The Association of Bisphenol A and Phthalates with Risk of Breast Cancer: A Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Literature Search and Selection
3.2. Characteristics of Included Studies
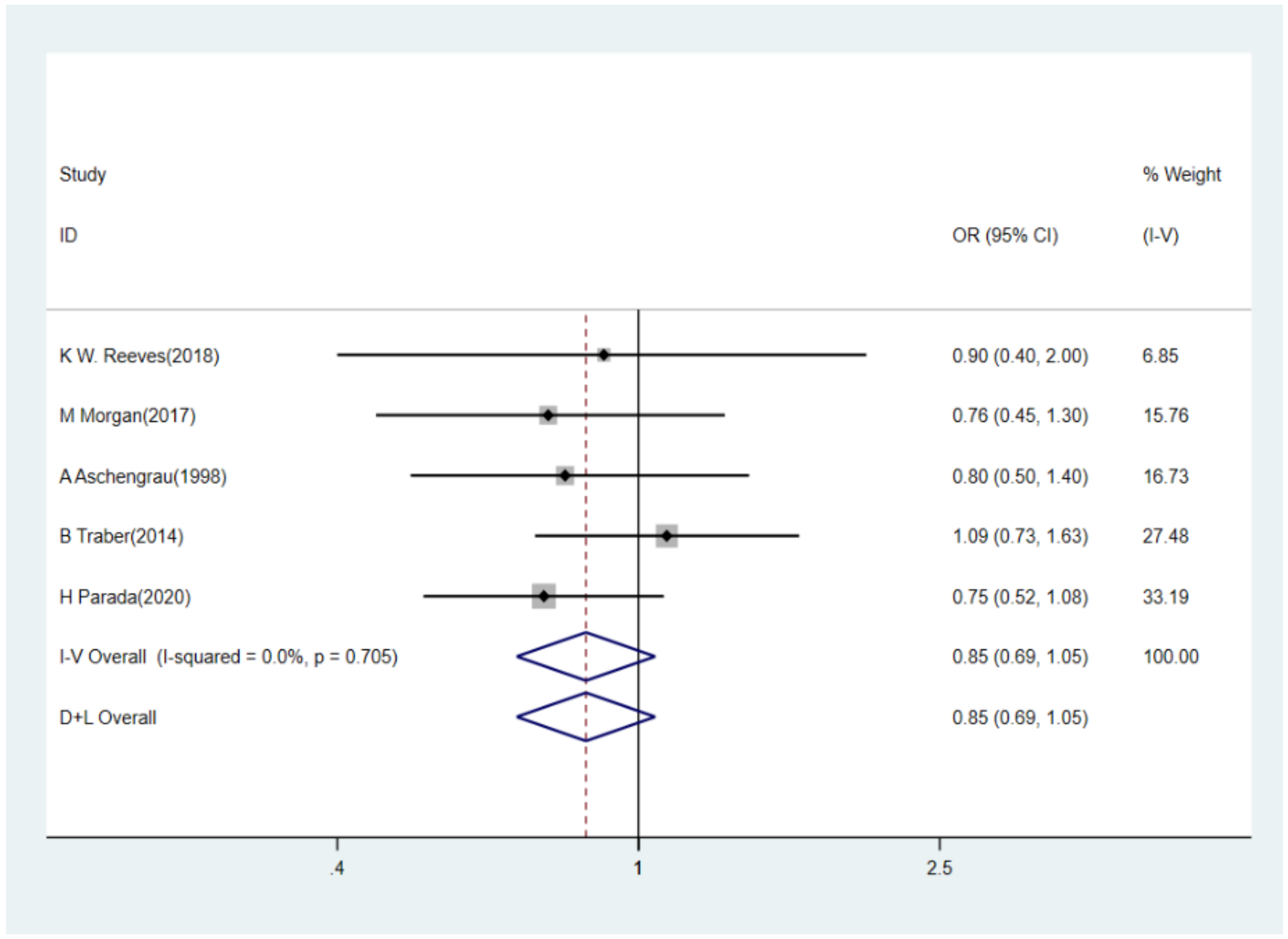
3.3. BPA Levels and Risk of Breast Cancer
3.4. Urinary Phthalate Metabolite Levels and Risk of Breast Cancer
3.5. Sensitivity Analyses
3.6. Subgroup Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statements
Conflicts of Interest
References
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Sifakis, S.; Androutsopoulos, V.P.; Tsatsakis, A.M.; Spandidos, D.A. Human exposure to endocrine disrupting chemicals: Effects on the male and female reproductive systems. Environ. Toxicol. Phar. 2017, 51, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Marie, C.; Vendittelli, F.; Sauvant-Rochat, M.P. Obstetrical outcomes and biomarkers to assess exposure to phthalates: A review. Environ. Int. 2015, 83, 116–136. [Google Scholar] [CrossRef]
- Cheng, B.J.; Xu, P.R.; Wei, R.; Li, X.D.; Sheng, J.; Wang, S.F.; Liu, K.Y.; Chen, G.M.; Tao, F.B.; Wang, Q.N.; et al. Levels and determinants of urinary phthalate metabolites in Chinese community-dwelling older adults. Sci. Total Environ. 2021, 762, 144173. [Google Scholar] [CrossRef] [PubMed]
- Talsness, C.E.; Andrade, A.J.; Kuriyama, S.N.; Taylor, J.A.; Vom, S.F. Components of plastic: Experimental studies in animals and relevance for human health. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2079–2096. [Google Scholar] [CrossRef]
- Hannon, P.R.; Flaws, J.A. The effects of phthalates on the ovary. Front. Endocrinol. 2015, 6, 8. [Google Scholar] [CrossRef]
- Rocha, B.A.; Asimakopoulos, A.G.; Barbosa, F.; Kannan, K. Urinary concentrations of 25 phthalate metabolites in Brazilian children and their association with oxidative DNA damage. Sci. Total Environ. 2017, 586, 152–162. [Google Scholar] [CrossRef]
- Hernandez-Diaz, S.; Mitchell, A.A.; Kelley, K.E.; Calafat, A.M.; Hauser, R. Medications as a potential source of exposure to phthalates in the U.S. population. Environ. Health Perspect. 2009, 117, 185–189. [Google Scholar] [CrossRef]
- Kelley, K.E.; Hernandez-Diaz, S.; Chaplin, E.L.; Hauser, R.; Mitchell, A.A. Identification of phthalates in medications and dietary supplement formulations in the United States and Canada. Environ. Health Perspect. 2012, 120, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Dodson, R.E.; Nishioka, M.; Standley, L.J.; Perovich, L.J.; Brody, J.G.; Rudel, R.A. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ. Health Perspect. 2012, 120, 935–943. [Google Scholar] [CrossRef]
