Physiological Resonance in Empathic Stress: Insights from Nonlinear Dynamics of Heart Rate Variability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
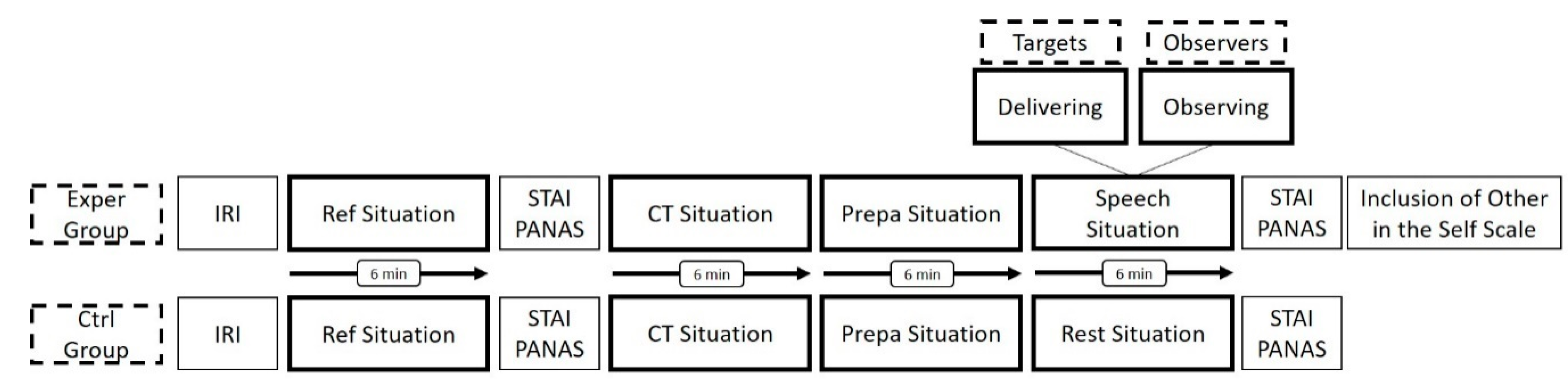
2.2. Protocol
2.3. Psychological Characteristics
2.4. Physiological Characteristics
2.4.1. Time-Domain and Frequency-Domain Cardiac Autonomic Markers
2.4.2. Complexity Marker of Heart Rate Variability Time Series
- (1)
- The RR time series is coarse grained considering overlapping windows to represent the initial time series on several time scales . Overlapping windows lead to coarse-grained series at each scale factor of .
- (2)
- At each scale factor of , the matched vector pairs, and are counted for all coarse-grained series, with ( in the present study) which corresponds to the sequence length chosen. This operation refers to the probability that vectors (sequences) of samples that are similar, remain similar with the increase of the sequence length to .
- (3)
- At a scale factor of , RCMSE is computed as follows, with , the tolerance value allowing to consider that vectors are matched. In the present work, of the standard deviation of the original time series:
2.5. Statistical Analyses
3. Results
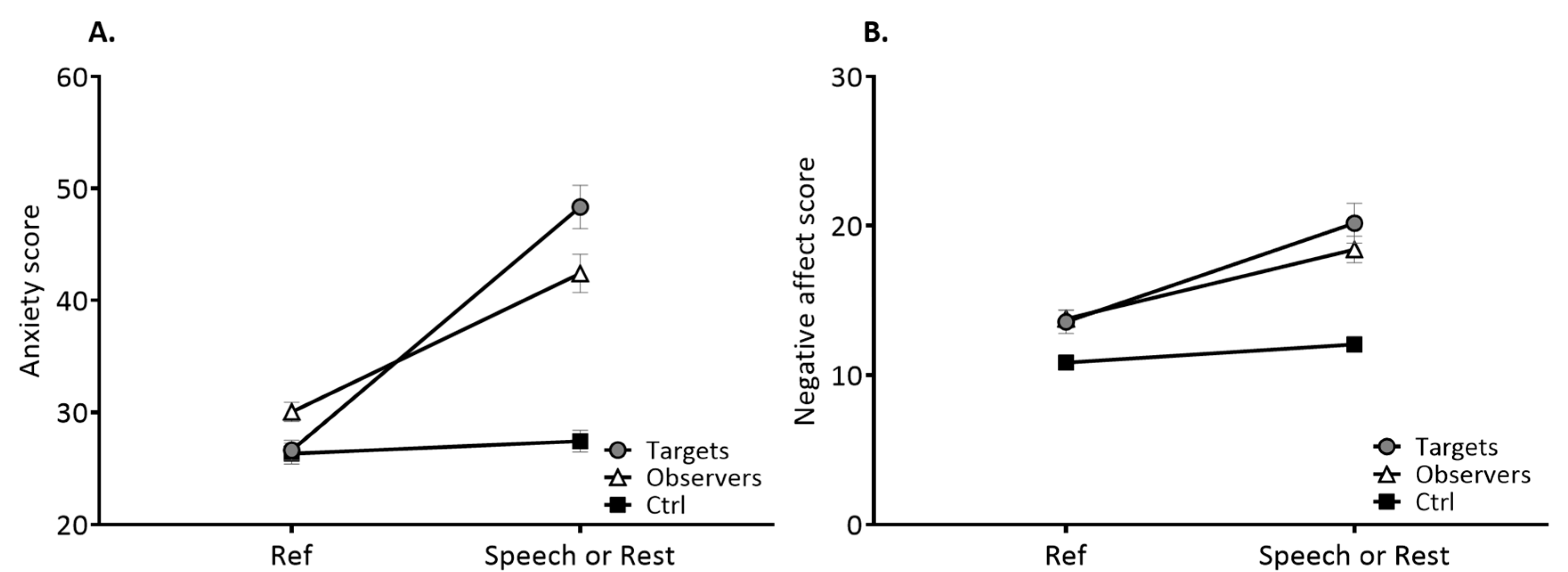
3.1. Psychological Characteristics
3.2. Physiological Characteristics
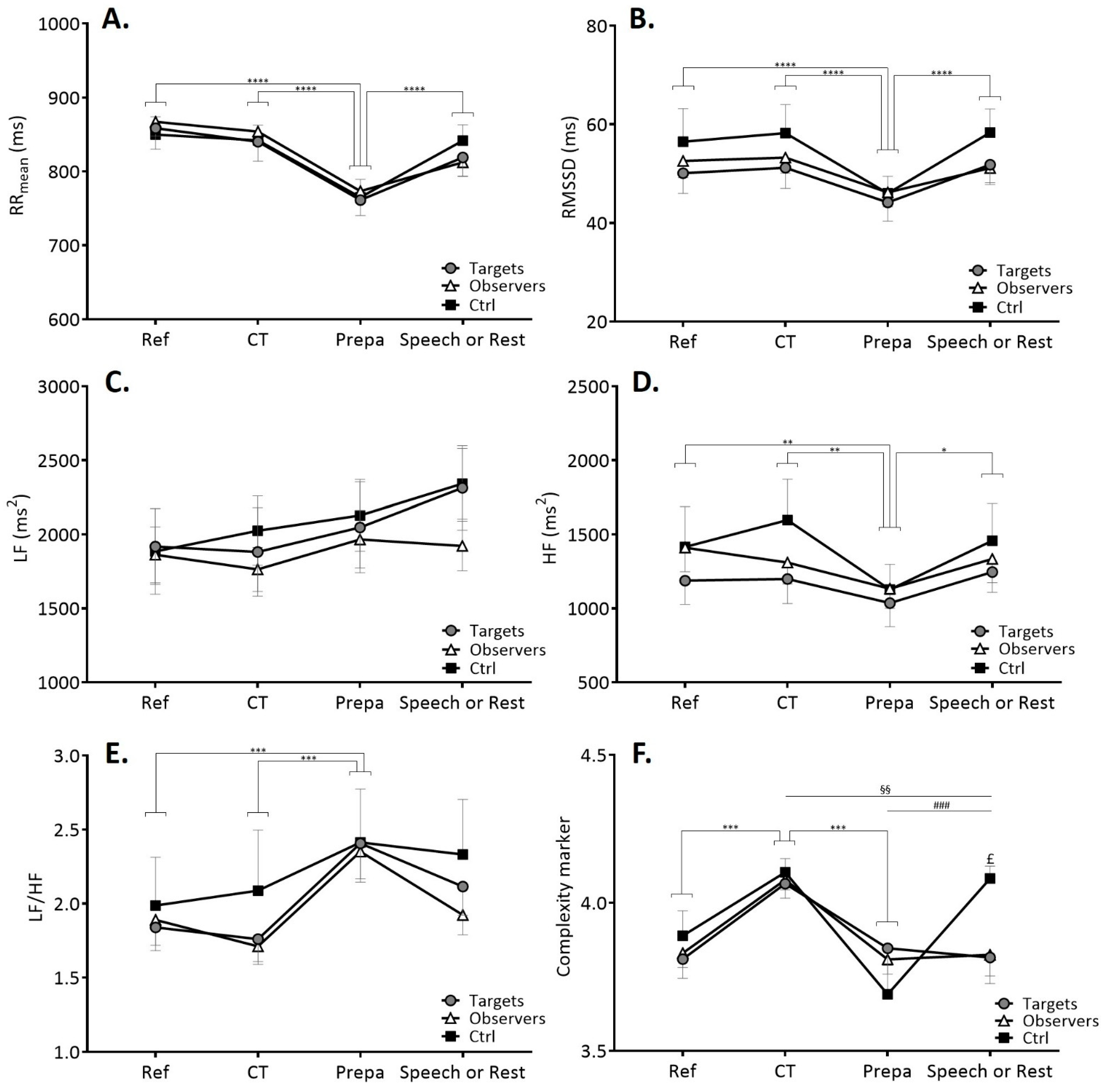
3.2.1. Time-Domain and Frequency-Domain Cardiac Autonomic Markers
3.2.2. Complexity in Heart Rate Variability Time Series
3.3. Psychophysiological Keys in Observers-Targets Linkage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Engert, V.; Linz, R.; Grant, J.A. Embodied stress: The physiological resonance of psychosocial stress. Psychoneuroendocrinology 2019, 105, 138–146. [Google Scholar] [CrossRef]
- White, C.N.; Buchanan, T.W. Empathy for the Stressed. Adapt. Hum. Behav. Physiol. 2016, 2, 311–324. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, T.W.; Bagley, S.L.; Stansfield, R.B.; Preston, S.D. The empathic, physiological resonance of stress. Soc. Neurosci. 2012, 7, 191–201. [Google Scholar] [CrossRef]
- Ebisch, S.J.; Aureli, T.; Bafunno, D.; Cardone, D.; Romani, G.L.; Merla, A. Mother and child in synchrony: Thermal facial imprints of autonomic contagion. Biol. Psychol. 2012, 89, 123–129. [Google Scholar] [CrossRef]
- Zaki, J.; Ochsner, K.N. The neuroscience of empathy: Progress, pitfalls and promise. Nat. Neurosci. 2012, 15, 675–680. [Google Scholar] [CrossRef]
