Coastline Levels of Dissolved Heavy Metals in the Estuarine Water–System of Vigo
Abstract
1. Introduction
2. Materials and Methods
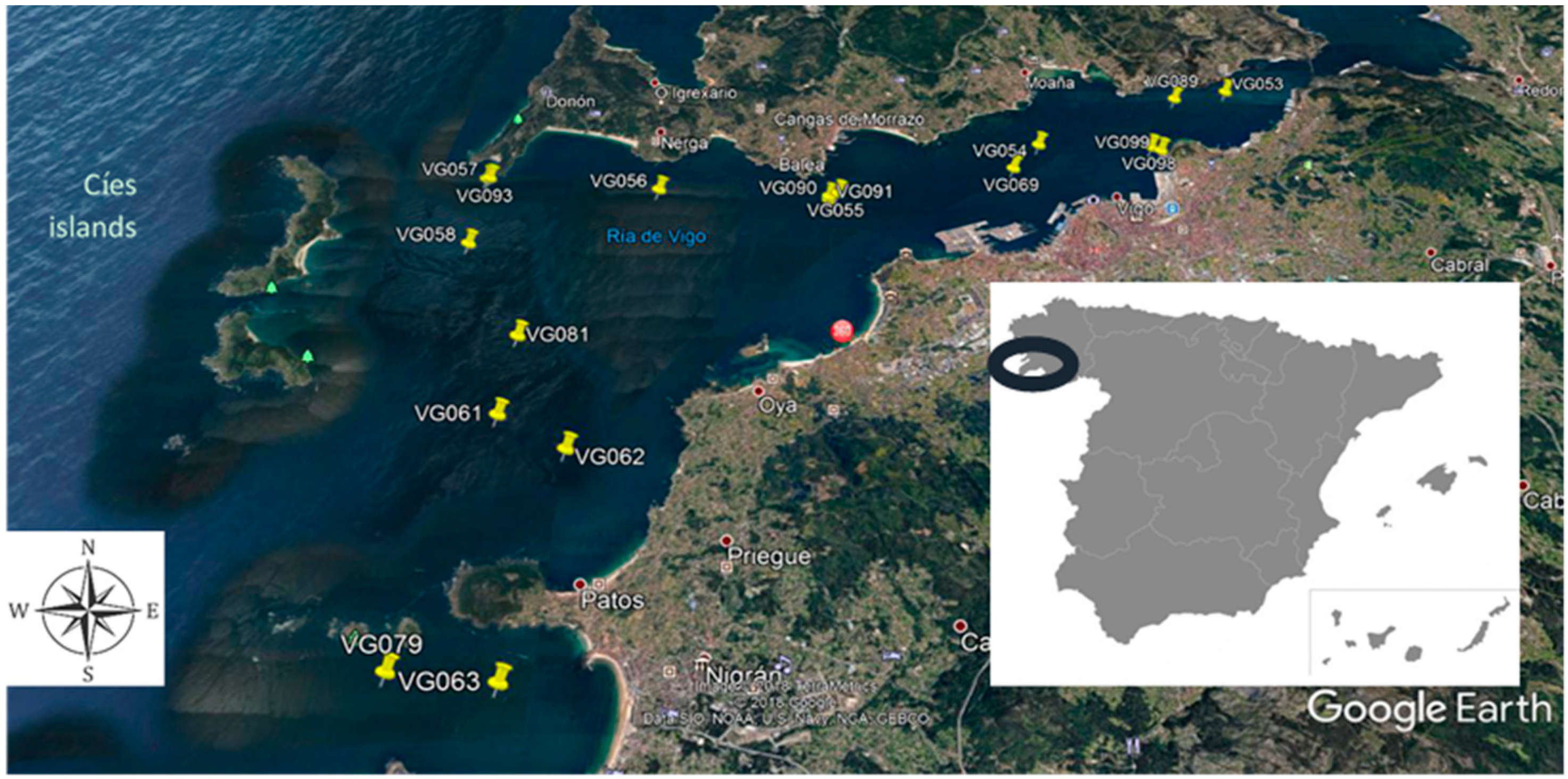
2.1. Water Sampling and Monitoring Campaign
2.2. Metal and Metalloids Detection and Determination
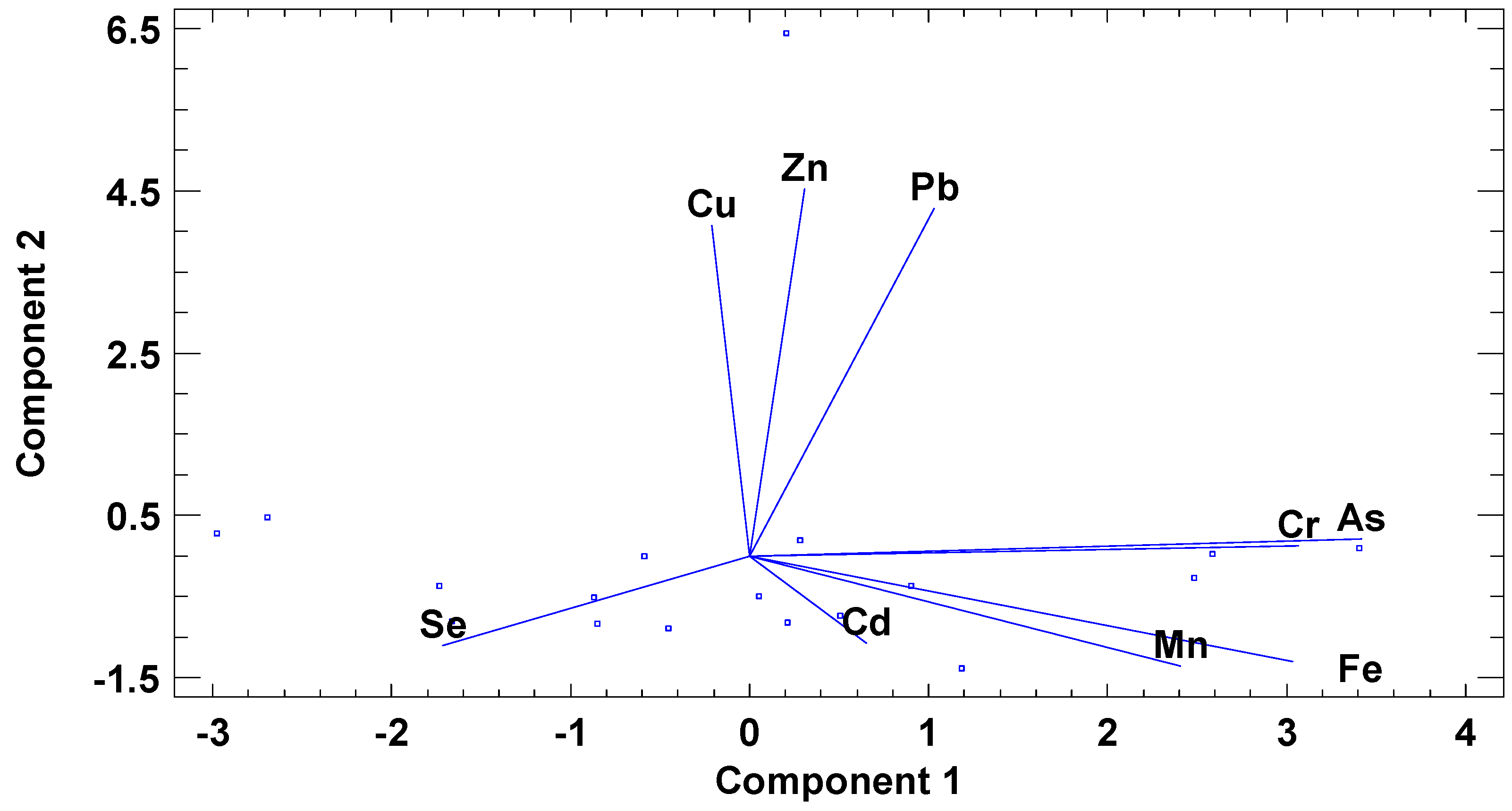
2.3. Statistical Analysis
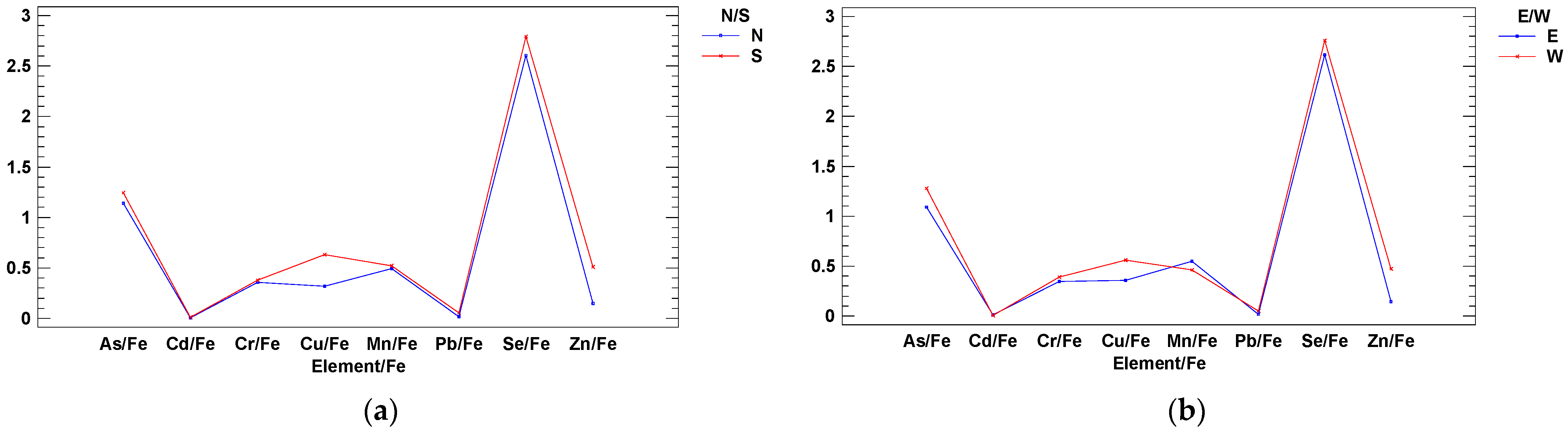
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Springer: Berlin, Germany, 2012; Volume 3. [Google Scholar]
- Martino, M.; Turner, A.; Nimmo, M.; Millward, G.E. Resuspension, reactivity and recycling of trace metals in the Mersey Estuary, UK. Mar. Chem. 2002, 77, 171–186. [Google Scholar] [CrossRef]
