Pavement Overrides the Effects of Tree Species on Soil Bacterial Communities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Field Sampling and Soil Chemical Analysis
2.3. DNA Extraction, Illumina MiSeq Sequencing and Bioinformatics Analysis
2.4. Statistical Analysis
3. Results
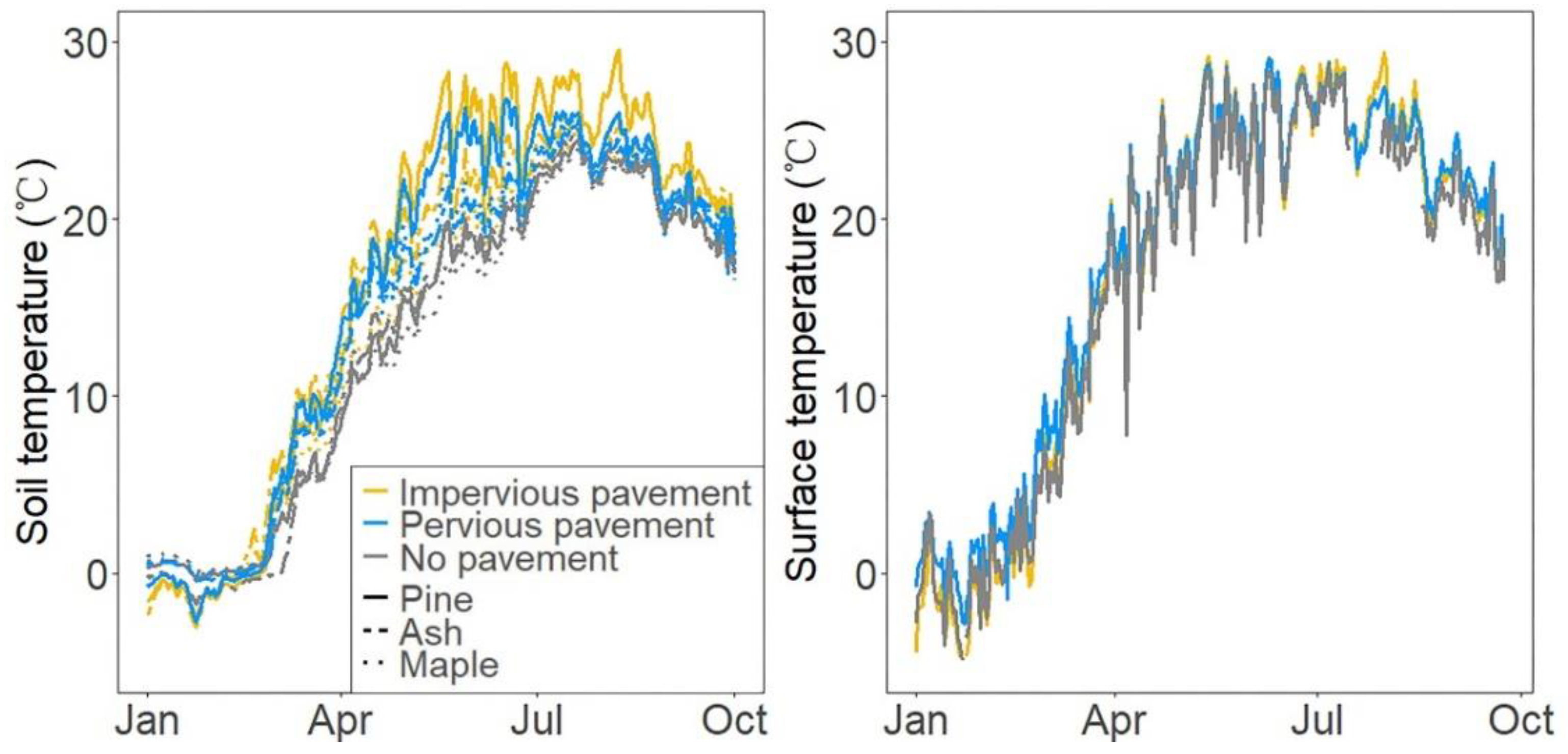
3.1. Soil Properties
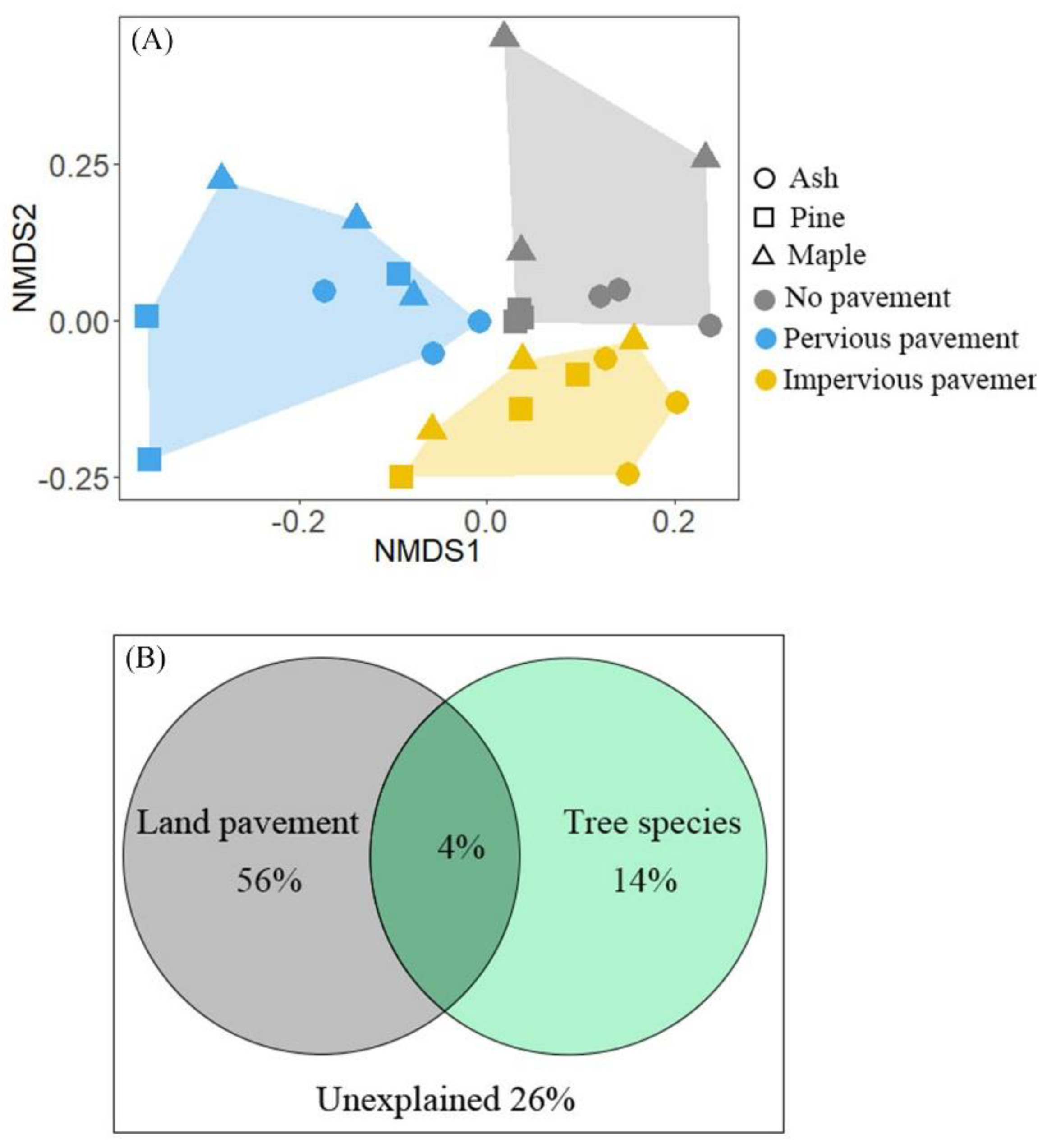
3.2. Bacterial Community Composition and Diversity
3.3. Soil Bacterial Diversity under Different Pavements
3.4. Relations between Soil Characters and Bacterial Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- UN-DESA. Department of Economic and Social Affairs, Population Division World Urbanization Prospects: The 2019 Revision, Highlights. 2019. Available online: https://population.un.org/wpp/Publications/ (accessed on 23 February 2021).
