Cultivation of the Acidophilic Microalgae Galdieria phlegrea with Wastewater: Process Yields
Abstract
:1. Introduction
2. Materials and Methods
2.1. Algal Culture and Growth Media
2.2. Experimental Setup
2.2.1. Ammonium and Phosphate
2.2.2. Biomass Concentration and Phycocyanin Content
2.2.3. Lipid Content
2.2.4. C and N Content and Evaluation of δ13C and δ15N
2.2.5. Statistical Analysis
3. Results and Discussion
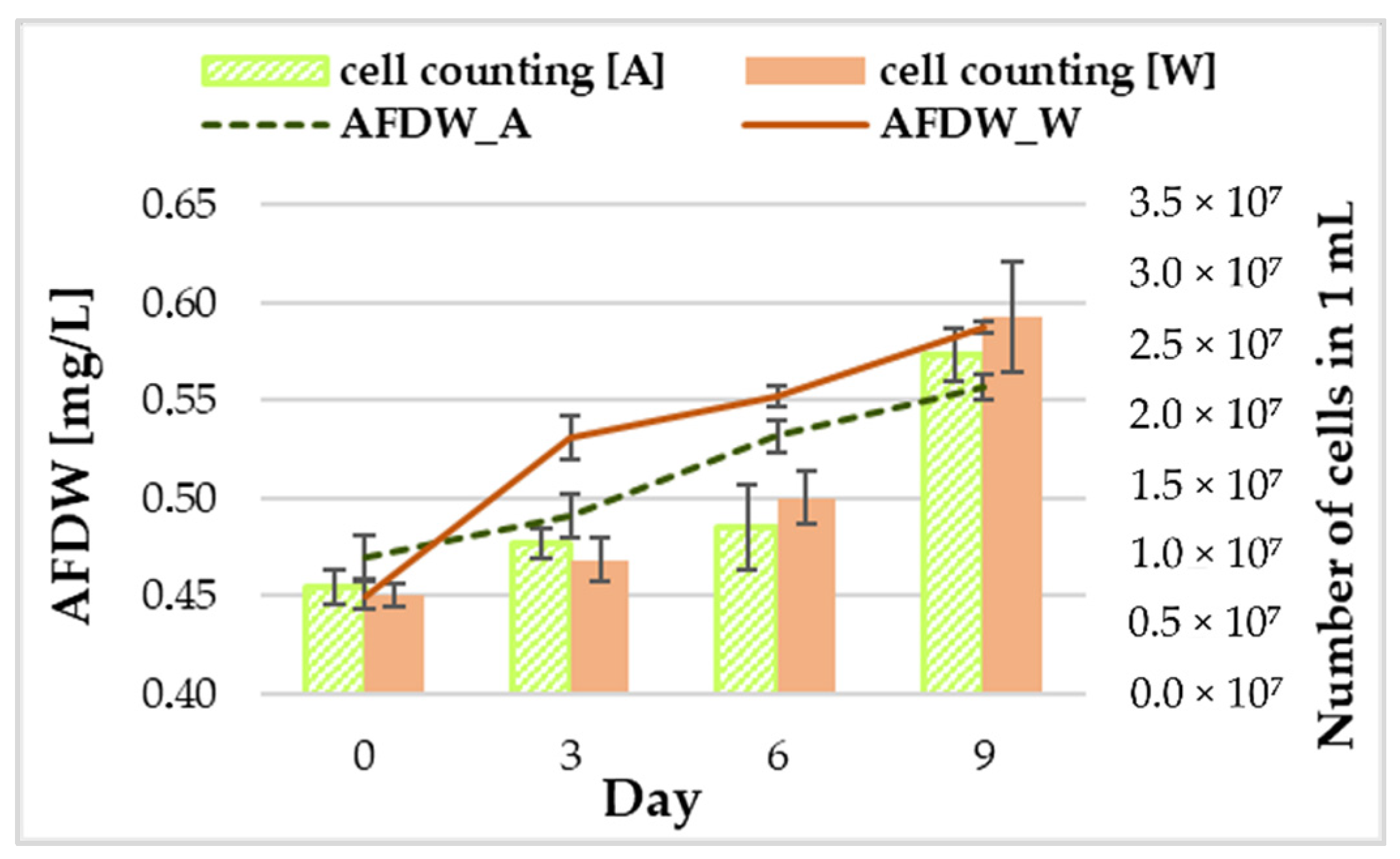
3.1. Biomass Growth and Phycocyanin Content
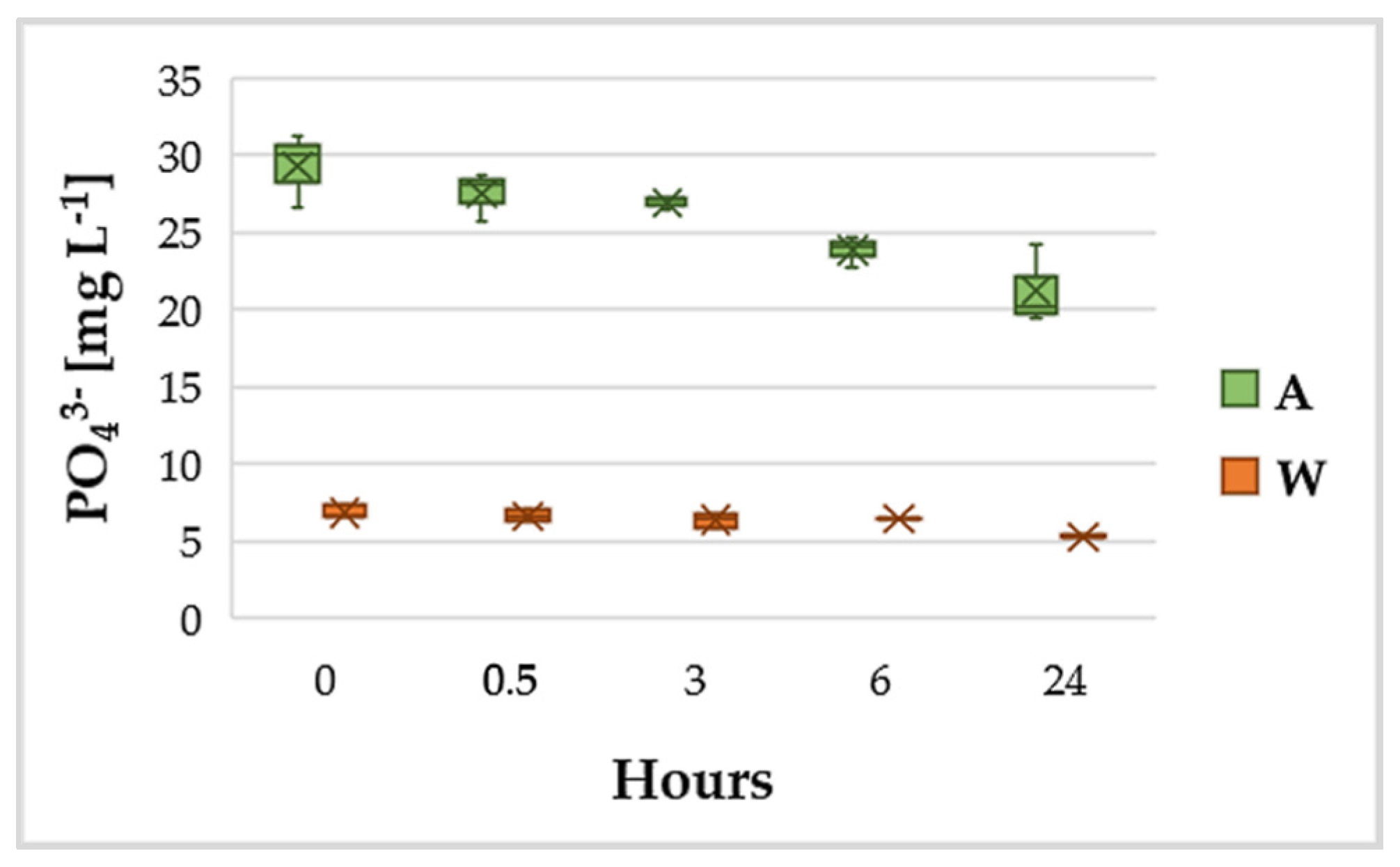
3.2. Variation of NH4+ and PO43−
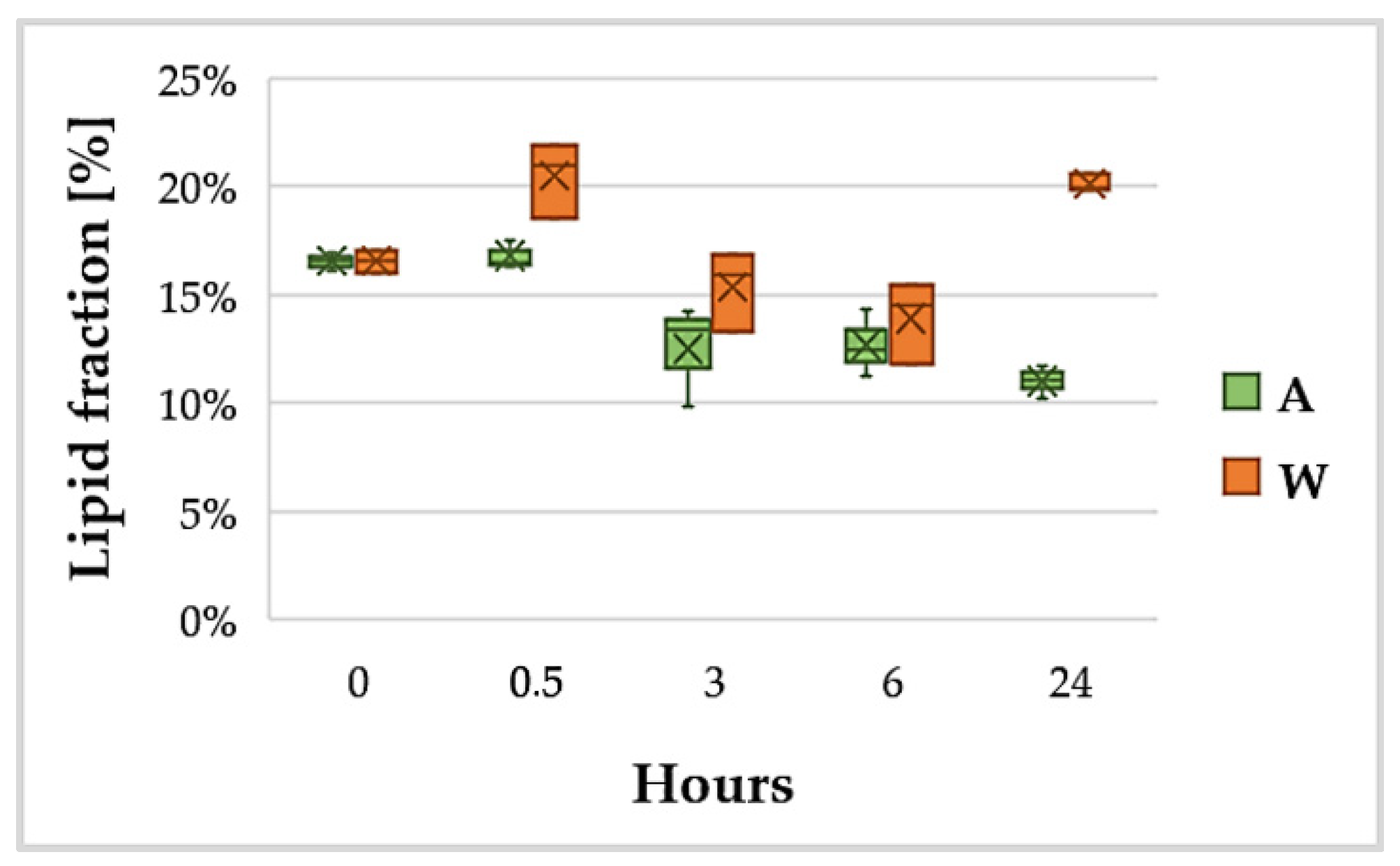
3.3. Lipid Content
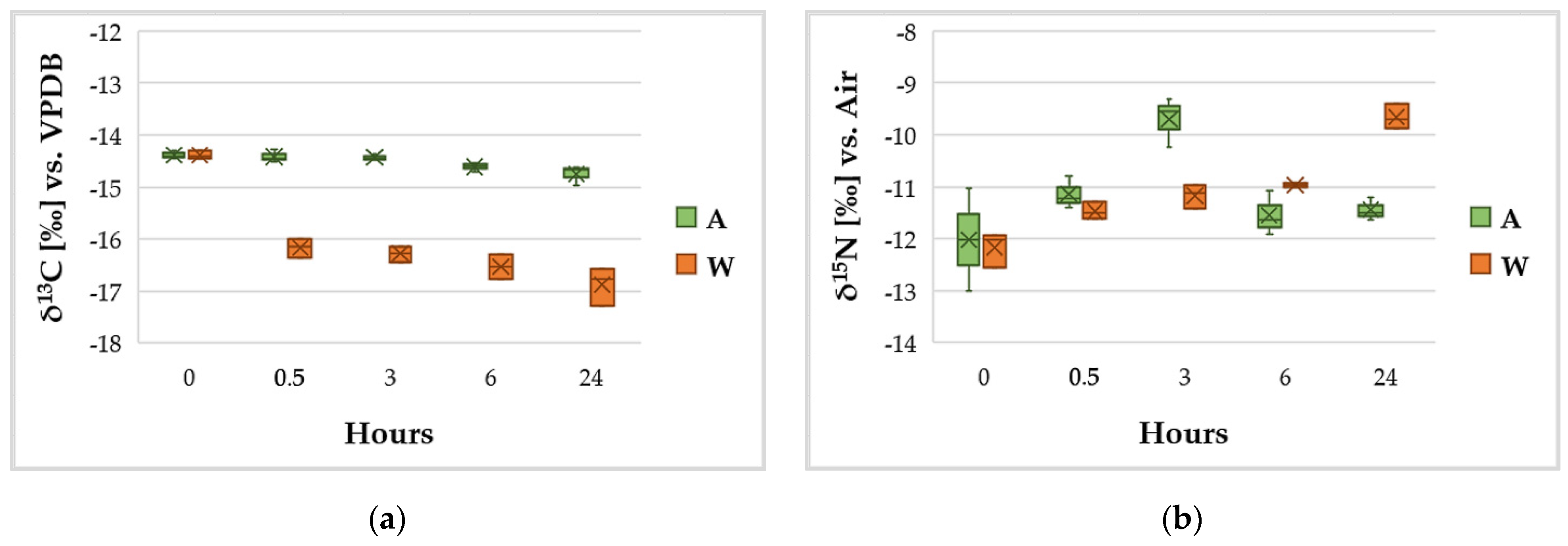
3.4. C and N Content and Variation of the Isotopic Ratios δ13C and δ15N
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kehrein, P.; van Loosdrecht, M.; Osseweijer, P.; Garfí, M.; Dewulf, J.; Posada, J. A critical review of resource recovery from municipal wastewater treatment plants–market supply potentials, technologies and bottlenecks. Environ. Sci. Water Res. Technol. 2020, 6, 877–910. [Google Scholar] [CrossRef] [Green Version]
- Capodaglio, A.G.; Olsson, G. Energy issues in sustainable urban wastewater management: Use, demand reduction and recovery in the urban water cycle. Sustainability 2020, 12, 266. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef] [PubMed]
- Cabanelas, I.T.D.; Arbib, Z.; Chinalia, F.A.; Souza, C.O.; Perales, J.A.; Almeida, P.F.; Druzian, J.I.; Nascimento, I.A. From waste to energy: Microalgae production in wastewater and glycerol. Appl. Energy 2013, 109, 283–290. [Google Scholar] [CrossRef]
- Jeyanayagam, S. True Confessions of the Biological Nutrient Removal Process. Fla. Water Resour. J. 2005, 1, 37–46. [Google Scholar]
- Clarens, A.F.; Resurreccion, E.P.; White, M.A.; Colosi, L.M. Environmental Life Cycle Comparison of Algae to Other Bioenergy Feedstocks. Environ. Sci. Technol. 2010, 44, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Heidrich, E.S.; Curtis, T.P.; Dolfing, J. Determination of the internal chemical energy of wastewater. Environ. Sci. Technol. 2011, 45, 827–832. [Google Scholar] [CrossRef]
- Tyler, H.; Fallgren, P.H.; Song, J.; Zhiyong Jason, R. Energy and Performance Comparison of Microbial Fuel Cell and Conventional Aeration Treating of Wastewater. J. Microb. Biochem. Technol. 2013, S6. [Google Scholar] [CrossRef] [Green Version]
- Chrispim, M.C.; Scholz, M.; Nolasco, M.A. Phosphorus recovery from municipal wastewater treatment: Critical review of challenges and opportunities for developing countries. J. Environ. Manag. 2019, 248, 109268. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ngo, H.H.; Guo, W.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Liang, H.; Wang, J. A critical review on ammonium recovery from wastewater for sustainable wastewater management. Bioresour. Technol. 2018, 268, 749–758. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Hwang, I.S.; Min, K.S. ANAMMOX and partial denitritation in anaerobic nitrogen removal from piggery waste. Water Sci. Technol. 2004, 49, 145–153. [Google Scholar] [CrossRef]