- Förstner, U.; Wittmann, G.T. Metal Pollution in the Aquatic Environment; Springer: London, UK, 1983; 486p. [Google Scholar]
- Ramírez-Pérez, A.M.; de Blas, E.; García-Gil, S. Sulfur, iron, and manganese speciation in anoxic sediments with methane (Ría de Vigo, NW Spain). Clean 2017, 45, 1600700. [Google Scholar] [CrossRef]
- Alkan, N.; Tüfekçi, M. Distributions of dissolved forms of manganese and iron in the water column of the Southeastern Black Sea. Turk. J. Fish Aquat. Sci. 2009, 9, 159–164. [Google Scholar] [CrossRef]
- Souto, X.M.; Méndez, G.; Collazo, C.; Ferrero, F.; López, E. Atlas Básico de Vigo e a Súa Área; Instituto de Estudios Vigueses: Vigo, Spain; Edicións Xerais de Galicia: Vigo, Spain, 1999; 59p. [Google Scholar]
- Prego, R.; Cobelo-García, A.; Santos-Echeandía, J.; de Castro, M.; Ospina-Álvarez, N.; García-Pérez, M. Estuary-ria exchange of cadmium, lead and zinc in the coastal system of the Ría of Vigo (NW Iberian Peninsula). Sci. Mar. 2010, 74, 77–87. [Google Scholar] [CrossRef]
- Besada, V.; Quelle, C.; Andrade, J.M.; Gutiérrez, N.; Gómez-Carracedo, M.P.; Schultze, F. A 10-year survey of trace metals in sediments using self-organizing maps. J. Chem. 2014, 28, 558–566. [Google Scholar] [CrossRef]
- Museo do Mar de Galicia. Xunta de Galicia. Available online: http://museodomar.xunta.gal/en/visita/277/the-bay-of-vigo (accessed on 27 January 2021).