- Li, H.; Li, L.; Chen, L.Q.; Zhou, X.S.; Cui, Y.F.; Liu, Y.Q.; Liu, W.Q. Mapping and characterizing spatiotemporal dynamics of impervious surfaces using Landsat images: A case study of Xuzhou, East China from 1995 to 2018. Sustainability 2019, 11, 1224. [Google Scholar] [CrossRef] [Green Version]
- Gong, P.; Li, X.; Wang, J.; Bai, Y.; Chen, B.; Hu, T.; Liu, X.; Xu, B.; Yang, J.; Zhang, W.; et al. Annual maps of global artificial impervious area (GAIA) between 1985 and 2018. Remote Sens. Environ. 2020, 236, 111510. [Google Scholar] [CrossRef]
- Yu, H.F.; Zhao, Y.L.; Fu, Y.C.; Li, L. Spatiotemporal variance assessment of urban rainstorm waterlogging affected by impervious surface expansion: A case study of Guangzhou, China. Sustainability 2018, 10, 3761. [Google Scholar] [CrossRef] [Green Version]
- Bao, T.L.G.; Li, X.M.; Zhang, J.; Zhang, Y.J.; Tian, S.Z. Assessing the distribution of urban green spaces and its anisotropic cooling distance on urban heat island pattern in Baotou, China. ISPRS Int. Geo-Inf. 2016, 5, 12. [Google Scholar] [CrossRef]
- Gao, C.; Liu, J.; Cui, H.; Wang, Z.W.; He, S. Optimized water surface ratio and pervious surface proportion in urbanized riverside areas. Environ. Earth Sci. 2014, 72, 569–576. [Google Scholar] [CrossRef]
- Souza, F.L.; Valente-Neto, F.; Severo-Neto, F.; Bueno, B.; Ochoa-Quintero, J.M.; Laps, R.R.; Bolzan, F.; Roque, F.D. Impervious surface and heterogeneity are opposite drivers to maintain bird richness in a Cerrado city. Landsc. Urban Plan. 2019, 192, 10. [Google Scholar] [CrossRef]
- Yan, Z.G.; Teng, M.J.; He, W.; Liu, A.Q.; Li, Y.R.; Wang, P.C. Impervious surface area is a key predictor for urban plant diversity in a city undergone rapid urbanization. Sci. Total Environ. 2019, 650, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Domeignoz-Horta, L.A.; Pold, G.; Liu, X.-J.A.; Frey, S.D.; Melillo, J.M.; DeAngelis, K.M. Microbial diversity drives carbon use efficiency in a model soil. Nat. Commun. 2020, 11, 3684. [Google Scholar] [CrossRef] [PubMed]
- Raciti, S.M.; Hutyra, L.R.; Finzi, A.C. Depleted soil carbon and nitrogen pools beneath impervious surfaces. Environ. Pollut. 2012, 164, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, F.; Wang, R.S.; Yang, Q.R.; Ni, H.S. Effect of soil sealing on the microbial biomass, N transformation and related enzyme activities at various depths of soils in urban area of Beijing, China. J. Soils Sediments 2012, 12, 519–530. [Google Scholar] [CrossRef]
- Wei, Z.Q.; Wu, S.H.; Zhou, S.L.; Lin, C. Installation of impervious surface in urban areas affects microbial biomass, activity (potential C mineralisation), and functional diversity of the fine earth. Soil Res. 2013, 51, 59–67. [Google Scholar] [CrossRef]
- Hu, Y.; Dou, X.; Li, J.; Li, F. Impervious surfaces alter soil bacterial communities in urban areas: A case study in Beijing, China. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Sanders, N.J.; Shi, Y.; Chu, H.; Classen, A.T.; Zhao, K.; Chen, L.; Shi, Y.; Jiang, Y.; He, J.-S. The links between ecosystem multifunctionality and above- and belowground biodiversity are mediated by climate. Nat. Commun. 2015, 6, 8159. [Google Scholar] [CrossRef]