- Hein, G.; Singer, T. I feel how you feel but not always: The empathic brain and its modulation. Curr. Opin. Neurobiol. 2008, 18, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Frith, U.; Frith, C.D. Development and neurophysiology of mentalizing. Philos. Trans. R. Soc. B Biol. Sci. 2003, 358, 459–473. [Google Scholar] [CrossRef] [Green Version]
- Frith, C.D.; Frith, U. The Neural Basis of Mentalizing. Neuron 2006, 50, 531–534. [Google Scholar] [CrossRef] [Green Version]
- Singer, T.; Lamm, C. The Social Neuroscience of Empathy. Ann. New York Acad. Sci. 2009, 1156, 81–96. [Google Scholar] [CrossRef]
- Decety, J.; Lamm, C. Human Empathy Through the Lens of Social Neuroscience. Sci. World J. 2006, 6, 1146–1163. [Google Scholar] [CrossRef] [Green Version]
- De Vignemont, F.; Singer, T. The empathic brain: How, when and why? Trends Cogn. Sci. 2006, 10, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Singer, T.; Seymour, B.; O’Doherty, J.P.; Stephan, K.E.; Dolan, R.J.; Frith, C.D. Empathic neural responses are modulated by the perceived fairness of others. Nature 2006, 439, 466–469. [Google Scholar] [CrossRef]
- Lamm, C.; Decety, J.; Singer, T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage 2011, 54, 2492–2502. [Google Scholar] [CrossRef]
- Jackson, P.L.; Meltzoff, A.N.; Decety, J. How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage 2005, 24, 771–779. [Google Scholar] [CrossRef] [Green Version]
- Wicker, B.; Keysers, C.; Plailly, J.; Royet, J.-P.; Gallese, V.; Rizzolatti, G. Both of Us Disgusted in My Insula. Neuron 2003, 40, 655–664. [Google Scholar] [CrossRef] [Green Version]
- Jabbi, M.; Swart, M.; Keysers, C. Empathy for positive and negative emotions in the gustatory cortex. NeuroImage 2007, 34, 1744–1753. [Google Scholar] [CrossRef]
- Manini, B.; Cardone, D.; Ebisch, S.J.H.; Bafunno, D.; Aureli, T.; Merla, A. Mom feels what her child feels: Thermal signatures of vicarious autonomic response while watching children in a stressful situation. Front. Hum. Neurosci. 2013, 7, 299. [Google Scholar] [CrossRef] [Green Version]
- Engert, V.; Plessow, F.; Miller, R.; Kirschbaum, C.; Singer, T. Cortisol increase in empathic stress is modulated by emotional closeness and observation modality. Psychoneuroendocrinology 2014, 45, 192–201. [Google Scholar] [CrossRef]