- Schecter, A.; Lorber, M.; Guo, Y.; Wu, Q.; Yun, S.H.; Kannan, K.; Hommel, M.; Imran, N.; Hynan, L.S.; Cheng, D.; et al. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ. Health Perspect. 2013, 121, 473–479. [Google Scholar] [CrossRef]
- Guo, Y.; Kannan, K. Comparative Assessment of Human Exposure to Phthalate Esters from House Dust in China and the United States. Environ. Sci. Technol. 2011, 45, 3788–3794. [Google Scholar] [CrossRef]
- Rudel, R.A.; Camann, D.E.; Spengler, J.D.; Korn, L.R.; Brody, J.G. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ. Sci. Technol. 2003, 37, 4543–4553. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.M.; Kannan, K. Occurrence of phthalate diesters in particulate and vapor phases in indoor air and implications for human exposure in Albany, New York, USA. Arch. Environ. Contam. Toxicol. 2015, 68, 489–499. [Google Scholar] [CrossRef]
- Martínez-Ibarra, A.; Martínez-Razo, L.D.; MacDonald-Ramos, K.; Morales-Pacheco, M.; Vázquez-Martínez, E.R.; López-López, M.; Rodríguez Dorantes, M.; Cerbón, M. Multisystemic alterations in humans induced by bisphenol A and phthalates: Experimental, epidemiological and clinical studies reveal the need to change health policies. Environ. Pollut. 2021, 271, 116380. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, S.; Jeong, M.; Choi, J.C.; Kim, M. Fast and simple determination and exposure assessment of bisphenol A, phenol, p-tert-butylphenol, and diphenylcarbonate transferred from polycarbonate food-contact materials to food simulants. Chemosphere 2018, 203, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Mendoza, M.; Gómez De León, C.T.; García-Becerra, R.; Ambrosio, J.; Nava-Castro, K.E.; Morales-Montor, J. The chemical environmental pollutants BPA and BPS induce alterations of the proteomic profile of different phenotypes of human breast cancer cells: A proposed interactome. Environ. Res. 2020, 191, 109960. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Yolton, K.; Dietrich, K.N.; Hornung, R.; Ye, X.; Calafat, A.M.; Lanphear, B.P. Prenatal bisphenol A exposure and early childhood behavior. Environ. Health Perspect. 2009, 117, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sarma, D.K.; Shubham, S.; Kumawat, M.; Verma, V.; Prakash, A.; Tiwari, R. Environmental Endocrine-Disrupting Chemical Exposure: Role in Non-Communicable Diseases. Front. Public Health 2020, 8, 553850. [Google Scholar] [CrossRef]
- Kwon, J.A.; Shin, B.; Kim, B. Urinary bisphenol A and thyroid function by BMI in the Korean National Environmental Health Survey (KoNEHS) 2012–2014. Chemosphere 2020, 240, 124918. [Google Scholar] [CrossRef]