- Cui, Y.-X.; Biswal, B.K.; Guo, G.; Deng, Y.-F.; Huang, H.; Chen, G.-H.; Wu, D. Biological nitrogen removal from wastewater using sulphur-driven autotrophic denitrification. Appl. Microbiol. Biotechnol. 2019, 103, 6023–6039. [Google Scholar] [CrossRef]
- González, I.; Herrero, N.; Siles, J.Á.; Chica, A.F.; Martín, M.; Izquierdo, C.G.; Gómez, J.M. Wastewater nutrient recovery using twin-layer microalgae technology for biofertilizer production. Water Sci. Technol. 2020, 82, 1044–1061. [Google Scholar] [CrossRef]
- Henkanatte-Gedera, S.M.; Selvaratnam, T.; Caskan, N.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Algal-based, single-step treatment of urban wastewaters. Bioresour. Technol. 2015, 189, 273–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, D.B.; Schideman, L.C.; Canam, T.; Hudson, R.J.M. Pilot-scale demonstration of efficient ammonia removal from a high-strength municipal wastewater treatment sidestream by algal-bacterial biofilms affixed to rotating contactors. Algal Res. 2018, 34, 143–153. [Google Scholar] [CrossRef]
- Wollmann, F.; Dietze, S.; Ackermann, J.-U.; Bley, T.; Walther, T.; Steingroewer, J.; Krujatz, F. Microalgae wastewater treatment: Biological and technological approaches. Eng. Life Sci. 2019, 19, 860–871. [Google Scholar] [CrossRef] [Green Version]
- Goswami, R.K.; Mehariya, S.; Verma, P.; Lavecchia, R.; Zuorro, A. Microalgae-based biorefineries for sustainable resource recovery from wastewater. J. Water Process. Eng. 2020, 101747. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Mehariya, S.; Bhatia, R.K.; Kumar, M.; Pugazhendhi, A.; Awasthi, M.K.; Atabani, A.E.; Kumar, G.; Kim, W.; Seo, S.-O.; et al. Wastewater based microalgal biorefinery for bioenergy production: Progress and challenges. Sci. Total Environ. 2021, 751, 141599. [Google Scholar] [CrossRef] [PubMed]
- Sydney, E.B.; Schafranski, K.; Barretti, B.R.V.; Sydney, A.C.N.; Zimmerman, J.F.D.A.; Cerri, M.L.; Mottin Demiate, I. Biomolecules from extremophile microalgae: From genetics to bioprocessing of a new candidate for large-scale production. Process. Biochem. 2019, 87, 37–44. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.; Kormpa, A.; van der Maarel, M.J.E.C. The glycogen of Galdieria sulphuraria as alternative to starch for the production of slowly digestible and resistant glucose polymers. Carbohydr. Polym. 2017, 169, 75–82. [Google Scholar] [CrossRef]
- Carfagna, S.; Landi, V.; Coraggio, F.; Salbitani, G.; Vona, V.; Pinto, G.; Pollio, A.; Ciniglia, C. Different characteristics of C-phycocyanin (C-PC) in two strains of the extremophilic Galdieria phlegrea. Algal Res. 2018, 31, 406–412. [Google Scholar] [CrossRef]