- Engert, V.; Ragsdale, A.M.; Singer, T. Cortisol stress resonance in the laboratory is associated with inter-couple diurnal cortisol covariation in daily life. Horm. Behav. 2018, 98, 183–190. [Google Scholar] [CrossRef]
- Waters, S.F.; West, T.V.; Mendes, W.B. Stress Contagion. Psychol. Sci. 2014, 25, 934–942. [Google Scholar] [CrossRef] [Green Version]
- Waters, S.F.; West, T.V.; Karnilowicz, H.R.; Mendes, W.B. Affect contagion between mothers and infants: Examining valence and touch. J. Exp. Psychol. Gen. 2017, 146, 1043–1051. [Google Scholar] [CrossRef]
- Dimitroff, S.J.; Kardan, O.; Necka, E.A.; Decety, J.; Berman, M.G.; Norman, G.J. Physiological dynamics of stress contagion. Sci. Rep. 2017, 7, 6168. [Google Scholar] [CrossRef] [Green Version]
- Benarroch, E.E. The Central Autonomic Network: Functional Organization, Dysfunction, and Perspective. Mayo Clin. Proc. 1993, 68, 988–1001. [Google Scholar] [CrossRef]
- Thayer, J.F.; Lane, R.D. A Model of Neurovisceral Integration in Emotion Regulation and Dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart–brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef]
- Lane, R.D.; McRae, K.; Reiman, E.M.; Chen, K.; Ahern, G.L.; Thayer, J.F. Neural correlates of heart rate variability during emotion. NeuroImage 2009, 44, 213–222. [Google Scholar] [CrossRef]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers, J.J.; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef]
- Park, G.; Thayer, J.F. From the heart to the mind: Cardiac vagal tone modulates top-down and bottom-up visual perception and attention to emotional stimuli. Front. Psychol. 2014, 5, 278. [Google Scholar] [CrossRef] [Green Version]
- Lehrer, P.M.; Vaschillo, B.; Zucker, T.; Graves, J.; Katsamanis, M.; Aviles, M.; Wamboldt, F.S. Protocol for Heart Rate Variability Biofeedback Training. Biofeedback 2013, 41, 98–109. [Google Scholar] [CrossRef] [Green Version]
- Lehrer, P.M.; Gevirtz, R. Heart rate variability biofeedback: How and why does it work? Front. Psychol. 2014, 5, 756. [Google Scholar] [CrossRef] [Green Version]
- Deschodt-Arsac, V.; Blons, E.; Gilfriche, P.; Spiluttini, B.; Arsac, L.M. Entropy in Heart Rate Dynamics Reflects How HRV-Biofeedback Training Improves Neurovisceral Complexity during Stress-Cognition Interactions. Entropy 2020, 22, 317. [Google Scholar] [CrossRef] [Green Version]
- Mather, M.; Thayer, J.F. How heart rate variability affects emotion regulation brain networks. Curr. Opin. Behav. Sci. 2018, 19, 98–104. [Google Scholar] [CrossRef]