- Park, C.; Choi, W.; Hwang, M.; Lee, Y.; Kim, S.; Yu, S.; Lee, I.; Paek, D.; Choi, K. Associations between urinary phthalate metabolites and bisphenol A levels, and serum thyroid hormones among the Korean adult population—Korean National Environmental Health Survey (KoNEHS) 2012–2014. Sci. Total Environ. 2017, 584–585, 950–957. [Google Scholar] [CrossRef]
- Srilanchakon, K.; Thadsri, T.; Jantarat, C.; Thengyai, S.; Nosoognoen, W.; Supornsilchai, V. Higher phthalate concentrations are associated with precocious puberty in normal weight Thai girls. J. Pediatr. Endocrinol. Metab. 2017, 30, 1293–1298. [Google Scholar] [CrossRef]
- Supornsilchai, V.; Jantarat, C.; Nosoognoen, W.; Pornkunwilai, S.; Wacharasindhu, S.; Soder, O. Increased levels of bisphenol A (BPA) in Thai girls with precocious puberty. J. Pediatr. Endocrinol. Metab. 2016, 29, 1233–1239. [Google Scholar] [CrossRef]
- Kandaraki, E.; Chatzigeorgiou, A.; Livadas, S.; Palioura, E.; Economou, F.; Koutsilieris, M.; Palimeri, S.; Panidis, D.; Diamanti-Kandarakis, E. Endocrine disruptors and polycystic ovary syndrome (PCOS): Elevated serum levels of bisphenol A in women with PCOS. J. Clin. Endocrinol. Metab. 2011, 96, E480–E484. [Google Scholar] [CrossRef] [PubMed]
- Hossein, R.B.; Amanlou, M.; Behrouzi, L.T.; Ghazizadeh, M.; Haghollahi, F.; Bagheri, M.; Eslami, B. The Association Between Bisphenol A and Polycystic Ovarian Syndrome: A Case-Control Study. Acta Med. Iran. 2017, 55, 759–764. [Google Scholar]
- Akgül, S.; Sur, Ü.; Düzçeker, Y.; Balcı, A.; Kızılkan, M.P.; Kanbur, N.; Bozdağ, G.; Erkekoğlu, P.; Gümüş, E.; Kocer-Gumusel, B.; et al. Bisphenol A and phthalate levels in adolescents with polycystic ovary syndrome. Gynecol. Endocrinol. 2019, 35, 1084–1087. [Google Scholar] [CrossRef]
- Chou, Y.; Chen, Y.; Chen, M.; Chang, C.; Lai, G.; Tzeng, C. Exposure to Mono-n-Butyl Phthalate in Women with Endometriosis and Its Association with the Biological Effects on Human Granulosa Cells. Int. J. Mol. Sci. 2020, 21, 1794. [Google Scholar] [CrossRef]
- Richardson, K.A.; Hannon, P.R.; Johnson-Walker, Y.J.; Myint, M.S.; Flaws, J.A.; Nowak, R.A. Di (2-ethylhexyl) phthalate (DEHP) alters proliferation and uterine gland numbers in the uteri of adult exposed mice. Reprod. Toxicol. 2018, 77, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Cho, S.; Ihm, H.J.; Oh, Y.S.; Heo, S.; Chun, S.; Im, H.; Chae, H.D.; Kim, C.; Kang, B.M. Possible Role of Phthalate in the Pathogenesis of Endometriosis: In Vitro, Animal, and Human Data. J. Clin. Endocrinol. Metab. 2015, 100, E1502–E1511. [Google Scholar] [CrossRef] [PubMed]
- Zahra, A.; Sisu, C.; Silva, E.; Sophie-Christine, D.A.G.; Randeva, H.S.; Chatha, K.; Kyrou, I.; Karteris, E. Is There a Link between Bisphenol A (BPA), a Key Endocrine Disruptor, and the Risk for SARS-CoV-2 Infection and Severe COVID-19? J. Clin. Med. 2020, 9, 3296. [Google Scholar] [CrossRef] [PubMed]
