Imaging the Functional Neuroanatomy of Parkinson’s Disease: Clinical Applications and Future Directions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Structural Magnetic Resonance Imaging (sMRI)
2.2. Single Photon Emission Computed Tomography (SPECT) and Positron Emission Tomography (PET)
2.3. Functional Magnetic Risonance Imaging (fMRI)
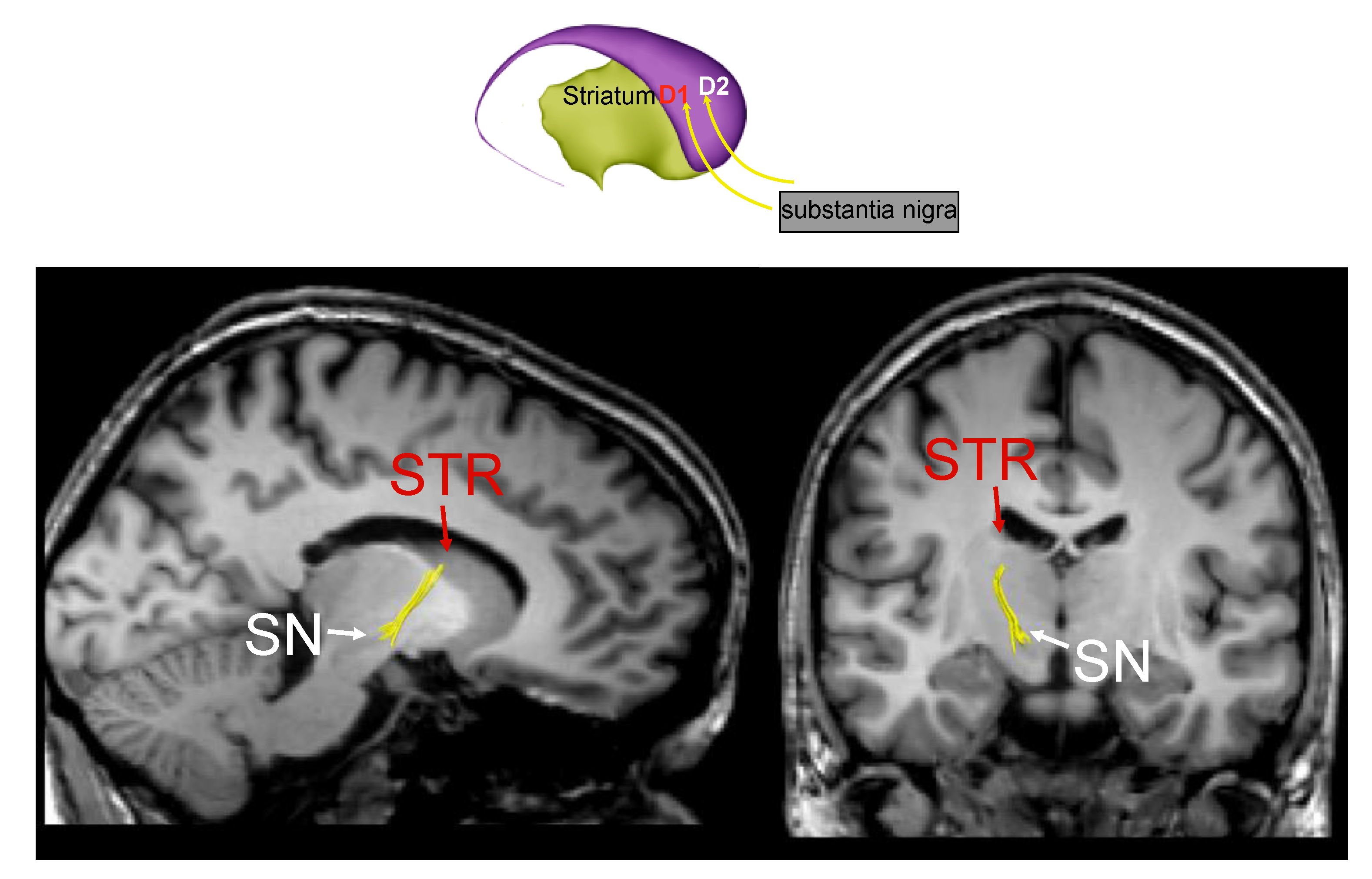
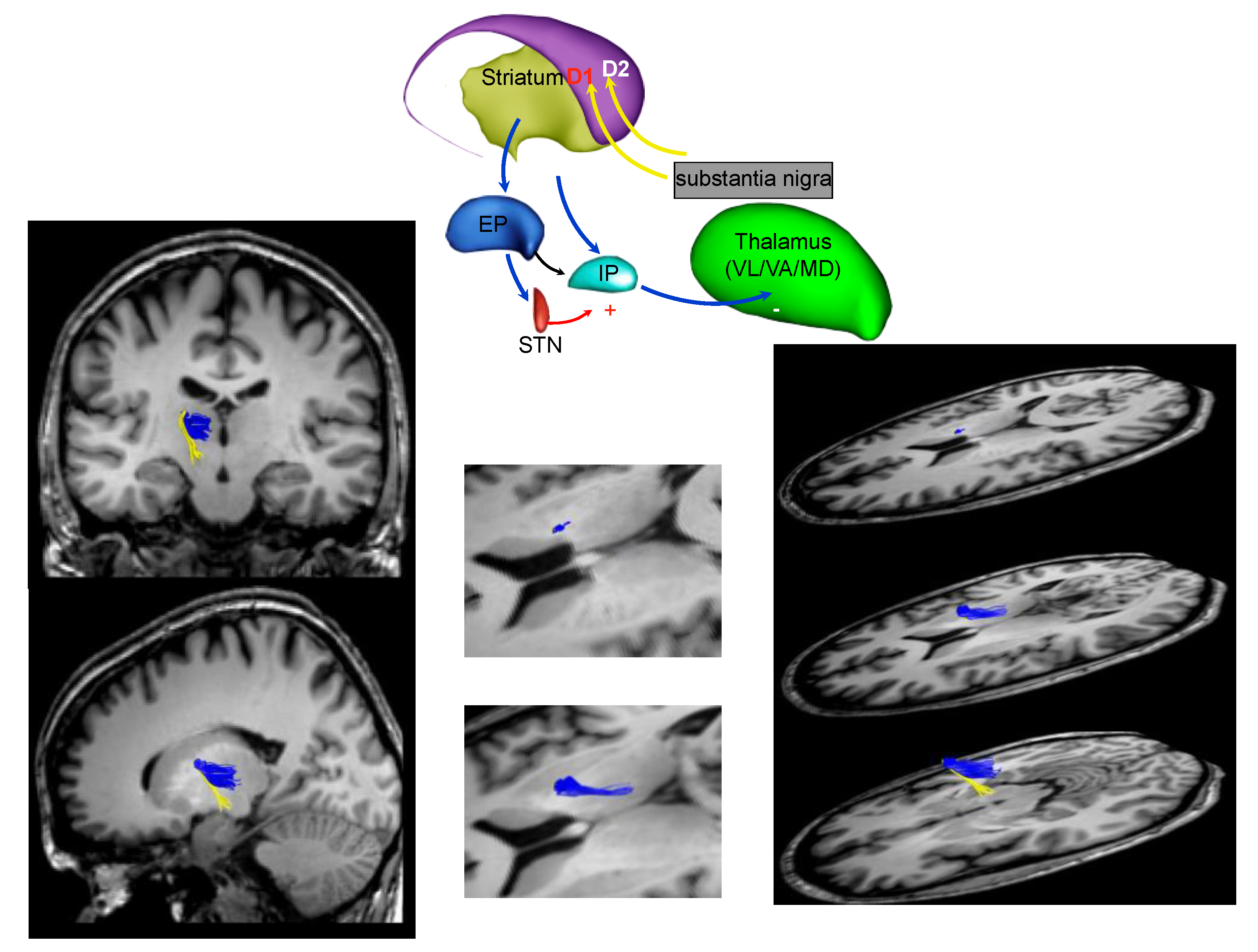
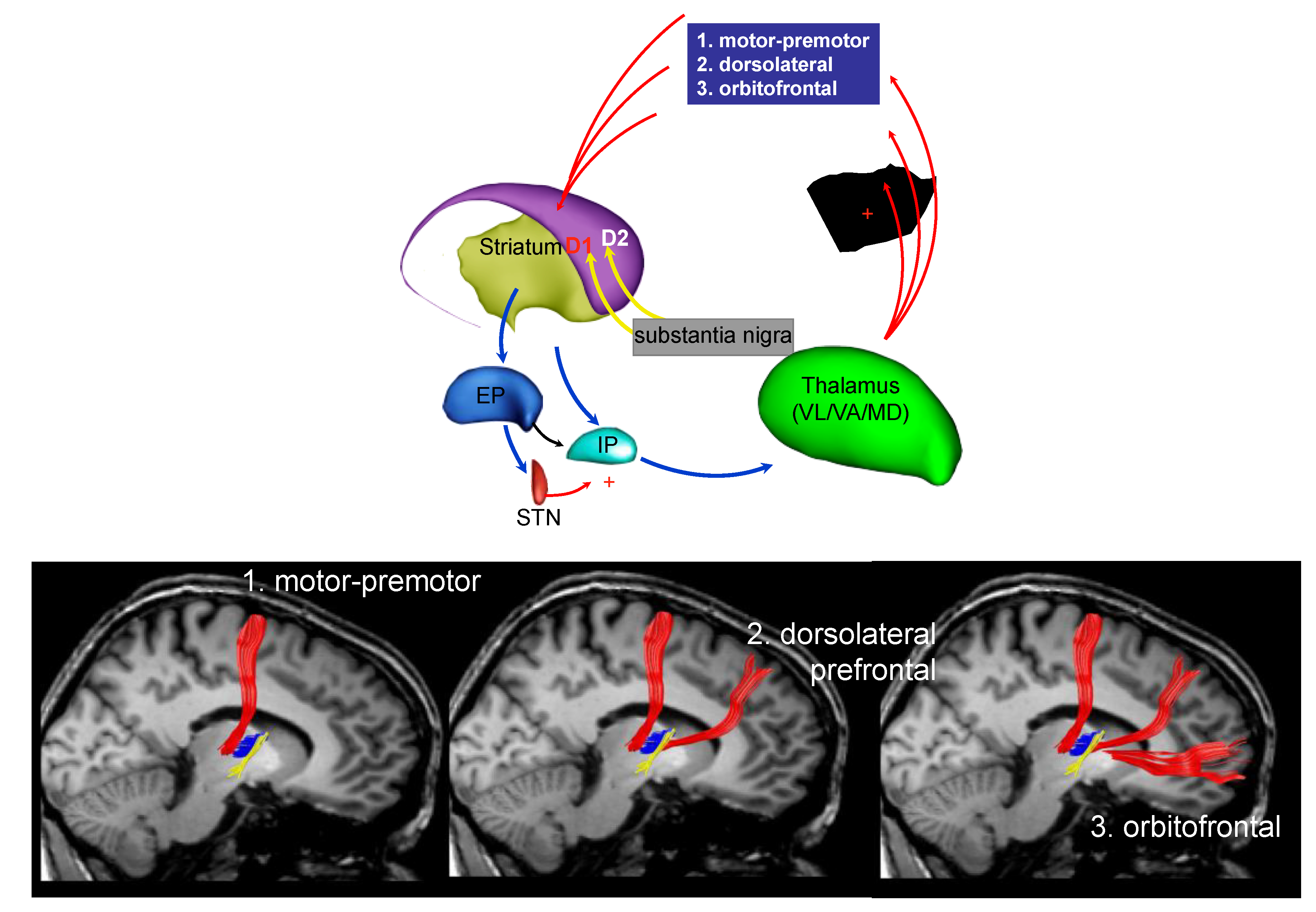
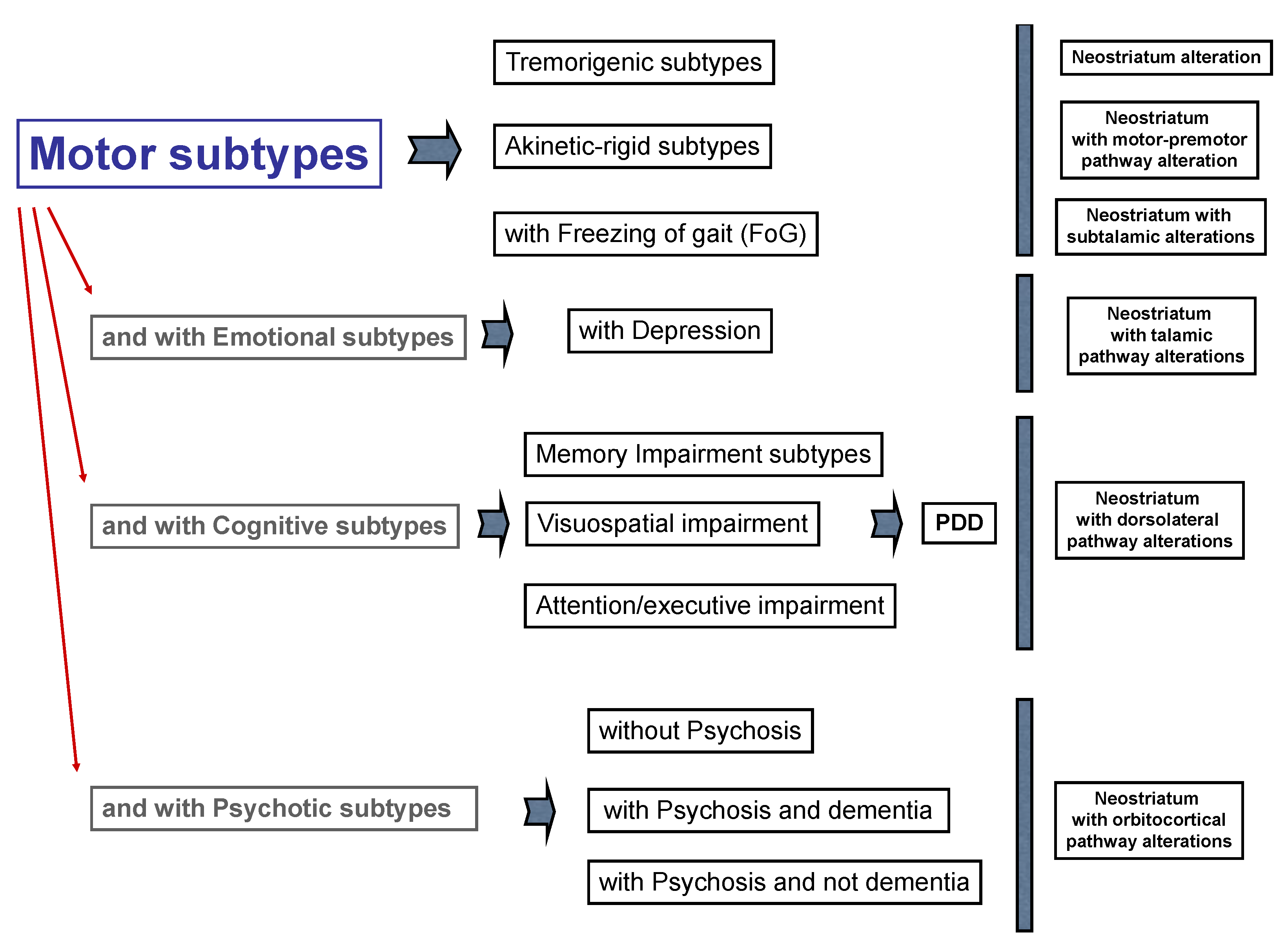
3. Parkinson’s Disease Subtypes Identified by Tractography
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.; Jansen Steur, E.N.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Lauretani, F.; Maggio, M.; Silvestrini, C.; Nardelli, A.; Saccavini, M.; Ceda, G.P. Parkinson’s disease (PD) in the elderly: An example of geriatric syndrome (GS)? Arch. Gerontol. Geriatr. 2012, 54, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Steinberger, J.D.; Warren Olanow, C.; Naidich, T.P.; Yousry, T.A. Milestones in magnetic resonance imaging and transcranial sonography of movement disorders. Mov. Disord. 2011, 26, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Saeed, U.; Lang, A.E.; Masellis, M. Neuroimaging Advances in Parkinson’s Disease and Atypical Parkinsonian Syndromes. Front. Neurol. 2020, 11, 572976. [Google Scholar] [CrossRef] [PubMed]
- Grubić Kezele, T.; Ćurko-Cofek, B. Age-Related Changes and Sex-Related Differences in Brain Iron Metabolism. Nutrients 2020, 12, 2601. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Ruffini, L.; Scarlattei, M.; Maggio, M. Relationship between comprehensive geriatric assessment and amyloid PET in older persons with MCI. BMC Geriatr. 2020, 20, 337. [Google Scholar] [CrossRef]
