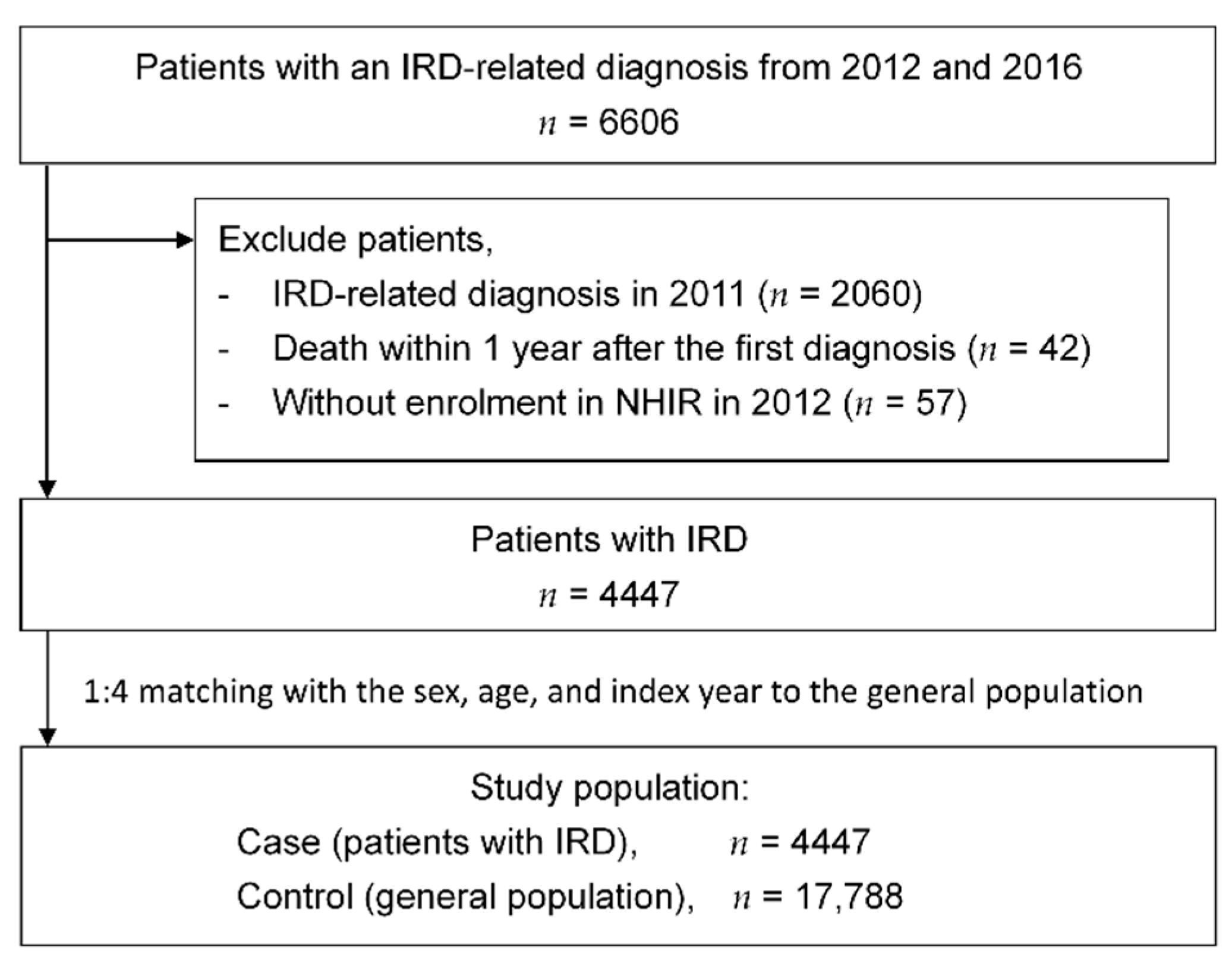
2.1.2. Study Population, Inclusion Criteria, and Exclusion Criteria
2.1.3. Study Period
2.2. Variable Definitions
2.2.1. Urbanization Level of City
2.2.2. Level of Health-Care Utilities
2.2.3. Comorbidities
2.2.4. Medications and Managements of Metabolic Syndrome
2.2.5. Medical Cost
2.2.6. Specialties of Medical Services
2.2.7. Disability Level and Percentage Calculations
2.3. Statistical Analysis
3. Results
3.1. Demographic Data of Study Subjects
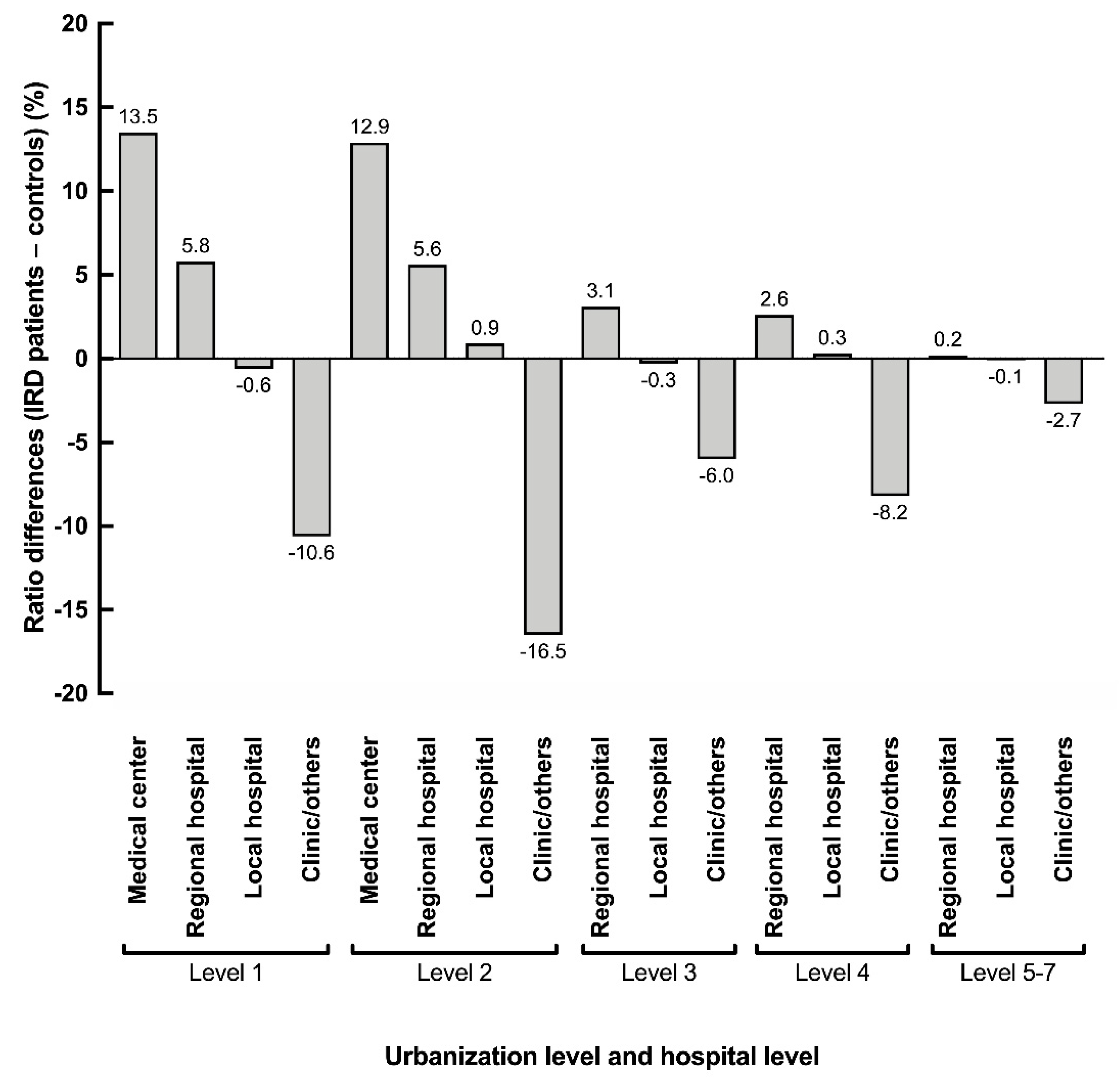
3.2. Healthcare Utilization and Relationship with the Urbanization Level
3.3. Comorbidities Accompanying IRDs
3.4. Medications for Metabolic Syndromes in Patients with IRD
3.5. Mean Official Medical Cost in Patients with IRD
3.6. Increased Medical Needs for Some Specialties in Patients with IRD
3.7. Limitations of Physical Activities and Working Abilities in Patients with IRD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hohman, T.C. Hereditary Retinal Dystrophy. In Pharmacologic Therapy of Ocular Disease; Handbook of Experimental Pharmacology Series; Whitcup, S., Azar, D., Eds.; Springer: Cham, Switzerland, 2016; Volume 242, pp. 337–367. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Q.; Zhao, K.; Wang, L.; Traboulsi, E.I. Update on the molecular genetics of retinitis pigmentosa. Ophthalmic Genet. 2001, 22, 133–154. [Google Scholar] [CrossRef]