- Ferreira, G.F.; Ríos Pinto, L.F.; Maciel Filho, R.; Fregolente, L.V. A review on lipid production from microalgae: Association between cultivation using waste streams and fatty acid profiles. Renew. Sustain. Energy Rev. 2019, 109, 448–466. [Google Scholar] [CrossRef]
- Chakraborty, M.; Miao, C.; McDonald, A.; Chen, S.L. Concomitant extraction of bio-oil and value added polysaccharides from Chlorella sorokiniana using a unique sequential hydrothermal extraction technology. Fuel 2012, 95, 63–70. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pegallapati, A.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Lammers, P.J.; van Voorhies, W. Feasibility of algal systems for sustainable wastewater treatment. Renew. Energy 2015, 82, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Hena, S.; Znad, H.; Heong, K.; Judd, S. Dairy farm wastewater treatment and lipid accumulation by Arthrospira platensis. Water Res. 2018, 128, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Moreno Osorio, J.H.; Luongo, V.; Del Mondo, A.; Pinto, G.; Pollio, A.; Frunzo, L.; Lens, P.N.L.; Esposito, G. Nutrient removal from high strength nitrate containing industrial wastewater using Chlorella sp. strain ACUF_802. Ann. Microbiol. 2018, 68, 899–913. [Google Scholar] [CrossRef]
- Martínez, M.E.; Sánchez, S.; Jiménez, J.M.; El Yousfi, F.; Muñoz, L. Nitrogen and phosphorus removal from urban wastewater by the microalga Scenedesmus obliquus. Bioresour. Technol. 2000, 73, 263–272. [Google Scholar] [CrossRef]
- Zhai, J.; Li, X.; Li, W.; Rahaman, M.H.; Zhao, Y.; Wei, B.; Wei, H. Optimization of biomass production and nutrients removal by Spirulina platensis from municipal wastewater. Ecol. Eng. 2017, 108, 83–92. [Google Scholar] [CrossRef]
- Čížková, M.; Vítová, M.; Zachleder, V. The Red Microalga Galdieria as a Promising Organism for Applications in Biotechnology. In Biotechnology, Microalgae–From Physiology to Application; Vítová, M., Ed.; IntechOpen: London, UK, 2019; pp. 105–122. [Google Scholar] [CrossRef] [Green Version]
- Biswal, B.K.; Mazza, A.; Masson, L.; Gehr, R.; Frigon, D. Impact of wastewater treatment processes on antimicrobial resistance genes and their co-occurrence with virulence genes in Escherichia coli. Water Res. 2014, 50, 245–253. [Google Scholar] [CrossRef]
- Cho, C.H.; Park, S.I.; Ciniglia, C.; Yang, E.C.; Graf, L.; Bhattacharya, D.; Yoon, H.S. Potential causes and consequences of rapid mitochondrial genome evolution in thermoacidophilic Galdieria (Rhodophyta). BMC Evol. Biol. 2020, 20, 1–15. [Google Scholar] [CrossRef]
- Ciniglia, C.; Cennamo, P.; De Natale, A.; De Stefano, M.; Sirakov, M.; Iovinella, M.; Yoon, H.S.; Pollio, A. Cyanidium chilense (Cyanidiophyceae, Rhodophyta) from tuff rocks of the archeological site of Cuma, Italy. Phycol. Res. 2019, 67, 311–319. [Google Scholar] [CrossRef]