- Beauchaine, T.P.; Thayer, J.F. Heart rate variability as a transdiagnostic biomarker of psychopathology. Int. J. Psychophysiol. 2015, 98, 338–350. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale entropy analysis of biological signals. Phys. Rev. E 2005, 71, 021906. [Google Scholar] [CrossRef] [Green Version]
- Wayne, P.M.; Manor, B.; Novak, V.; Costa, M.D.; Hausdorff, J.M.; Goldberger, A.L.; Ahn, A.C.; Yeh, G.Y.; Peng, C.-K.; Lough, M.; et al. A systems biology approach to studying Tai Chi, physiological complexity and healthy aging: Design and rationale of a pragmatic randomized controlled trial. Contemp. Clin. Trials 2013, 34, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Goldberger, A.L.; Giles, F. Filley Lecture. Complex Systems. Proc. Am. Thorac. Soc. 2006, 3, 467–471. [Google Scholar] [CrossRef] [Green Version]
- Lipsitz, L.A. Dynamics of Stability: The Physiologic Basis of Functional Health and Frailty. Journals Gerontol. Ser. A Boil. Sci. Med Sci. 2002, 57, B115–B125. [Google Scholar] [CrossRef] [Green Version]
- Sleimen-Malkoun, R.; Temprado, J.-J.; Hong, S.L. Aging induced loss of complexity and dedifferentiation: Consequences for coordination dynamics within and between brain, muscular and behavioral levels. Front. Aging Neurosci. 2014, 6, 140. [Google Scholar] [CrossRef] [Green Version]
- Dimitriev, D.A.; Saperova, E.V.; Dimitriev, A.D. State Anxiety and Nonlinear Dynamics of Heart Rate Variability in Students. PLoS ONE 2016, 11, e0146131. [Google Scholar] [CrossRef] [Green Version]
- Young, H.; Benton, D. We should be using nonlinear indices when relating heart-rate dynamics to cognition and mood. Sci. Rep. 2015, 5, 16619. [Google Scholar] [CrossRef] [Green Version]
- Goldberger, A. Non-linear dynamics for clinicians: Chaos theory, fractals, and complexity at the bedside. Lancet 1996, 347, 1312–1314. [Google Scholar] [CrossRef]
- Blons, E.; Arsac, L.M.; Gilfriche, P.; McLeod, H.; Lespinet-Najib, V.; Grivel, E.; Deschodt-Arsac, V. Alterations in heart-brain interactions under mild stress during a cognitive task are reflected in entropy of heart rate dynamics. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Blons, E.; Arsac, L.M.; Gilfriche, P.; Deschodt-Arsac, V. Multiscale Entropy of Cardiac and Postural Control Reflects a Flexible Adaptation to a Cognitive Task. Entropy 2019, 21, 1024. [Google Scholar] [CrossRef] [Green Version]