- Ríos, A.F.; Pérez, F.F.; Fraga, F. Water masses in the upper and middle North Atlantic Ocean east of the Azores. Deep Sea Res. Part A 1992, 39, 645–658. [Google Scholar] [CrossRef]
- Fernández, E.; Alvarez-Salgado, X.A.; Beiras, R.; Ovejero, A.; Méndez, G. Coexistence of urban uses and shellfish production in an upwelling-driven, highly productive marine environment: The case of the Ría de Vigo (Galicia, Spain). Reg. Stud. Mar. Sci. 2016, 8, 362–370. [Google Scholar] [CrossRef]
- Birch, G.F.; Lee, J.H.; Tanner, E.; Fortune, J.; Munksgaard, N.; Whitehead, J.; Coughanowr, C.; Agius, J.; Chrsipijn, J.; Taylor, U.; et al. Sediment metal enrichment and ecological risk assessment of ten ports and estuaries in the World Harbors Project. Mar. Pollut. Bull. 2020, 155, 111129. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Iglesias, P.; Quintana, B.; Rubi, B.; Pérez-Arlucea, M. Sedimentation rates and trace metal input history in intertidal sediments from San Simón Bay (Ría de Vigo, NW Spain) derived from 210Pb and 137Cs chronology. J. Environ. Radioact. 2007, 98, 229–250. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.; Howarth, R.J.; Nombela, M.A. Metals in the sediments of Ensenada de San Simón (inner Ría de Vigo), Galicia, NW Spain. Appl. Geochem. 2003, 18, 973–996. [Google Scholar] [CrossRef]
- García, A.; Bernárdez, P.; Prego, R. Copper in Galician ría sediments: Natural levels and harbor contamination. Sci. Mar. 2013, 77, 91–99. [Google Scholar]
- Rubio, B.; Mombela, M.A.; Vilas, F. Heavy metal pollution in the Galician Rías Baixas: New background values for Ría de Vigo (NW Spain). J. Iber. Geol. 2000, 26, 121–149. [Google Scholar]
- Pérez-López, M.; Alonso, J.; Nóvoa-Valiñas, M.C.; Melgar, M.J. Assessment of heavy metal contamination of seawater and marine Limpet, Patella vulgata L., from Northwest Spain. J. Environ. Sci. Health Part A Tox. Hazard. Subst. Environ. Eng. 2003, 38, 2845–2856. [Google Scholar] [CrossRef]
- Santos-Echeandía, J.; Laglera, L.M.; Prego, R.; van den Berg, C.M.G. Dissolved copper speciation behaviour during estuarine mixing in the San Simon Inlet (wet season, Galicia). Influence of particulate matter. Estuar. Coast. Shelf Sci. 2008, 76, 447–453. [Google Scholar] [CrossRef]
- Santos-Echeandía, J.; Prego, R.; Cobelo-García, A. Intra-annual variation and baseline concentrations of dissolved trace metals in the Vigo Ría and adjacent coastal waters (NE Atlantic Coast). Mar. Pollut. Bull. 2009, 58, 299–304. [Google Scholar] [CrossRef]
- Erdoğrul, Ö.; Erbilir, F. Heavy metal and trace elements in various fish samples from Sir Dam Lake, Kahramanmaraş, Turkey. Environ. Monit. Assess. 2007, 130, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Kumar-Singh, R.; Chavan, S.L.; Sapkale, P.H. Heavy metal concentrations in water, sediments and body tissues of red worm (Tubifex sp.) collected from natural habitats in Mumbai, India. Environ. Monit. Assess. 2007, 129, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Reisenhofer, E.; Adami, G.; Favretto, A. Heavy metals and nutrients in coastal, surface seawaters (Gulf of Trieste, Northern Adriatic Sea): An environmental study by factor analysis. Anal. Bioanal. Chem. 1996, 354, 729–734. [Google Scholar] [CrossRef]