- Delfosse, V.; Maire, A.L.; Balaguer, P.; Bourguet, W. A structural perspective on nuclear receptors as targets of environmental compounds. Acta Pharmacol. Sin. 2015, 36, 88–101. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ahern, T.P.; Broe, A.; Lash, T.L.; Cronin-Fenton, D.P.; Ulrichsen, S.P.; Christiansen, P.M.; Cole, B.F.; Tamimi, R.M.; Sorensen, H.T.; Damkier, P. Phthalate Exposure and Breast Cancer Incidence: A Danish Nationwide Cohort Study. J. Clin. Oncol. 2019, 37, 1800–1809. [Google Scholar] [CrossRef]
- Yang, P.J.; Hou, M.F.; Tsai, E.M.; Liang, S.S.; Chiu, C.C.; Ou-Yang, F.; Kan, J.Y.; Peng, C.Y.; Wang, T.N. Breast cancer is associated with methylation and expression of the a disintegrin and metalloproteinase domain 33 (ADAM33) gene affected by endocrinedisrupting chemicals. Oncol. Rep. 2018, 40, 2766–2777. [Google Scholar] [CrossRef]
- Binder, A.M.; Corvalan, C.; Pereira, A.; Calafat, A.M.; Ye, X.; Shepherd, J.; Michels, K.B. Prepubertal and Pubertal Endocrine-Disrupting Chemical Exposure and Breast Density among Chilean Adolescents. Cancer Epidemiol. Biomarkers Prev. 2018, 27, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Crobeddu, B.; Ferraris, E.; Kolasa, E.; Plante, I. Di(2-ethylhexyl) phthalate (DEHP) increases proliferation of epithelial breast cancer cells through progesterone receptor dysregulation. Environ. Res. 2019, 173, 165–173. [Google Scholar] [CrossRef]
- Holmes, A.K.; Koller, K.R.; Kieszak, S.M.; Sjodin, A.; Calafat, A.M.; Sacco, F.D.; Varner, D.W.; Lanier, A.P.; Rubin, C.H. Case-control study of breast cancer and exposure to synthetic environmental chemicals among Alaska Native women. Int. J. Circumpolar Health 2014, 73, 25760. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Carrillo, L.; Hernandez-Ramirez, R.U.; Calafat, A.M.; Torres-Sanchez, L.; Galvan-Portillo, M.; Needham, L.L.; Ruiz-Ramos, R.; Cebrian, M.E. Exposure to phthalates and breast cancer risk in northern Mexico. Environ. Health Perspect. 2010, 118, 539–544. [Google Scholar] [CrossRef]
- Morgan, M.; Deoraj, A.; Felty, Q.; Roy, D. Environmental estrogen-like endocrine disrupting chemicals and breast cancer. Mol. Cell. Endocrinol. 2017, 457, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid. Based Healthc. 2018, 16, 195–203. [Google Scholar] [CrossRef]
- Reeves, K.W.; Schneider, S.; Xue, J.; Kannan, K.; Mason, H.; Johnson, M.; Makari-Judson, G.; Santana, M.D. Bisphenol-A in breast adipose tissue of breast cancer cases and controls. Environ. Res. 2018, 167, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Aschengrau, A.; Coogan, P.F.; Quinn, M.; Cashins, L.J. Occupational exposure to estrogenic chemicals and the occurrence of breast cancer: An exploratory analysis. Am. J. Ind. Med. 1998, 34, 6–14. [Google Scholar] [CrossRef]