- Schoofs, D.; Preuss, D.; Wolf, O.T. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology 2008, 33, 643–653. [Google Scholar] [CrossRef]
- Kirschbaum, C.; Pirke, K.-M.; Hellhammer, D.H. The ‘Trier Social Stress Test’—A Tool for Investigating Psychobiological Stress Responses in a Laboratory Setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar] [CrossRef]
- Spielberger, C.D. Manual for the State-Trail Anxiety Inventory (Form Y); Consulting Psychologist Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Davis, M.H. Measuring individual differences in empathy: Evidence for a multidimensional approach. J. Pers. Soc. Psychol. 1983, 44, 113–126. [Google Scholar] [CrossRef]
- Aron, A.; Aron, E.N.; Smollan, D. Inclusion of Other in the Self Scale and the structure of interpersonal closeness. J. Pers. Soc. Psychol. 1992, 63, 596–612. [Google Scholar] [CrossRef]
- The European Society of Cardiology; The North American Society of Pacing and Electrophysiology. Heart Rate Variability. Standards of Measurement, Physiological Interpretation and Clinical Use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar]
- Wu, S.-D.; Wu, C.-W.; Lin, S.-G.; Lee, K.-Y.; Peng, C.-K. Analysis of complex time series using refined composite multiscale entropy. Phys. Lett. A 2014, 378, 1369–1374. [Google Scholar] [CrossRef]
- Gow, B.J.; Peng, C.-K.; Wayne, P.M.; Ahn, A.C. Multiscale Entropy Analysis of Center-of-Pressure Dynamics in Human Postural Control: Methodological Considerations. Entropy 2015, 17, 7926–7947. [Google Scholar] [CrossRef]
- Batson, C.D. How social an animal? The human capacity for caring. Am. Psychol. 1990, 45, 336–346. [Google Scholar] [CrossRef]
- Hatfield, E.; Cacioppo, J.T.; Rapson, R.L. Emotional Contagion. Curr. Dir. Psychol. Sci. 1993, 2, 96–100. [Google Scholar] [CrossRef]
- Preston, S.D.; de Waal, F.B.M. Empathy: Its Ultimate and Proximate Bases. Behav. Brain Sci. 2002, 25, 1–71. [Google Scholar] [CrossRef] [Green Version]
- Butler, E.A. Temporal Interpersonal Emotion Systems. Pers. Soc. Psychol. Rev. 2011, 15, 367–393. [Google Scholar] [CrossRef]
- Gallese, V. The Roots of Empathy: The Shared Manifold Hypothesis and the Neural Basis of Intersubjectivity. Psychopathol. 2003, 36, 171–180. [Google Scholar] [CrossRef]
- Gallese, V.; Keysers, C.; Rizzolatti, G. A unifying view of the basis of social cognition. Trends Cogn. Sci. 2004, 8, 396–403. [Google Scholar] [CrossRef]