- Eren, A.; Iovinella, M.; Yoon, H.S.; Cennamo, P.; de Stefano, M.; de Castro, O.; Ciniglia, C. Genetic structure of Galdieria populations from Iceland. Polar Biol. 2018, 41, 1681–1691. [Google Scholar] [CrossRef]
- Ciniglia, C.; Yoon, H.S.; Pollio, A.; Pinto, G.; Bhattacharya, D. Hidden biodiversity of the extremophilic Cyanidiales red algae. Mol. Ecol. 2004, 13, 1827–1838. [Google Scholar] [CrossRef]
- Iovinella, M.; Carbone, D.A.; Diana, C.; Seth, J.D.; Michele, I.; Esposito, S.; Ciniglia, C. Prevalent pH Controls the Capacity of Galdieria maxima to Use Ammonia and Nitrate as a Nitrogen Source. Plants 2020, 9, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salbitani, G.; Cipolletta, S.; Vona, V.; Di Martino, C.; Carfagna, S. Heterotrophic Cultures of Galdieria phlegrea Shift to Autotrophy in the Presence or Absence of Glycerol. J. Plant. Growth Regul. 2020, 1–8. [Google Scholar] [CrossRef]
- Minoda, A.; Sawada, H.; Suzuki, S.; Miyashita, S.; Inagaki, K.; Yamamoto, T.; Tsuzuki, M. Recovery of rare earth elements from the sulfothermophilic red alga Galdieria sulphuraria using aqueous acid. Appl. Microbiol. Biotechnol. 2015, 99, 1513–1519. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pegallapati, A.K.; Reddy, H.; Kanapathipillai, N.; Nirmalakhandan, N.; Deng, S.; Lammers, P.J. Algal biofuels from urban wastewaters: Maximizing biomass yield using nutrients recycled from hydrothermal processing of biomass. Bioresour. Technol. 2015, 182, 232–238. [Google Scholar] [CrossRef] [Green Version]
- Tchinda, D.; Henkanatte-Gedera, S.M.; Abeysiriwardana-Arachchige, I.S.A.; Delanka-Pedige, H.M.K.; Munasinghe-Arachchige, S.P.; Zhang, Y.; Nirmalakhandan, N. Single-step treatment of primary effluent by Galdieria sulphuraria: Removal of biochemical oxygen demand, nutrients, and pathogens. Algal Res. 2019, 42, 101578. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Reddy, H.; Muppaneni, T.; Holguin, F.O.; Nirmalakhandan, N.; Lammers, P.J.; Deng, S. Optimizing energy yields from nutrient recycling using sequential hydrothermal liquefaction with Galdieria sulphuraria. Algal Res. 2015, 12, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Henkanatte-Gedera, S.M.; Selvaratnam, T.; Karbakhshravari, M.; Myint, M.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Removal of dissolved organic carbon and nutrients from urban wastewaters by Galdieria sulphuraria: Laboratory to field scale demonstration. Algal Res. 2017, 24, 450–456. [Google Scholar] [CrossRef]
- Cheng, F.; Jarvis, J.M.; Yu, J.; Jena, U.; Nirmalakhandan, N.; Schaub, T.M.; Brewer, C.E. Bio-crude oil from hydrothermal liquefaction of wastewater microalgae in a pilot-scale continuous flow reactor. Bioresour. Technol. 2019, 294, 122184. [Google Scholar] [CrossRef]
- Li, Y.; Slouka, S.A.; Henkanatte-Gedera, S.M.; Nirmalakhandan, N.; Strathmann, T.J. Seasonal treatment and economic evaluation of an algal wastewater system for energy and nutrient recovery. Environ. Sci. Water Res. Technol. 2019, 5, 1545–1557. [Google Scholar] [CrossRef]