- Torres-Palenzuela, J.M.; González-Vilas, L.; Bellas-Alaez, F.M.; Pazos, Y. Potential application of the new sentinel satellites for monitoring of harmful algal blooms in the Galician aquaculture. Thalass. Int. J Mar. Sci. 2020, 36, 85–93. [Google Scholar] [CrossRef]
- Nebot, C.; González, L.; Pazos, Y.; Miranda, J.M.; Bellas, F.M.; Cepeda, A.; Franco, C.; Spyrakos, E.; Torres, J. Estudio de Presencia de Fármacos de Aguas en la Ria de Vigo en Proyecto de Indicadores de HABs con Imágenes de Sentinel; Red BioAuga: Galicia, Spain, 2019; Chapter 2; pp. 21–31. ISBN 978-84-8158-816-3. [Google Scholar]
- Rodríguez, J.; Pérez, B.; Nebot, C.; Falqué, E.; Simal-Gandara, J. Food production link to underground waters quality in A Limia river basin. Agric. Ecosyst. Environ. 2020, 297, 106969. [Google Scholar] [CrossRef]
- Ho, H.H.; Swennen, R.; Cappuyns, V.; Vassilieva, E.; Tran, T.V. Necessity of normalization to aluminum to assess the contamination by heavy metals and arsenic in sediments near Haiphong Harbor, Vietnam. J. Asian Earth Sci. 2012, 56, 229–239. [Google Scholar] [CrossRef]
- Ytreberg, E.; Karlsson, J.; Eklund, B. Comparison of toxicity and release rates of Cu and Zn from anti-fouling paints leached in natural and artificial brackish seawater. Sci. Total Environ. 2010, 408, 2459–2466. [Google Scholar] [CrossRef]
- Monaco, D.; Chianese, E.; Riccio, A.; Delgado-Sánchez, A.; Lacorte, S. Spatial distribution of heavy hydrocarbons, PAHs and metals in polluted areas. The case of “Galicia”, Spain. Mar. Pollut. Bull. 2017, 121, 230–237. [Google Scholar] [CrossRef]
- Sánchez-Marín, P.; Besada, V.; Beiras, R. Use of whole mussels and mussel gills in metal pollution biomonitoring. Cienc. Mar. 2018, 44, 279–294. [Google Scholar] [CrossRef]
- Okoro, H.K.; Fatoki, O.S.; Adekola, F.A.; Ximba, B.J.; Snyman, R.G. Fractionation, mobility and multivariate statistical evaluation of metals in marine sediments of Cape Town Harbor, South Africa. Chem. Speciat. Bioavailab. 2014, 26, 126–138. [Google Scholar] [CrossRef]
- Meixner, T.; Hibbs, B.; Sjolin, J.; Walker, J. Sources of Selenium, Arsenic and Nutrients in the Newport Bay Watershed; SWRCB: Sacramento, CA, USA, 2004. [Google Scholar]
- Van Hulten, M.; Dutay, J.-C.; Middag, R.; De Baar, H.; Roy-Barman, M.; Gehlen, M.; Tagliabue, A.; Sterl, A. Manganese in the world ocean: A first global model. Biogeosci. Discuss. 2016. [Google Scholar] [CrossRef]
- EPA (Environmental Protection Agency). National Recommended Water Quality Criteria—Aquatic Life Criteria Table. 2019. Available online: https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table (accessed on 10 December 2020).
- Pei, D.-S.; Junaid, M. Marine Ecology: Current and Future Developments. In Marine Pollution: Current Status, Impacts and Remedies; Bentham Science Publishers: Singapore, 2019; Volume 1. [Google Scholar]