- Xu, L.; Hu, L.; Ma, K.; Li, J.; Jonas, J. Prevalence of retinitis pigmentosa in urban and rural adult Chinese: The Beijing Eye Study. Eur. J. Ophthalmol. 2006, 16, 865–866. [Google Scholar] [CrossRef]
- Moore, A.T. Cone and cone-rod dystrophies. J. Med. Genet. 1992, 29, 289–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, D.C.; Traboulsi, E.I. Leber congenital amaurosis: Clinical correlations with genotypes, gene therapy trials update, and future directions. J. AAPOS 2009, 13, 587–592. [Google Scholar] [CrossRef]
- Frick, K.D.; Roebuck, M.C.; Feldstein, J.I.; Mccarty, C.A.; Grover, L.L. Health Services Utilization and Cost of Retinitis Pigmentosa. Arch. Ophthalmol. 2012, 130, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Bertelsen, M.; Linneberg, A.; Rosenberg, T. Socio-economic characteristics of patients with generalized retinal dystrophy in Denmark. Acta Ophthalmol. 2015, 93, 134–140. [Google Scholar] [CrossRef] [PubMed]
- National Health Insurance Administration, Ministry of Health and Welfare, Executive Yuan, Taiwan. How Premiums Are Calculated; National Health Insurance Administration, Ministry of Health and Welfare, Executive Yuan: Taipei, Taiwan, 2020. Available online: https://www.nhi.gov.tw/english/Content_List.aspx?n=B9C9C690524F2543&topn=46FA76EB55BC2CB8 (accessed on 8 October 2020).