- Selvaratnam, T.; Pegallapati, A.K.; Montelya, F.; Rodriguez, G.; Nirmalakhandan, N.; Van Voorhies, W.; Lammers, P.J. Evaluation of a thermo-tolerant acidophilic alga, Galdieria sulphuraria, for nutrient removal from urban wastewaters. Bioresour. Technol. 2014, 156, 395–399. [Google Scholar] [CrossRef]
- Pinto, G.; Ciniglia, C.; Cascone, C.; Pollio, A. Species Composition of Cyanidiales Assemblages in Pisciarelli (Campi Flegrei, Italy) and Description of Galdieria Phlegrea SP. NOV. In Algae and Cyanobacteria in Extreme Environments; Seckbach, J., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 487–502. [Google Scholar] [CrossRef]
- Barcytė, D.; Elster, J.; Nedbalová, L. Plastid-encoded rbcL phylogeny suggests widespread distribution of Galdieria phlegrea (Cyanidiophyceae, Rhodophyta). Nord. J. Bot. 2018, 36, e01794. [Google Scholar] [CrossRef]
- Di Cicco, M.R.; Spagnuolo, A.; Masiello, A.; Vetromile, C.; Nappa, M.; Corbo, G.; Lubritto, C. Energy Monitoring of a Wastewater Treatment Plant in Salerno, Campania Region (Southern Italy). In Frontiers in Water-Energy-Nexus—Nature-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability, Proceedings of the 2nd WaterEnergyNEXUS Conference, 14–17 November 2018, Salerno, Italy; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Di Cicco, M.R.; Spagnuolo, A.; Masiello, A.; Vetromile, C.; Nappa, M.; Corbo, G.; Lubritto, C. Assessing energy performance and critical issues of a large wastewater treatment plant through full-scale data Benchmarking. Water Sci. Technol. 2019, 80, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Di Cicco, M.R.; Spagnuolo, A.; Masiello, A.; Vetromile, C.; Nappa, M.; Lubritto, C. Energetic and environmental analysis of a wastewater treatment plant through static and dynamic monitoring activities. Int. J. Environ. Sci. Technol. 2020, 17, 4299–4312. [Google Scholar] [CrossRef]
- Allen, M.B. Studies with cyanidium caldarium, an anomalously pigmented chlorophyte. Arch. Mikrobiol. 1959, 32, 270–277. [Google Scholar] [CrossRef] [PubMed]
- LeGresley, M.; McDermott, G. Counting chamber methods for quantitative phytoplankton analysis—haemocytometer, Palmer-Maloney cell and Sedgewick-Rafter cell. UNESCO (IOC Man. Guides) 2010, 55, 25–30. [Google Scholar]
- Hernández-López, J.; Vargas-Albores, F. A microplate technique to quantify nutrients (NO₂−, NO₃−,NH₄⁺ and PO₄3−) in seawater. Aquac. Res. 2003, 34, 1201–1204. [Google Scholar] [CrossRef]
- Silveira, S.T.; Burkert, J.F.; Costa, J.A.; Burkert, C.A.; Kalil, S.J. Optimization of phycocyanin extraction from Spirulina platensis using factorial design. Bioresour. Technol. 2007, 98, 1629–1634. [Google Scholar] [CrossRef] [PubMed]