- Samuel, A.O.; Madukwe, H.Y.; Imoleayo, F. Heavy metals concentration and pollution assessment of the beach sediments in Lagos, Southwestern Nigeria. J. Earth Sci. Environ. Stud. 2019, 4, 567–578. [Google Scholar]
- Beiras, R.; Durán, I.; Parra, S.; Urrutia, M.B.; Besada, V.; Bellas, J.; Viñas, L.; Sánchez-Marín, P.; González-Quijano, A.; Franco, M.A.; et al. Linking chemical contamination to biological effects in coastal pollution monitoring. Ecotoxicology 2012, 21, 9–17. [Google Scholar] [CrossRef]
- Gautam, R.K.; Sharma, S.K.; Mahiya, S.; Chattopadhyaya, M.C. Contamination of heavy metals in aquatic media: Transport, toxicity and technologies for remediation. In Heavy Metals in Water: Presence, Removal and Safety; The Royal Society of Chemistry: Cambridge, UK, 2015; Chapter 1. [Google Scholar]
- Mello, L.C.; Claudino, A.; Rizzatti, I.; Bortoluzzi, R.L.; Zanette, D.R. Analysis of trace metals Cu2+, Pb2+ and Zn2+ in coastal marine water samples from Florianopolis, Santa Catarina State, Brazil. J. Braz. Chem. Soc. 2005, 16, 308–315. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, X.; Jin, H.; Feng, H.; Shen, G.; Cao, Y.; Yu, C.; Lu, Z.; Zhang, Q. Spatiotemporal variation and potential risks of seven heavy metals in seawater, sediment, and seafood in Xiangshan Bay, China (2011–2016). Chemosphere 2018, 212, 1163–1171. [Google Scholar] [CrossRef]
- Tian, K.; Wu, Q.; Liu, P.; Hu, W.; Huang, B.; Shi, B.; Zhou, Y.; Kwon, B.O.; Choi, K.; Ryu, J.; et al. Ecological risk assessment of heavy metals in sediments and water from the coastal areas of the Bohai Sea and the Yellow Sea. Environ. Int. 2020, 136, 105512. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Zhao, L.; Xu, H.; Zhang, X. Assessment of dissolved heavy metals in the Laoshan Bay, China. Mar. Pollut. Bull. 2019, 149, 110608. [Google Scholar] [CrossRef]
- González-Ortegón, E.; Laiz, I.; Sánchez-Quiles, D.; Cobelo-Garcia, A.; Tovar-Sánchez, A. Trace metal characterization and fluxes from the Guadiana, Tinto-Odiel and Guadalquivir estuaries to the Gulf of Cadiz. Sci. Total Environ. 2019, 650, 2454–2466. [Google Scholar] [CrossRef] [PubMed]
- Armid, A.; Shinjo, R.; Ruslan, R. Distributions and pollution assessment of heavy metals Pb, Cd and Cr in the water system of Kendari Bay, Indonesia. IOP Conf. Ser. Mater. Sci. Eng. 2017, 172, 012002. [Google Scholar] [CrossRef]
- Crosley Munksgaard, N.; Livingstone Parry, D. Trace metals, arsenic and lead isotopes in dissolved and particulate phases of North Australian coastal and estuarine seawater. Mar. Chem. 2001, 75, 165–184. [Google Scholar] [CrossRef]
- Pavoni, E.; Crosera, M.; Petranich, E.; Adami, G.; Faganeli, J.; Covelli, S. Partitioning and mixing behaviour of trace elements at the Isonzo/Soča River mouth (Gulf of Trieste, Northern Adriatic Sea). Mar. Chem. 2020, 223, 103800. [Google Scholar] [CrossRef]
- Cutter, G.A.; Cutter, L.S. Behavior of dissolved antimony, arsenic, and selenium in the Atlantic Ocean. Mar. Chem. 1995, 49, 295–306. [Google Scholar] [CrossRef]