- Kwon, D.H.; Kim, J.M.; Oh, S.H.; Jeong, H.J.; Park, S.Y.; Oh, E.S.; Chi, J.G.; Kim, Y.B.; Jeon, B.S.; Cho, Z.H. Seven-Tesla magnetic resonance images of the substantia nigra in Parkinson disease. Ann. Neurol. 2012, 71, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Cho, Z.H.; Oh, S.H.; Kim, J.M.; Park, S.Y.; Kwon, D.H.; Jeong, H.J.; Kim, Y.B.; Chi, J.G.; Park, C.W.; Huston, J., 3rd; et al. Direct visualization of Parkinson’s disease by in vivo human brain imaging using 7.0T magnetic resonance imaging. Mov. Disord. 2011, 26, 713–718. [Google Scholar] [CrossRef]
- Sofic, E.; Paulus, W.; Jellinger, K.; Riederer, P.; Youdim, M.B. Selective increase of iron in substantia nigra zona compacta of parkinsonian brains. J. Neurochem. 1991, 56, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Fedeli, M.P.; Contarino, V.E.; Siggillino, S.; Samoylova, N.; Calloni, S.; Melazzini, L.; Conte, G.; Sacilotto, G.; Pezzoli, G.; Triulzi, F.M.; et al. Iron deposition in Parkinsonisms: A Quantitative Susceptibility Mapping study in the deep grey matter. Eur. J. Radiol. 2020, 133, 109394. [Google Scholar] [CrossRef]
- Nolze-Charron, G.; Mouiha, A.; Duchesne, S.; Bocti, C. Alzheimer’s Disease Neuroimaging Initiative1. White Matter Hyperintensities in Mild Cognitive Impairment and Lower Risk of Cognitive Decline. J. Alzheimers Dis. 2015, 46, 855–862. [Google Scholar] [CrossRef]
- Lauretani, F.; Caffarra, P.; Ruffini, L.; Nardelli, A.; Ceda, G.P.; Maggio, M.; Scaglioni, A. Brief practical clinical diagnostic criteria for the neurodegenerative diseases in the elderly. Drugs Ther. Stud. 2011, 1, e6. [Google Scholar] [CrossRef] [Green Version]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [Green Version]
- Lees, A.J. The Parkinson chimera. Neurology 2009, 7, S2–S11. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Ben-Shlomo, Y.; Lees, A.J. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain 2002, 125, 861–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhawan, V.; Ishikawa, T.; Patlak, C.; Chaly, T.; Robeson, W.; Belakhlef, A.; Margouleff, C.; Mandel, F.; Eidelberg, D. Combined FDOPA and 3OMFD PET studies in Parkinson’s disease. J. Nucl. Med. 1996, 37, 209–216. [Google Scholar] [PubMed]