- Lu, J.-F.R.; Hsiao, W.C. Does Universal Health Insurance Make Health Care Unaffordable? Lessons from Taiwan. Heal. Aff. 2003, 22, 77–88. [Google Scholar] [CrossRef] [PubMed]
- National Health Insurance Administration, Ministry of Health and Welfare, Executive Yuan, Taiwan. 2017–2018 National Health Insurance Annual Report; National Health Insurance Administration, Ministry of Health and Welfare, Executive Yuan: Taipei, Taiwan, 2017. Available online: https://www.nhi.gov.tw/DL.aspx?sitessn=293&u=LzAwMS9VcGxvYWQvMjkzL3JlbGZpbGUvMC8xNjEwNi8yMDE3LTIwMTjlhajmsJHlgaXlurfkv53pmqrlubTloLHngI%2fopr3mqpQucGRm&n=MjAxNy0yMDE45YWo5rCR5YGl5bq35L%2bd6Zqq5bm05aCx54CP6Ka95qqULnBkZg%3d%3d (accessed on 21 October 2020).
- Liu, C.-Y.; Hung, Y.-T.; Chuang, Y.-L.; Chen, Y.J.; Weng, W.S.; Liu, J.S. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J. Health Manag. 2006, 4, 1–22. [Google Scholar]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Ministry of Labor, Executive Yuan, Taiwan. Labor Insurance Disability Benefit Payment Standards; Council of Labor Affairs of Executive Yuan: Taipei, Taiwan, 2015.