- Rahman, D.Y.; Sarian, F.D.; van Wijk, A.; Martinez-Garcia, M.; van der Maarel, M. Thermostable phycocyanin from the red microalga Cyanidioschyzon merolae, a new natural blue food colorant. J. Appl. Phycol. 2017, 29, 1233–1239. [Google Scholar] [CrossRef] [Green Version]
- Onay, M.; Sonmez, C.; Oktem, H.A.; Yucel, M. Evaluation of various extraction techniques for efficient lipid recovery from thermo-resistant microalgae, Hindakia, Scenedesmus and Micractinium Species. Am. J. Anal. Chem. 2016, 07, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Scirè-Calabrisotto, C.; Webb, J.M.; Frankel, D.; Ricci, P.; Altieri, S.; Lubritto, C. New evidence for diet and subsistence economy in Early and Middle Bronze Age Cyprus. J. Archaeol. Sci. Rep. 2020, 33, 102518. [Google Scholar] [CrossRef]
- Tiwari, M.; Singh, A.K.; Sinha, D.K. Chapter 3–Stable Isotopes: Tools for Understanding Past Climatic Conditions and Their Applications in Chemostratigraphy. In Chemostratigraphy; Ramkumar, M., Ed.; Elsevier: Oxford, UK, 2015; pp. 65–92. [Google Scholar] [CrossRef]
- Kounaves, S.P.; Oberlin, E.A. Chapter 9–Volatiles Measured by the Phoenix Lander at the Northern Plains of Mars. In Volatiles in the Martian Crust; Filiberto, J., Schwenzer, S.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 265–283. [Google Scholar] [CrossRef]
- Condie, K.C. 7–Living Systems. In Earth as an Evolving Planetary System; Condie, K.C., Ed.; Academic Press: Burlington, NJ, USA, 2005; pp. 223–263. [Google Scholar] [CrossRef]
- Coplen, T.B.; Brand, W.A.; Gehre, M.; Gröning, M.; Meijer, H.A.J.; Toman, B.; Verkouteren, R.M. New Guidelines for δ13C Measurements. Anal. Chem. 2006, 78, 2439–2441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohlke, J.K.; Gwinn, C.J.; Coplen, T.B. New reference materials for nitrogen isotope ratio measurements. Geostand. Newsl. 1993, 17, 159–164. [Google Scholar] [CrossRef]
- Fuggi, A.; Di Martino Rigano, V.; Vona, V.; Rigano, C. Nitrate and ammonium assimilation in algal cell-suspensions and related pH variations in the external medium, monitored by electrodes. Plant. Sci. Lett. 1981, 23, 129–138. [Google Scholar] [CrossRef]
- Fuggi, A.; Rigano, V.D.M.; Vona, V.; Rigano, C. Pattern of inhibition of nitrate utilization by ammonium in the acidophilic thermophilic unicellular alga Cyanidium caldarium. Arch. Microbiol. 1981, 130, 349–352. [Google Scholar] [CrossRef]
- Rigano, C.; Di Martino Rigano, V.; Vona, V.; Fuggi, A. Nitrate reductase and glutamine synthetase activities, nitrate and ammonia assimilation, in the unicellular alga Cyanidium caldarium. Arch. Microbiol. 1981, 129, 110–114. [Google Scholar] [CrossRef]