- Kägi, G.; Bhatia, K.P.; Tolosa, E. The role of DAT-SPECT in movement disorders. J. Neurol. Neurosurg. Psychiatry 2010, 81, 5–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajaj, N.; Hauser, R.A.; Grachev, I.D. Clinical utility of dopamine transporter single photon emission CT (DaT-SPECT) with (123I) ioflupane in diagnosis of parkinsonian syndromes. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1288–1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherfler, C.; Schwarz, J.; Antonini, A.; Grosset, D.; Valldeoriola, F.; Marek, K.; Oertel, W.; Tolosa, E.; Lees, A.J.; Poewe, W. Role of DAT-SPECT in the diagnostic work up of parkinsonism. Mov. Disord. 2007, 22, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Ruffini, L.; Scaglioni, A.; Cicca, C.; Guareschi, C.; Nardelli, A.; Ceda, G.P.; Maggio, M. Utilization of the DaT-SCAN SPECT in the Diagnosis of Parkinsons Disease in Older Subjects. Lett. Des. Discov. 2015, 12, 614–621. [Google Scholar] [CrossRef]
- Benamer, H.T.; Patterson, J.; Wyper, D.J.; Hadley, D.M.; Macphee, G.J.; Grosset, D.G. Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov. Disord. 2000, 15, 692–698. [Google Scholar] [CrossRef]
- Saeed, U.; Compagnone, J.; Aviv, R.I.; Strafella, A.P.; Black, S.E.; Lang, A.E.; Masellis, M. Imaging biomarkers in Parkinson’s disease and Parkinsonian syndromes: Current and emerging concepts. Transl. Neurodegener. 2017, 6, 8. [Google Scholar] [CrossRef] [Green Version]
- Zijlmans, J.C. The role of imaging in the diagnosis of vascular parkinsonism. Neuroimaging Clin. N. Am. 2010, 20, 69–76. [Google Scholar] [CrossRef]