- Tseng, L.H. Explaining the Law of Damages in Detail; San Min Book Co., Ltd.: Taipei, Taiwan, 2008; p. 346. (In Chinese) [Google Scholar]
- Crewe, J.M.; Spilsbury, K.; Morlet, N.; Morgan, W.H.; Mukhtar, A.; Clark, A.; Semmens, J.B. Health Service Use and Mortality of the Elderly Blind. Ophthalmology 2015, 122, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.-P.; Janssen, I.; Heymsfield, S.B. Metabolic syndrome in normal-weight Americans: New definition of the metabolically obese, normal-weight individual. Diabetes Care 2004, 27, 2222–2228. [Google Scholar] [CrossRef] [Green Version]
- Hadaegh, F.; Zabetian, A.; Harati, H.; Azizi, F. Metabolic syndrome in normal-weight Iranian adults. Ann. Saudi Med. 2007, 27, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Suliga, E.; Kozieł, D.; Głuszek, S. Prevalence of metabolic syndrome in normal weight individuals. Ann. Agric. Environ. Med. 2016, 23, 631–635. [Google Scholar] [CrossRef] [Green Version]
- Whitson, H.E.; Malhotra, R.; Chan, A.; Matchar, D.B.; Østbye, T. Comorbid visual and cognitive impairment: Relationship with disability status and self-rated health among older Singaporeans. Asia Pac. J. Public Health 2014, 26, 310–319. [Google Scholar] [CrossRef]
- Patel, N.; Stagg, B.C.; Swenor, B.K.; Zhou, Y.; Talwar, N.; Ehrlich, J.R. Association of Co-occurring Dementia and Self-reported Visual Impairment with Activity Limitations in Older Adults. JAMA Ophthalmol. 2020, 138, 756. [Google Scholar] [CrossRef]
- Moon, J.H.; Choo, S.R.; Kim, J.S. Relationship between low muscle mass and metabolic syndrome in elderly people with normal body mass index. J. Bone Metab. 2015, 22, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Shin, D.W.; An, A.R.; Lee, C.H.; Park, J.H.; Oh, M.K.; Hwang, S.H.; Kim, Y.; Cho, B.; Lee, H.K. Mental Health of People with Retinitis Pigmentosa. Optom. Vis. Sci. 2013, 90, 488–493. [Google Scholar] [CrossRef] [Green Version]
- Chaumet-Riffaud, A.E.; Chaumet-Riffaud, P.; Cariou, A.; Devisme, C.; Audo, I.; Sahel, J.-A.; Mohand-Said, S. Impact of Retinitis Pigmentosa on Quality of Life, Mental Health, and Employment Among Young Adults. Am. J. Ophthalmol. 2017, 177, 169–174. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, X.; Lin, X.; Lin, H. The Prevalence of Depression and Depressive Symptoms among Eye Disease Patients: A Systematic Review and Meta-analysis. Sci. Rep. 2017, 7, 46453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levenstein, S.; Ackerman, S.; Kiecolt-Glaser, J.K.; Dubois, A. Stress and peptic ulcer disease. JAMA 1999, 281, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Ryu, H.; Kang, H.-Y.; Lee, H.; Kwon, J.-W. Clinical and Economic Burden of Visual Impairment in an Aging Society of South Korea. Asia Pac. J. Public Health 2015, 27, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.; Swenor, B.K.; Canner, J.K.; Singh, M.S. Healthcare Cost of Stargardt Disease. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Woung, L.-C.; Tsai, C.-Y.; Chou, H.-K.; Tsai, M.-T.; Tsai, W.-H.; Chou, P.; Shen, S.-T. Healthcare costs associated with progressive diabetic retinopathy among National Health Insurance enrollees in Taiwan, 2000–2004. BMC Heal. Serv. Res. 2010, 10, 136. [Google Scholar] [CrossRef] [Green Version]
- Bainbridge, J.W.; Smith, A.J.; Barker, S.S.; Robbie, S.; Henderson, R.; Balaggan, K.; Viswanathan, A.; Holder, G.E.; Stockman, A.; Tyler, N.; et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N. Engl. J. Med. 2008, 358, 2231–2239. [Google Scholar] [CrossRef]