- Berg, G.; Smalla, K. Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. Fems Microbiol. Ecol. 2009, 68, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-M.; Wang, X.-K.; Su, Y.-B.; Zhang, H.-X. Land pavement depresses photosynthesis in urban trees especially under drought stress. Sci. Total Environ. 2019, 653, 120–130. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Wang, X.K.; Jiang, B.; Li, L. The leaf phenophase of deciduous species altered by land pavements. Int. J. Biometeorol. 2018, 62, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, F.; Wang, X.; Xu, C.; Zhang, J.; Liu, X.; Zhang, H. The effects of urban impervious surfaces on eco-physiological characteristics of Ginkgo biloba: A case study from Beijing, China. Urban Green. 2015, 14, 1102–1109. [Google Scholar] [CrossRef]
- Yu, W.; Chen, Y.; Wang, X.; Wang, X. Effects of land pavement on the structure and function of soil microbial community under different tree species. Sheng Tai Xue Bao 2019, 39, 3575–3585. [Google Scholar]
- Liu, Y.; Li, T.; Yu, L. Urban heat island mitigation and hydrology performance of innovative permeable pavement: A pilot-scale study. J. Clean. Prod. 2020, 244, 118938. [Google Scholar] [CrossRef]
- Shuster, W.D.; Pappas, E.; Zhang, Y. Laboratory-scale simulation of runoff response from pervious-impervious systems. J. Hydrol. Eng. 2008, 13, 886–893. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, X.; Jiang, B.; Wen, Z.; Yang, N.; Li, L. Tree survival and growth are impacted by increased surface temperature on paved land. Landsc. Urban Plan. 2017, 162, 68–79. [Google Scholar] [CrossRef]
- Yu, W.; Hu, Y.; Cui, B.; Chen, Y.; Wang, X. The effects of pavement types on soil bacterial communities across different depths. Int. J. Environ. Res. Public Health 2019, 16, 1805. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ding, J.; Peng, Y.; Li, F.; Yang, G.; Liu, L.; Qin, S.; Fang, K.; Yang, Y. Patterns and drivers of soil microbial communities in Tibetan alpine and global terrestrial ecosystems. J. Biogeogr. 2016, 43, 2027–2039. [Google Scholar] [CrossRef]
- Li, X.; He, H.; Zhang, X.; Yan, X.; Six, J.; Cai, Z.; Barthel, M.; Zhang, J.; Necpalova, M.; Ma, Q.; et al. Distinct responses of soil fungal and bacterial nitrate immobilization to land conversion from forest to agriculture. Soil Biol. Biochem. 2019, 134, 81–89. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, C.; Jiang, L.; Song, M.; Zhang, D.; Li, J.; Li, Y.; Ostle, N.J.; Zhang, G. Land-use changes alter soil bacterial composition and diversity in tropical forest soil in China. Sci. Total Environ. 2020, 712, 136526. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.-J.; Li, S.; Su, J.-Q.; Nie Sa Gibson, V.; Li, H.; Zhu, Y.-G. Does urbanization shape bacterial community composition in urban park soils? A case study in 16 representative Chinese cities based on the pyrosequencing method. FEMS Microbiol. Ecol. 2014, 87, 182–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, B.; Li, J.; Xiao, N.; Qi, Y.; Fu, G.; Liu, G.; Qiao, M. Urban-development-induced changes in the diversity and composition of the soil bacterial community in Beijing. Sci. Rep. 2016, 6, 38811. [Google Scholar] [CrossRef] [Green Version]
- Prescott, C.E.; Grayston, S.J. Tree species influence on microbial communities in litter and soil: Current knowledge and research needs. Ecol. Manag. 2013, 309, 19–27. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef] [Green Version]
- Pandey, R.R.; Sharma, G.; Tripathi, S.K.; Singh, A.K. Litterfall, litter decomposition and nutrient dynamics in a subtropical natural oak forest and managed plantation in northeastern India. Ecol. Manag. 2007, 240, 96–104. [Google Scholar] [CrossRef]