- Trabert, B.; Falk, R.T.; Figueroa, J.D.; Graubard, B.I.; Garcia-Closas, M.; Lissowska, J.; Peplonska, B.; Fox, S.D.; Brinton, L.A. Urinary bisphenol A-glucuronide and postmenopausal breast cancer in Poland. Cancer Causes Control 2014, 25, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Parada, H.; Gammon, M.D.; Ettore, H.L.; Chen, J.; Calafat, A.M.; Neugut, A.I.; Santella, R.M.; Wolff, M.S.; Teitelbaum, S.L. Urinary concentrations of environmental phenols and their associations with breast cancer incidence and mortality following breast cancer. Environ. Int. 2019, 130, 104890. [Google Scholar] [CrossRef] [PubMed]
- Reeves, K.W.; Díaz Santana, M.; Manson, J.E.; Hankinson, S.E.; Zoeller, R.T.; Bigelow, C.; Sturgeon, S.R.; Spiegelman, D.; Tinker, L.; Luo, J.; et al. Urinary Phthalate Biomarker Concentrations and Postmenopausal Breast Cancer Risk. JNCI J. Natl. Cancer Inst. 2019, 111, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Parada, H.; Gammon, M.D.; Chen, J.; Calafat, A.M.; Neugut, A.I.; Santella, R.M.; Wolff, M.S.; Teitelbaum, S.L. Urinary Phthalate Metabolite Concentrations and Breast Cancer Incidence and Survival following Breast Cancer: The Long Island Breast Cancer Study Project. Environ. Health Persp. 2018, 126, 47013. [Google Scholar] [CrossRef]
- Skakkebaek, N.E.; Rajpert-De, M.E.; Main, K.M. Testicular dysgenesis syndrome: An increasingly common developmental disorder with environmental aspects. Hum. Reprod. 2001, 16, 972–978. [Google Scholar] [CrossRef]
- Piazza, M.J.; Urbanetz, A.A. Environmental toxins and the impact of other endocrine disrupting chemicals in women’s reproductive health. JBRA Assist. Reprod. 2019, 23, 154–164. [Google Scholar] [CrossRef]
- Rodprasert, W.; Main, K.M.; Toppari, J.; Virtanen, H.E. Associations between male reproductive health and exposure to endocrine-disrupting chemicals. Curr. Opin. Endocr. Metab. Res. 2019, 7, 49–61. [Google Scholar] [CrossRef]
- Desai, M.; Jellyman, J.K.; Ross, M.G. Epigenomics, gestational programming and risk of metabolic syndrome. Int. J. Obes. 2015, 39, 633–641. [Google Scholar] [CrossRef]
- Sun, L.; Fan, J.; Song, G.; Cai, S.; Fan, C.; Zhong, Y.; Li, Y. Exposure to phthalates is associated with grip strength in US adults. Ecotoxicol. Environ. Saf. 2020, 209, 111787. [Google Scholar] [CrossRef]
- Takashima, K.; Ito, Y.; Gonzalez, F.J.; Nakajima, T. Different mechanisms of DEHP-induced hepatocellular adenoma tumorigenesis in wild-type and Ppar alpha-null mice. J. Occup. Health 2008, 50, 169–180. [Google Scholar] [CrossRef]