- Santos-Echeandía, J.; Caetano, M.; Brito, P.; Canario, J.; Vale, C. The relevance of defining trace metal baselines in coastal waters at a regional scale: The case of the Portuguese coast (SW Europe). Mar. Environ. Res. 2012, 79, 86–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cobelo-García, A.; Prego, R.; De Castro, M. Metal distributions and their fluxes at the coastal boundary of a semi-enclosed ria. Mar. Chem. 2005, 97, 277–292. [Google Scholar] [CrossRef]

| 52Cr | 55Mn | 56Fe | 63Cu | 66Zn | 75As | 78Se | 111Cd | 202Hg | 208Pb | |
|---|---|---|---|---|---|---|---|---|---|---|
| Range | 0–50 | 0–50 | 0–100 | 0–100 | 0–500 | 0–50 | 0–100 | 0–50 | 0–5 | 0–50 |
| a | 0.0206 | 0.0105 | 0.6011 | 0.0181 | 0.0240 | 4.29 × 10−4 | 0.0216 | 1.43 × 10−4 | 0.0042 | 0.0231 |
| b | 0.1224 | 0.1118 | 0.1185 | 0.0795 | 0.0210 | 0.0195 | 0.0021 | 0.0244 | 0.0376 | 0.3589 |
| LOD | 0.050 | 0.012 | 0.155 | 0.084 | 0.294 | 0.050 | 2.548 | 0.009 | 0.350 | 0.007 |
| LOQ | 0.163 | 0.040 | 0.515 | 0.281 | 0.981 | 0.168 | 8.493 | 0.029 | 1.165 | 0.023 |
| r2 | 0.9998 | 0.9996 | 0.9997 | 1.0 | 0.9990 | 0.9999 | 0.9997 | 0.9990 | 0.9999 | 0.9991 |
| Subzone | Statistics | Cr | Mn | Fe | Cu | Zn | As | Se | Cd | Pb |
|---|---|---|---|---|---|---|---|---|---|---|
| North | Mean ± SD | 1.54 ± 0.18 | 2.13 ± 0.43 | 4.28 ± 0.56 | 1.34 ± 0.74 | 0.62 ± 0.41 | 4.90 ± 0.53 | 11.5 ± 3.9 | 0.01 ± 0.03 | 0.06 ± 0.10 |
| Min | 1.4 | 1.5 | 3.3 | 0.7 | ND | 4.3 | ND | ND | ND | |
| Max | 2.0 | 2.8 | 5.2 | 3.2 | 1.6 | 5.7 | 17.2 | 0.1 | 0.3 | |
| Median ± IR | 1.50 ± 0 | 2.00 ± 0.40 | 4.40 ± 0.60 | 1.10 ± 0.40 | 0.50 ± 0.30 | 4.80 ± 1.00 | 12.3 ± 7.1 | 0 ± 0 | 0 ± 0.1 | |
| Lower Q | 1.5 | 1.9 | 4.0 | 0.9 | ND | 4.4 | ND | ND | ND | |
| Upper Q | 1.5 | 2.3 | 4.6 | 1.3 | ND | 5.4 | 15.2 | ND | 0.1 | |
| South | Mean ± SD | 1.43 ± 0.12 | 2.06 ± 0.54 | 4.00 ± 0.81 | 2.23 ± 1.83 | 1.71 ± 3.23 | 4.76 ± 0.46 | 10.0 ± 2.4 | 0.01 ± 0.03 | 0.20 ± 0.36 |
| Min | 1.2 | 1.3 | 2.7 | 0.8 | ND | 3.9 | ND | ND | ND | |
| Max | 1.6 | 2.8 | 5.5 | 6.9 | 10.3 | 5.5 | 12.7 | 0.1 | 1.1 | |
| Median ± IR | 1.50 ± 0.10 | 2.10 ± 0.80 | 4.10 ± 0.90 | 1.60 ± 1.00 | 0.60 ± 0.60 | 4.90 ± 0.50 | 10.0 ± 2.0 | 0 ± 0 | 0 ± 0.20 | |
| Lower Q | 1.4 | 1.7 | 3.6 | 1.3 | ND | 4.5 | 9.5 | ND | ND | |
| Upper Q | 1.5 | 2.5 | 4.5 | 2.3 | 1.0 | 5.0 | 11.5 | ND | 0.2 | |
| East | Mean ± SD | 1.57 ± 0.22 | 2.62 ± 0.19 | 4.60 ± 0.46 | 1.73 ± 0.79 | 0.72 ± 0.51 | 5.00 ± 0.44 | 10.6 ± 3.7 | 0.03 ± 0.05 | 0.10 ± 0.13 |
| Min | 1.4 | 2.3 | 4.2 | 0.9 | ND | 4.5 | ND | ND | ND | |
| Max | 2.0 | 2.8 | 5.5 | 3.2 | 1.6 | 5.5 | 15.6 | 0.1 | 0.3 | |
| Median ± IR | 1.50 ± 0.20 | 2.65 ± 0.30 | 4.50 ± 0.10 | 1.55 ± 0.60 | 0.60 ± 0.50 | 4.95 ± 0.90 | 11.3 ± 5.5 | 0 ± 0.10 | 0.05 ± 0.20 | |
| Lower Q | 1.4 | 2.5 | 4.4 | 1.3 | ND | 4.6 | ND | ND | ND | |
| Upper Q | 1.6 | 2.8 | 4.5 | 1.9 | 0.9 | 5.5 | 12.7 | 0.1 | 0.2 | |
| West | Mean ± SD | 1.45 ± 0.11 | 1.83 ± 0.32 | 3.91 ± 0.69 | 1.82 ± 1.70 | 1.39 ± 2.82 | 4.74 ± 0.50 | 10.8 ± 3.2 | ND | 0.14 ± 0.32 |
| Min | 1.2 | 1.3 | 2.7 | 0.7 | ND | 3.9 | ND | ND | ND | |
| Max | 1.6 | 2.3 | 5.2 | 6.9 | 10.3 | 5.7 | 17.2 | ND | 1.1 | |
| Median ± IR | 1.50 ± 0.10 | 1.85 ± 0.45 | 3.90 ± 0.90 | 1.25 ± 0.95 | 0.50 ± 0.45 | 4.75 ± 0.60 | 10.6 ± 3.7 | ND | 0 ± 0.10 | |