- Kalra, S.; Grosset, D.G.; Benamer, H.T. Differentiating vascular parkinsonism from idiopathic Parkinson’s disease: A systematic review. Mov. Disord. 2010, 23, 149–156. [Google Scholar] [CrossRef]
- Zijlmans, J.C.; Daniel, S.E.; Hughes, A.J.; Révész, T.; Lees, A.J. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov. Disord. 2004, 19, 630–640. [Google Scholar] [CrossRef]
- Foster, N.L.; Heidebrink, J.L.; Clark, C.M.; Jagust, W.J.; Arnold, S.E.; Barbas, N.R.; DeCarli, C.S.; Turner, R.S.; Koeppe, R.A.; Higdon, R.; et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain 2007, 130 Pt 10, 2616–2635. [Google Scholar] [CrossRef]
- Morrish, P.K.; Sawle, G.V.; Brooks, D.J. Clinical and [18F] dopa PET findings in early Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1995, 59, 597–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohnen, N.I.; Kaufer, D.I.; Ivanco, L.S.; Lopresti, B.; Koeppe, R.A.; Davis, J.G.; Mathis, C.A.; Moore, R.Y.; DeKosky, S.T. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: An in vivo positron emission tomographic study. Arch. Neurol. 2003, 60, 1745–1748. [Google Scholar] [CrossRef] [Green Version]
- Lauretani, F.; Galuppo, L.; Costantino, C.; Ticinesi, A.; Ceda, G.; Ruffini, L.; Nardelli, A.; Maggio, M. Parkinson’s disease (PD) with dementia and falls is improved by AChEI? A preliminary study report. Aging Clin. Exp. Res. 2016, 28, 551–555. [Google Scholar] [CrossRef]
- Goedert, M. NEURODEGENERATION. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science 2015, 349, 1255555. [Google Scholar] [CrossRef] [PubMed]
- Donaghy, P.C.; Firbank, M.; Petrides, G.; Lloyd, J.; Barnett, N.; Olsen, K.; Thomas, A.J.; O’Brien, J.T. Diffusion imaging in dementia with Lewy bodies: Associations with amyloid burden, atrophy, vascular factors and clinical features. Parkinsonism Relat. Disord. 2020, 78, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Parmera, J.B.; Coutinho, A.M.; Aranha, M.R.; Studart-Neto, A.; de Godoi Carneiro, C.; de Almeida, I.J.; Fontoura Solla, D.J.; Ono, C.R.; Barbosa, E.R.; Nitrini, R.; et al. FDG-PET Patterns Predict Amyloid Deposition and Clinical Profile in Corticobasal Syndrome. Mov. Disord. 2020. [Google Scholar] [CrossRef]