- Shi, X.Y.; Wang, Z.; Liu, L.; Feng, L.M.; Li, N.; Liu, S.; Gao, H. Low concentrations of bisphenol A promote human ovarian cancer cell proliferation and glycolysis-based metabolism through the estrogen receptor-alpha pathway. Chemosphere 2017, 185, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Kalkbrenner, A.E.; Calafat, A.M.; Yolton, K.; Ye, X.; Dietrich, K.N.; Lanphear, B.P. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 2011, 128, 873. [Google Scholar] [CrossRef]
- Latini, G.; De Felice, C.; Presta, G.; Del, V.A.; Paris, I.; Ruggieri, F.; Mazzeo, P. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ. Health Perspect. 2003, 111, 1783–1785. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.J.; Sanchez, B.N.; Tellez-Rojo, M.M.; Lee, J.M.; Mercado-Garcia, A.; Blank-Goldenberg, C.; Peterson, K.E.; Meeker, J.D. Phthalate and bisphenol A exposure during in utero windows of susceptibility in relation to reproductive hormones and pubertal development in girls. Environ. Res. 2017, 159, 143–151. [Google Scholar] [CrossRef]
- Fenton, S.E. Endocrine-Disrupting Compounds and Mammary Gland Development: Early Exposure and Later Life Consequences. Endocrinology 2006, 147, s18–s24. [Google Scholar] [CrossRef]
- Manservisi, F.; Gopalakrishnan, K.; Tibaldi, E.; Hysi, A.; Iezzi, M.; Lambertini, L.; Teitelbaum, S.; Chen, J.; Belpoggi, F. Effect of maternal exposure to endocrine disrupting chemicals on reproduction and mammary gland development in female Sprague-Dawley rats. Reprod. Toxicol. 2015, 54, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, K.; Teitelbaum, S.L.; Lambertini, L.; Wetmur, J.; Manservisi, F.; Falcioni, L.; Panzacchi, S.; Belpoggi, F.; Chen, J. Changes in mammary histology and transcriptome profiles by low-dose exposure to environmental phenols at critical windows of development. Environ. Res. 2017, 152, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Hurst, C.H.; Waxman, D.J. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol. Sci. 2003, 74, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.; Sarraf, P.; Tontonoz, P.; Evans, R.M.; Martin, K.J.; Zhang, M.; Fletcher, C.; Singer, S.; Spiegelman, B.M. Terminal Differentiation of Human Breast Cancer through PPARγ. Mol. Cell 1998, 1, 465–470. [Google Scholar] [CrossRef]
- Elstner, E.; Muller, C.; Koshizuka, K.; Williamson, E.A.; Park, D.; Asou, H.; Shintaku, P.; Said, J.W.; Heber, D.; Koeffler, H.P. Ligands for peroxisome proliferator-activated receptorgamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc. Natl. Acad. Sci. USA 1998, 95, 8806–8811. [Google Scholar] [CrossRef] [PubMed]



| Study (Year) | Region | Study Design | Time Period | No. of Case/Control | Age | Categories of EDCs | Samples Determined |