- Wei, L.; Chen, H.; Wu, G.-R. Structural Covariance of the Prefrontal-Amygdala Pathways Associated with Heart Rate Variability. Front. Hum. Neurosci. 2018, 12. [Google Scholar] [CrossRef] [Green Version]
- Sakaki, M.; Yoo, H.J.; Nga, L.; Lee, T.-H.; Thayer, J.F.; Mather, M. Heart rate variability is associated with amygdala functional connectivity with MPFC across younger and older adults. NeuroImage 2016, 139, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Iyengar, N.; Peng, C.K.; Morin, R.; Goldberger, A.L.; Lipsitz, L.A. Age-related alterations in the fractal scaling of cardiac interbeat interval dynamics. Am. J. Physiol. Integr. Comp. Physiol. 1996, 271, R1078–R1084. [Google Scholar] [CrossRef] [Green Version]
- Vergotte, G.; Perrey, S.; Muthuraman, M.; Janaqi, S.; Torre, K. Concurrent Changes of Brain Functional Connectivity and Motor Variability When Adapting to Task Constraints. Front. Physiol. 2018, 9, 909. [Google Scholar] [CrossRef]
| Trait Empathy (Interpersonal Reactivity Index) | |
|---|---|
| Empathic concern | 20.5 ± 4.2 |
| Personal distress | 11.7 ± 5.4 |
| Perspective taking | 16.1 ± 4.5 |
| Fantasy | 15.1 ± 5.8 |
| Relationship closeness (inclusion of other in the self scale) | 2.6 ± 1.8 |
| β | p Value | |
|---|---|---|
| ΔCM targets between Prepa and speech situations | 0.42 | 0.0089 |
| ΔCM observers between CT and Prepa situations | −0.85 | 0.0003 |
| Relationship closeness of observers with targets | −3.96 | 0.0003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blons, E.; Arsac, L.M.; Grivel, E.; Lespinet-Najib, V.; Deschodt-Arsac, V. Physiological Resonance in Empathic Stress: Insights from Nonlinear Dynamics of Heart Rate Variability. Int. J. Environ. Res. Public Health 2021, 18, 2081. https://doi.org/10.3390/ijerph18042081
Blons E, Arsac LM, Grivel E, Lespinet-Najib V, Deschodt-Arsac V. Physiological Resonance in Empathic Stress: Insights from Nonlinear Dynamics of Heart Rate Variability. International Journal of Environmental Research and Public Health. 2021; 18(4):2081. https://doi.org/10.3390/ijerph18042081
Chicago/Turabian StyleBlons, Estelle, Laurent M. Arsac, Eric Grivel, Veronique Lespinet-Najib, and Veronique Deschodt-Arsac. 2021. "Physiological Resonance in Empathic Stress: Insights from Nonlinear Dynamics of Heart Rate Variability" International Journal of Environmental Research and Public Health 18, no. 4: 2081. https://doi.org/10.3390/ijerph18042081