- Li, K.; Su, W.; Li, S.H.; Jin, Y.; Chen, H.B. Resting State fMRI: A Valuable Tool for Studying Cognitive Dysfunction in PD. Parkinson’s Dis. 2018, 2018, 6278649. [Google Scholar] [CrossRef] [Green Version]
- Azeez, A.K.; Biswal, B.B. A Review of Resting-State Analysis Methods. Neuroimaging Clin. N. Am. 2017, 27, 581–592. [Google Scholar] [CrossRef]
- Tahmasian, M.; Bettray, L.M.; van Eimeren, T.; Drzezga, A.; Timmermann, L.; Eickhoff, C.R.; Eickhoff, S.B.; Eggers, C. A systematic review on the applications of resting-state fMRI in Parkinson’s disease: Does dopamine replacement therapy play a role? Cortex 2015, 73, 80–105. [Google Scholar] [CrossRef]
- Lopes, R.; Delmaire, C.; Defebvre, L.; Moonen, A.J.; Duits, A.A.; Hofman, P.; Leentjens, A.F.; Dujardin, K. Cognitive phenotypes in parkinson’s disease differ in terms of brain-network organization and connectivity. Hum. Brain Mapp. 2017, 38, 1604–1621. [Google Scholar] [CrossRef] [PubMed]
- Olde Dubbelink, K.T.; Schoonheim, M.M.; Deijen, J.B.; Twisk, J.W.; Barkhof, F.; Berendse, H.W. Functional connectivity and cognitive decline over 3 years in Parkinson disease. Neurology 2014, 83, 2046–2053. [Google Scholar] [CrossRef]
- Filippi, M.; Basaia, S.; Sarasso, E.; Stojkovic, T.; Stankovic, I.; Fontana, A.; Tomic, A.; Piramide, N.; Stefanova, E.; Markovic, V.; et al. Longitudinal brain connectivity changes and clinical evolution in Parkinson’s disease. Mol. Psychiatry 2020. [Google Scholar] [CrossRef] [PubMed]
- Rubinovi, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Li, D.C.; Malcolm, J.G.; Rindler, R.S.; Baum, G.R.; Rao, A.; Khurpad, S.N.; Ahmad, F.U. The role of diffusion tensor imaging in spinal pathology: A review. Neurol. India 2017, 65, 982–992. [Google Scholar]