- Kara, O.; Bolat, I.; Cakiroglu, K.; Ozturk, M. Plant canopy effects on litter accumulation and soil microbial biomass in two temperate forests. Biol. Fertil. Soils 2008, 45, 193–198. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, C.; Xu, Z.; Liu, Y. Effects of single and mixed species forest ecosystems on diversity and function of soil microbial community in subtropical China. J. Soils Sediments 2012, 12, 228–240. [Google Scholar] [CrossRef]
- Jangid, K.; Williams, M.A.; Franzluebbers, A.J.; Schmidt, T.M.; Coleman, D.C.; Whitman, W.B. Land-use history has a stronger impact on soil microbial community composition than aboveground vegetation and soil properties. Soil Biol. Biochem. 2011, 43, 2184–2193. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Shi, Y.-M.; Sun, G.-D.; Li, J.-T.; Chen, H.; Chow, A.T.; Yang, Z.-B.; Majidzadeh, H.; Wang, J.-J. Soil organic carbon signature under impervious surfaces. ACS Earth Space Chem. 2020, 4, 1785–1792. [Google Scholar] [CrossRef]
- Lu, C.Y.; Kotze, D.J.; Setala, H.M. Soil sealing causes substantial losses in C and N storage in urban soils under cool climate. Sci. Total Environ. 2020, 725, 7. [Google Scholar] [CrossRef] [PubMed]
- Dennis, P.G.; Newsham, K.K.; Rushton, S.P.; O’Donnell, A.G.; Hopkins, D.W. Soil bacterial diversity is positively associated with air temperature in the maritime Antarctic. Sci. Rep. 2019, 9, 2686. [Google Scholar] [CrossRef] [Green Version]


| Pavement | Pine | Ash | Maple | |||
|---|---|---|---|---|---|---|
| Chao 1 | Shannon | Chao 1 | Shannon | Chao 1 | Shannon | |
| IPP | 2063 ± 149 b | 10.11 ± 0.05 b | 1987 ± 337 b | 9.97 ± 0.05 a | 2310 ± 332 a | 10.09 ± 0.07 a |
| PP | 2507 ± 199 a | 10.24 ± 0.04 a | 2133 ± 271 b | 10.04 ± 0.04 a | 2196 ± 361 a | 10.24 ± 0.02 a |
| NP | 2672 ± 86 a | 10.26 ± 0.01 a | 3052 ± 132 a | 10.26 ± 0.11 a | 2822 ± 208 a | 10.26 ± 0.09 a |
| Variable | R | p |
|---|---|---|
| Temperature | 0.397 | 0.001 |
| AP | 0.286 | 0.004 |
| TC | 0.233 | 0.006 |
| TN | 0.198 | 0.037 |
| PH | 0.097 | 0.146 |
| NH4+ | 0.101 | 0.824 |
| NO3- | 0.040 | 0.525 |
| AK | 0.030 | 0.407 |
| Index | Fixed Effects | Estimate | Std Error | t-Value | p-Value |
|---|---|---|---|---|---|
| Chao 1 | (Intercept) | 0 | 0.128 | 0 | 1.000 |
| temperature | −0.387 | 0.173 | −2.236 | 0.041 | |
| PH | 0.411 | 0.155 | 2.646 | 0.013 | |
| TC | −1.302 | 0.437 | −2.979 | 0.006 | |
| TN | 1.364 | 0.502 | 2.718 | 0.012 | |
| NH4+ | 0.031 | 0.127 | 0.246 | 0.808 | |
| NO3− | −0.351 | 0.181 | −1.945 | 0.063 | |
| AP | −0.014 | 0.272 | −0.050 | 0.960 | |
| Shannon | (Intercept) | 0 | 0.151 | 0 | 1.000 |
| temperature | −0.143 | 0.201 | −0.711 | 0.488 | |
| PH | 0.414 | 0.177 | 2.343 | 0.027 | |
| NO3− | −0.969 | 0.495 | −1.957 | 0.062 | |
| TC | 1.039 | 0.568 | 1.829 | 0.080 | |
| TN | −0.030 | 0.144 | −0.211 | 0.834 | |
| NH4+ | −0.449 | 0.205 | −2.19 | 0.038 | |
| AP | 0.084 | 0.309 | 0.272 | 0.788 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Yu, W.; Cui, B.; Chen, Y.; Zheng, H.; Wang, X. Pavement Overrides the Effects of Tree Species on Soil Bacterial Communities. Int. J. Environ. Res. Public Health 2021, 18, 2168. https://doi.org/10.3390/ijerph18042168
Hu Y, Yu W, Cui B, Chen Y, Zheng H, Wang X. Pavement Overrides the Effects of Tree Species on Soil Bacterial Communities. International Journal of Environmental Research and Public Health. 2021; 18(4):2168. https://doi.org/10.3390/ijerph18042168
Chicago/Turabian StyleHu, Yinhong, Weiwei Yu, Bowen Cui, Yuanyuan Chen, Hua Zheng, and Xiaoke Wang. 2021. "Pavement Overrides the Effects of Tree Species on Soil Bacterial Communities" International Journal of Environmental Research and Public Health 18, no. 4: 2168. https://doi.org/10.3390/ijerph18042168