- Woertz, I.; Feffer, A.; Lundquist, T.; Nelson, Y. Algae Grown on Dairy and Municipal Wastewater for Simultaneous Nutrient Removal and Lipid Production for Biofuel Feedstock. J. Environ. Eng. 2009, 135, 1115–1122. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Mennerich, A.; Urban, B. Municipal wastewater treatment and biomass accumulation with a wastewater-born and settleable algal-bacterial culture. Water Res. 2011, 45, 3351–3358. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Min, M.; Zhou, W.; Li, Y.; Mohr, M.; Cheng, Y.; Lei, H.; Liu, Y.; Lin, X.; Chen, P.; et al. Influence of Exogenous CO2 on Biomass and Lipid Accumulation of Microalgae Auxenochlorella protothecoides Cultivated in Concentrated Municipal Wastewater. Appl. Biochem. Biotechnol. 2012, 166, 1661–1673. [Google Scholar] [CrossRef]
- Samorì, G.; Samorì, C.; Guerrini, F.; Pistocchi, R. Growth and nitrogen removal capacity of Desmodesmus communis and of a natural microalgae consortium in a batch culture system in view of urban wastewater treatment: Part I. Water Res. 2013, 47, 791–801. [Google Scholar] [CrossRef]
- Alishah Aratboni, H.; Rafiei, N.; Garcia-Granados, R.; Alemzadeh, A.; Morones-Ramírez, J.R. Biomass and lipid induction strategies in microalgae for biofuel production and other applications. Microb. Cell Factories 2019, 18, 178. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.D.; Li, Z.H.; Hiltunen, E. Strategies for Lipid Production Improvement in Microalgae as a Biodiesel Feedstock. Biomed Res. Int. 2016, 2016, 8792548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graziani, G.; Schiavo, S.; Nicolai, M.A.; Buono, S.; Fogliano, V.; Pinto, G.; Pollio, A. Microalgae as human food: Chemical and nutritional characteristics of the thermo-acidophilic microalga Galdieria sulphuraria. Food Funct. 2013, 4, 144–152. [Google Scholar] [CrossRef] [PubMed]
- López, G.; Yate, C.; Ramos, F.A.; Cala, M.P.; Restrepo, S.; Baena, S. Production of Polyunsaturated Fatty Acids and Lipids from Autotrophic, Mixotrophic and Heterotrophic cultivation of Galdieria sp. strain USBA-GBX-832. Sci. Rep. 2019, 9, 10791. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, T.; Aoki, M.; Ju, X.; Ueda, T.; Nakamura, Y.; Fujiwara, S.; Umemura, T.; Tsuzuki, M.; Minoda, A. Profiling of lipid and glycogen accumulations under different growth conditions in the sulfothermophilic red alga Galdieria sulphuraria. Bioresour. Technol. 2016, 200, 861–866. [Google Scholar] [CrossRef] [Green Version]
- Minoda, A.; Sato, N.; Nozaki, H.; Okada, K.; Takahashi, H.; Sonoike, K.; Tsuzuki, M. Role of sulfoquinovosyl diacylglycerol for the maintenance of photosystem II in Chlamydomonas reinhardtii. Eur. J. Biochem. 2002, 269, 2353–2358. [Google Scholar] [CrossRef]
- Weber, A.P.M.; Horst, R.J.; Barbier, G.G.; Oesterhelt, C. Metabolism and Metabolomics of Eukaryotes Living Under Extreme Conditions. In International Review of Cytology; Academic Press: Cambridge, MA, USA, 2007; Volume 256, pp. 1–34. [Google Scholar]
- Berto, D.; Calace, N.; Rampazzo, F.; Saccomandi, F.; Stellato, L. Isotopi: Dalla teoria alla pratica. Transl. “Isotopes: From theory to practice”; ISPRA–Istituto Superiore per la Protezione e la Ricerca Ambientale: Rome, Italy, 2018.