- Conturo, T.E.; Lori, N.F.; Cull, T.S.; Akbudak, E.; Snyder, A.Z.; Shimony, J.S.; McKinstry, R.C.; Burton, H.; Raichle, M.E. Tracking neuronal fiber pathways in the living human brain. Proc. Natl. Acad. Sci. USA 1999, 96, 10422–10427. [Google Scholar] [CrossRef] [Green Version]
- Sandrone, S.; Catani, M. Journal Club. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology 2013, 81, e172–e175. [Google Scholar] [CrossRef]
- Catani, M.; Thiebaut de Schotten, M. A diffusion tensor atlas for virtual in vivo dissections. Cortex 2008, 44, 1105–1132. [Google Scholar] [CrossRef]
- Lauretani, F.; Saginario, A.; Ceda, G.P.; Galuppo, L.; Ruffini, L.; Nardelli, A.; Maggio, M. Treatment of the motor and non-motor symptoms in Parkinson’s disease according to cluster symptoms presentation. Curr. Drug Targets 2014, 15, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s Disease: Biomarkers, Treatment, and Risk Factors. Front. Neurosci. 2018, 12, 612. [Google Scholar] [CrossRef]
- Erro, R.; Vitale, C.; Amboni, M.; Picillo, M.; Moccia, M.; Longo, K.; Santangelo, G.; De Rosa, A.; Allocca, R.; Giordano, F.; et al. The heterogeneity of early Parkinson’s disease: A cluster analysis on newly diagnosed untreated patients. PLoS ONE 2013, 8, e70244. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.J.; Glenmullen, J.; Mattison, D.R. Reports of pathological gambling, hypersexuality, and compulsive shopping associated with dopamine receptor agonist drugs. JAMA Intern. Med. 2014, 174, 1930–1933. [Google Scholar] [CrossRef] [Green Version]
- Berardelli, I.; Belvisi, D.; Pasquini, M.; Fabbrini, A.; Petrini, F.; Fabbrini, G. Treatment of psychiatric disturbances in hypokinetic movement disorders. Expert Rev. Neurother. 2019, 19, 965–981. [Google Scholar] [CrossRef]