| Lower Q | 1.4 | 1.6 | 3.45 | 1.0 | ND | 4.4 | 8.65 | ND | ND | |
| Upper Q | 1.5 | 2.05 | 4.35 | 1.95 | 0.85 | 5.0 | 12.35 | 0 | 0.1 | |
| Saltwater CMC | 1100 | 4.8 | 90 | 69 | 290 | 33 | 140 | |||
| (Acute) (µg L−1) | ||||||||||
| Saltwater CCC | 50 | 3.1 | 81 | 36 | 71 | 7.9 | 5.6 | |||
| (Chronic) (µg L−1) | ||||||||||
| Region | Sampling year | Cr | Mn | Fe | Cu | Zn | As | Se | Cd | Pb | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Laoshan Bay | 2017–2018 | 0.31−2.71 | 0.51–4.50 | 0.09−5.71 | 0.63−1.75 | 0.059−0.769 | 0.16−9.13 | [41] | |||
| (China) | |||||||||||
| Xiangshan Bay (China) | 2011–2016 | ND–2.0 | ND−44.5 | 0.7−65.9 | 0.6−8.5 | 0.01−1.61 | 0.06−8.08 | [39] | |||
| Yellow Sea | 2018 | 0.07−0.66 | 0.64−15.20 | 0.20−4.47 | 0.83−2.51 | 0.002−0.088 | 0.01−0.75 | [40] | |||
| (South Korea) | |||||||||||
| Kendari Bay | 2014 | 0.085−0.386 | 0.001−0.015 | 0.009−0.549 | [43] | ||||||
| (Indonesia) | |||||||||||
| North Australian (coast and estuaries) | 1996–2000 | 0.14−34.1 | 0.15−1.04 | 0.018−0.49 | 0.39−1.35 | 0.002−0.034 | <0.002−0.057 | [44] | |||
| Gulf of Trieste | 2018–2019 * | <0.08−0.31 | 6.09−16.9 | <0.9−3.51 | 0.41−1.74 | 6.93−31.2 | 2.10−2.31 | <0.03−0.08 | [45] | ||
| Adriatic Sea | |||||||||||
| (Italy) | |||||||||||
| Gulf of Cádiz(1) | 2016 | 0.15−54.4 | 0.45−10.9 | 0.59−55.3 | 0.015−0.81 | 0.006−0.83 | [42] | ||||
| three estuaries | |||||||||||
| (South Spain) | |||||||||||
| Atlantic Ocean (1) (North and South) | 1990 | 1.33−1.56 | 0.082−0.099 | [46] | |||||||
| Portuguese Coast (1) | 2010 | 0.057−2.86 | 0.091−4.05 | 0.001−0.10 | 0.002−0.031 | [47] | |||||
| Ferrol Ría (1)) | 2000–2001 | 0.43−0.58 | 1.11−1.57 | 0.010−0.011 | 0.048−0.063 | [48] | |||||
| (Northwest Spain | |||||||||||
| Vigo Ría (1) | 2002–2003 | 0.069−0.59 | 0.48−1.25 | 0.002−0.011 | 0.017−0.052 | [19] | |||||
| (Northwest Spain) | |||||||||||
| Vigo Ría | 2018 | 1.20−2.00 | 1.30−2.80 | 2.70−5.50 | 0.70−6.90 | 0.20–10.3 | 3.90−5.70 | 5.70−17.20 | ND−0.100 | ND−1.10 | This study |
| (Northwest Spain) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Cid, B.; Falqué, E.; Simal-Gandara, J. Coastline Levels of Dissolved Heavy Metals in the Estuarine Water–System of Vigo. Int. J. Environ. Res. Public Health 2021, 18, 2136. https://doi.org/10.3390/ijerph18042136
Pérez-Cid B, Falqué E, Simal-Gandara J. Coastline Levels of Dissolved Heavy Metals in the Estuarine Water–System of Vigo. International Journal of Environmental Research and Public Health. 2021; 18(4):2136. https://doi.org/10.3390/ijerph18042136
Chicago/Turabian StylePérez-Cid, Benita, Elena Falqué, and Jesus Simal-Gandara. 2021. "Coastline Levels of Dissolved Heavy Metals in the Estuarine Water–System of Vigo" International Journal of Environmental Research and Public Health 18, no. 4: 2136. https://doi.org/10.3390/ijerph18042136
APA StylePérez-Cid, B., Falqué, E., & Simal-Gandara, J. (2021). Coastline Levels of Dissolved Heavy Metals in the Estuarine Water–System of Vigo. International Journal of Environmental Research and Public Health, 18(4), 2136. https://doi.org/10.3390/ijerph18042136