- Yu, G.; Zhang, Y.; Schideman, L.; Funk, T.; Wang, Z. Distributions of carbon and nitrogen in the products from hydrothermal liquefaction of low-lipid microalgae. Energy Environ. Sci. 2011, 4, 4587–4595. [Google Scholar] [CrossRef]
- Toyoda, S.; Suzuki, Y.; Hattori, S.; Yamada, K.; Fujii, A.; Yoshida, N.; Kouno, R.; Murayama, K.; Shiomi, H. Isotopomer Analysis of Production and Consumption Mechanisms of N2O and CH4 in an Advanced Wastewater Treatment System. Environ. Sci. Technol. 2011, 45, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Oesterhelt, C.; Schmalzlin, E.; Schmitt, J.M.; Lokstein, H. Regulation of photosynthesis in the unicellular acidophilic red alga Galdieria sulphuraria. Plant. J. 2007, 51, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Price, D.C.; Weber, A.P.M.; Reeb, V.; Chan Yang, E.; Lee, J.M.; Kim, S.Y.; Yoon, H.S.; Bhattacharya, D. Adaptation through horizontal gene transfer in the cryptoendolithic red alga Galdieria phlegrea. Curr. Biol. 2013, 23, R865–R866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
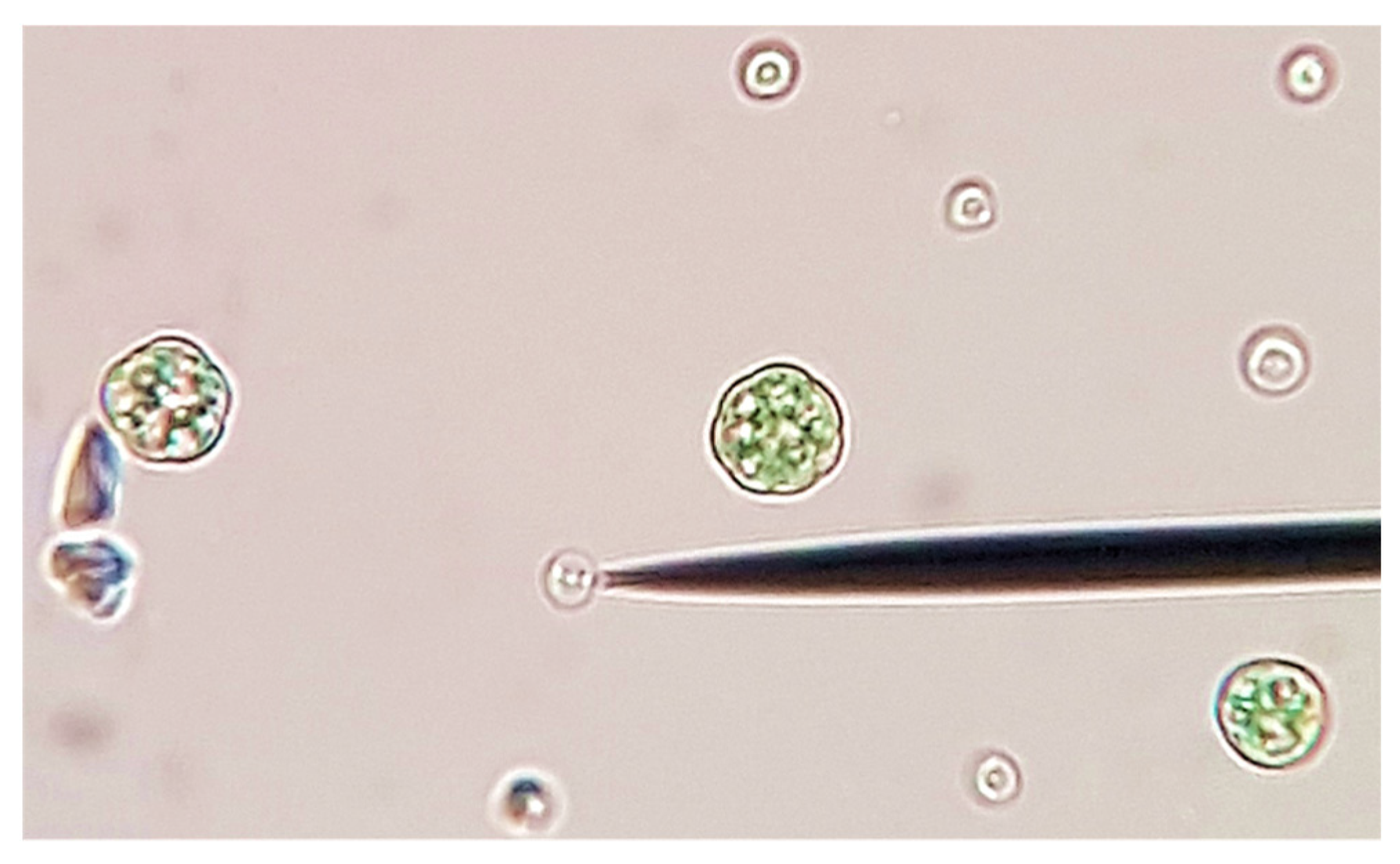

| Sampling Times | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 0.5 h | 3 h | 6 h | 24 h | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | ||
| Monitored parameters | OD750 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||
| Cell counting | ✔ | ✔ | ✔ | ✔ | ||||||||||
| NH4+ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||
| PO43− | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||
| Phycocyanin | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||
| Lipid fraction | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||
| [C]-[N] fraction | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||
| δ13C-δ15N | ✔ | ✔ | ✔ | ✔ | ✔ | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
di Cicco, M.R.; Palmieri, M.; Altieri, S.; Ciniglia, C.; Lubritto, C. Cultivation of the Acidophilic Microalgae Galdieria phlegrea with Wastewater: Process Yields. Int. J. Environ. Res. Public Health 2021, 18, 2291. https://doi.org/10.3390/ijerph18052291
di Cicco MR, Palmieri M, Altieri S, Ciniglia C, Lubritto C. Cultivation of the Acidophilic Microalgae Galdieria phlegrea with Wastewater: Process Yields. International Journal of Environmental Research and Public Health. 2021; 18(5):2291. https://doi.org/10.3390/ijerph18052291
Chicago/Turabian Styledi Cicco, Maria Rosa, Maria Palmieri, Simona Altieri, Claudia Ciniglia, and Carmine Lubritto. 2021. "Cultivation of the Acidophilic Microalgae Galdieria phlegrea with Wastewater: Process Yields" International Journal of Environmental Research and Public Health 18, no. 5: 2291. https://doi.org/10.3390/ijerph18052291