- Voelker, R. Parkinson disease guidelines aid diagnosis, management. JAMA 2006, 295, 2126–2128. [Google Scholar] [CrossRef]
- Olanow, C.W.; Stocchi, F. Levodopa: A new look at an old friend. Mov. Disord. 2018, 33, 859–866. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Balkrishnan, R.; Anderson, R.T.; Edin, H.M.; Kirsch, J.; Stacy, M.A. Medication adherence and associated outcomes in medicare health maintenance organization-enrolled older adults with Parkinson’s disease. Mov. Disord. 2008, 23, 359–365. [Google Scholar] [CrossRef]
- Shin, J.Y.; Habermann, B. Initiation of medications for Parkinson’s disease: A qualitative description. J. Clin. Nurs. 2016, 25, 127–133. [Google Scholar] [CrossRef]
- Patel, K. Optimising medication for Parkinson’s disease patients with dysphagia. Br. J. Community Nurs. 2015, 20, 322–326. [Google Scholar] [CrossRef]
- Lennaerts, H.; Groot, M.; Rood, B.; Gilissen, K.; Tulp, H.; van Wensen, E.; Munneke, M.; van Laar, T.; Bloem, B.R. A Guideline for Parkinson’s Disease Nurse Specialists, with Recommendations for Clinical Practice. J. Parkinsons Dis. 2017, 7, 749–754. [Google Scholar] [CrossRef]
- Radder, D.L.M.; Lennaerts, H.H.; Vermeulen, H.; van Asseldonk, T.; Delnooz, C.C.S.; Hagen, R.H.; Munneke, M.; Bloem, B.R.; de Vries, N.M. The cost-effectiveness of specialized nursing interventions for people with Parkinson’s disease: The NICE-PD study protocol for a randomized controlled clinical trial. Trials 2020, 21, 88. [Google Scholar] [CrossRef]
- Pretzer-Aboff, I.; Galik, E.; Resnick, B. Feasibility and impact of a function focused care intervention for Parkinson’s disease in the community. Nurs. Res. 2011, 60, 276–283. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Lin, W.C.; Chang, W.N.; Lin, T.K.; Tsai, N.W.; Huang, C.C.; Wang, H.C.; Huang, Y.C.; Chang, H.W.; Lin, Y.J.; et al. Factors associated with fall-related fractures in Parkinson’s disease. Parkinsonism Relat. Disord. 