- Smith, A.J.; Bainbridge, J.W.; Ali, R.R. Prospects for retinal gene replacement therapy. Trends Genet. 2009, 25, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Maguire, A.M.; Russell, S.; Wellman, J.A.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; Marshall, K.A.; et al. Efficacy, safety, and durability of voretigene neparvovec-rzyl in RPE65 mutation–associated inherited retinal dystrophy: Results of phase 1 and 3 trials. Ophthalmology 2019, 126, 1273–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65 -mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]





| IRD (n = 4447) | Control (n = 17,788) | p Value | |
|---|---|---|---|
| Age on the index date | |||
| Mean (SD) | 51.8 (18.1) | 51.7 (18.2) | - |
| Median (Q1, Q3) | 54 (40, 64) | 54 (40, 64) | |
| Age | |||
| 0–19, n (%) | 241 (5.42) | 968 (5.44) | - |
| 20–64, n (%) | 3100 (69.71) | 12454 (70.01) | |
| 65+, n (%) | 1106 (24.87) | 4366 (24.54) | |
| Sex | |||
| Male, n (%) | 2079 (46.75) | 8316 (46.75) | - |
| Female, n (%) | 2368 (53.25) | 9472 (53.25) | |
| Urbanization level of the registered city | |||
| Level 1, n (%) | 1432 (32.20) | 5388 (30.29) | 0.1323 |
| Level 2, n (%) | 1363 (30.65) | 5637 (31.69) | |
| Level 3, n (%) | 727 (16.35) | 2986 (16.79) | |
| Level 4, n (%) | 588 (13.22) | 2281 (12.82) | |
| Level 5, n (%) | 69 (1.55) | 314 (1.77) | |
| Level 6, n (%) | 130 (2.92) | 601 (3.38) | |
| Level 7, n (%) | 138 (3.10) | 581 (3.27) | |
| NHI monthly income bracket | |||
| <666.7 USD, n (%) | 1244 (27.97) | 4780 (26.87) | 0.1426 |
| 666.7–1000 USD, n (%) | 1619 (36.41) | 6809 (38.28) | |
| 1000–1333.3 USD, n (%) | 525 (11.81) | 2147 (12.07) | |
| 1333.3–1666.7 USD, n (%) | 479 (10.77) | 1821 (10.24) | |
| 1666.7+ USD, n (%) | 580 (13.04) | 2231 (12.54) | |
| Charlson comorbidity index | |||
| Mean (SD) | 0.74 (1.41) | 0.52 (1.21) | <0.001 |
| Median (Q1, Q3) | 0 (0, 1) | 0 (0, 1) | |
| Ophthalmology visit times from the index date to 1 year later | |||
| Mean (SD) | 6.80 (5.40) | 1.06 (2.69) | <0.001 |
| Median (Q1, Q3) | 5 (3, 9) | 0 (0, 1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiou, G.-J.; Huang, D.-S.; Hu, F.-R.; Yang, C.-M.; Yang, C.-H.; Huang, C.-W.; Lin, J.-W.; Lin, C.-W.; Ho, T.-C.; Hsieh, Y.-T.; et al. Metabolic Syndromes as Important Comorbidities in Patients of Inherited Retinal Degenerations: Experiences from the Nationwide Health Database and a Large Hospital-Based Cohort. Int. J. Environ. Res. Public Health 2021, 18, 2065. https://doi.org/10.3390/ijerph18042065
Chiou G-J, Huang D-S, Hu F-R, Yang C-M, Yang C-H, Huang C-W, Lin J-W, Lin C-W, Ho T-C, Hsieh Y-T, et al. Metabolic Syndromes as Important Comorbidities in Patients of Inherited Retinal Degenerations: Experiences from the Nationwide Health Database and a Large Hospital-Based Cohort. International Journal of Environmental Research and Public Health. 2021; 18(4):2065. https://doi.org/10.3390/ijerph18042065
Chicago/Turabian StyleChiou, Guann-Jye, Ding-Siang Huang, Fung-Rong Hu, Chung-May Yang, Chang-Hao Yang, Ching-Wen Huang, Jou-Wei Lin, Chao-Wen Lin, Tzyy-Chang Ho, Yi-Ting Hsieh, and et al. 2021. "Metabolic Syndromes as Important Comorbidities in Patients of Inherited Retinal Degenerations: Experiences from the Nationwide Health Database and a Large Hospital-Based Cohort" International Journal of Environmental Research and Public Health 18, no. 4: 2065. https://doi.org/10.3390/ijerph18042065