2014, 20, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Bellelli, G.; Pelà, G.; Morganti, S.; Tagliaferri, S.; Maggio, M. Treatment of Delirium in Older Persons: What We Should Not Do! Int. J. Mol. Sci. 2020, 21, 2397. [Google Scholar] [CrossRef] [Green Version]
- Belvisi, D.; Fabbrini, A.; De Bartolo, M.I.; Costanzo, M.; Manzo, N.; Fabbrini, G.; Defazio, G.; Conte, A.; Berardelli, A. The Pathophysiological Correlates of Parkinson’s Disease Clinical Subtypes. Mov. Disord. 2021, 36, 370–379. [Google Scholar] [CrossRef]
- Belvisi, D.; Pellicciari, R.; Fabbrini, A.; Costanzo, M.; Pietracupa, S.; De Lucia, M.; Modugno, N.; Magrinelli, F.; Dallocchio, C.; Ercoli, T.; et al. Risk factors of Parkinson disease: Simultaneous assessment, interactions, and etiologic subtypes. Neurology 2020, 95, e2500–e2508. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauretani, F.; Longobucco, Y.; Ravazzoni, G.; Gallini, E.; Salvi, M.; Maggio, M. Imaging the Functional Neuroanatomy of Parkinson’s Disease: Clinical Applications and Future Directions. Int. J. Environ. Res. Public Health 2021, 18, 2356. https://doi.org/10.3390/ijerph18052356
Lauretani F, Longobucco Y, Ravazzoni G, Gallini E, Salvi M, Maggio M. Imaging the Functional Neuroanatomy of Parkinson’s Disease: Clinical Applications and Future Directions. International Journal of Environmental Research and Public Health. 2021; 18(5):2356. https://doi.org/10.3390/ijerph18052356
Chicago/Turabian StyleLauretani, Fulvio, Yari Longobucco, Giulia Ravazzoni, Elena Gallini, Marco Salvi, and Marcello Maggio. 2021. "Imaging the Functional Neuroanatomy of Parkinson’s Disease: Clinical Applications and Future Directions" International Journal of Environmental Research and Public Health 18, no. 5: 2356. https://doi.org/10.3390/ijerph18052356