|---|---|---|---|---|---|---|---|
| A K. Holmes (2014) [38] | Alaska Native | case-control | 1999–2002 | 75/95 | 30–88 | MBzP, MEP, MBP, MEHHP, MEHP, MEOHP, MMP | Urine |
| L. López-Carrillo (2010) [39] | Northern Mexico | case-control | 2007–2008 | 233/221 | Cases: 53.41 ± 12.78 Controls:53.83 ± 12.54 | MBzP, MEP, MCPP, MBP, MEHHP, MEHP, MEOHP, MiBP, MECPP | Urine |
| M Morgan (2017) [40] | America | case-control | 2003–2010 | 91/2410 | ≥20 | MBzP/MZP, MEP, MBP, MEHHP, MEHP, MEOHP, MiBP, MnBP, MCCP, MCPP | Urine |
| M Morgan (2017) [40] | America | case-control | 2005–2010 | 78/2067 | ≥20 | BPA | Urine |
| K W. Reeves (2018) [43] | America | case-control | 2014–2015 | 36/14 | Case: 55.7 ± 10.5 Control: 42.1 ± 16.5 | BPA | Breast adipose tissue |
| A. Aschengrau (1998) [44] | America | case-control | 1983–1986 | 261/753 | ≥18 | BBzP | Urine |
| A Aschengrau (1998) [44] | America | case-control | 1983–1986 | 261/753 | ≥18 | BPA | NIOSH/NOES |
| B Traber (2014) [45] | Poland | case-control | 2000–2003 | 575/575 | 20–74 | BPA-glucuronide (BPA-G) | Urine |
| H Parada (2019) [46] | America | case-control | 1996–1997 | 711/598 | 22–96 | BPA | Urine |
| K W. Reeves (2019) [47] | America | case-control | NA | 404/768 | Case: 62.56; Control: 62.46 | BzP, MEP, MCPP, DBP, DiBP, MCNP, MCOP, DEHP | Urine |
| H Parada (2018) [48] | America | case-control | 1996–1997 | 710/598 | 22–96 | MBzP, MEP, MCPP, MEHHP, MEHP, MEOHP, MiBP, MnBP, MECPP, MCOP, MCNP | Urine |
| Scheme | No. of Studies | Meta-Analyses | Model | Heterogeneity | ||
|---|---|---|---|---|---|---|
| ORs (95% CIs) | p-Value | I2 | p-Value | |||
| Region | ||||||
| Non-America | 1 | 1.09 (0.73–1.63) | 0.674 | - | - | - |
| America | 4 | 0.78 (0.61–0.99) | 0.045 | Fixed | 0.0 | 0.980 |
| Source of controls | ||||||
| Clinical medical centre | 1 | 0.90 (0.40–2.01) | 0.797 | - | - | - |
| General population | 4 | 0.85 (0.68–1.06) | 0.142 | Fixed | 0.0 | 0.542 |
| Subgroups | No. of Studies | Meta-Analyses | Model | Heterogeneity | ||
|---|---|---|---|---|---|---|
| ORs (95% CIs) | p-Value | I2 | p-Value | |||
| Region | ||||||
| Non-America | 2 | 0.82 (0.25–2.64) | 0.734 | Random | 84.9 | 0.010 |
| America | 4 | 0.74 (0.59–0.93) | 0.010 | Fixed | 0.0 | 0.983 |
| Source of controls | ||||||
| Clinical medical center | 2 | 1.00 (0.51–1.95) | 0.996 | Random | 63.4 | 0.098 |
| General population | 4 | 0.66 (0.51–0.85) | 0.001 | Fixed | 0.0 | 0.503 |
| Subgroups | No. of Studies | Meta-Analyses | Model | Heterogeneity | ||
|---|---|---|---|---|---|---|
| ORs (95% CIs) | p-Value | I2 | p-Value | |||
| Region | ||||||
| Non-America | 2 | 1.13 (0.29–4.40) | 0.856 | Random | 88.8 | 0.003 |
| America | 3 | 0.87 (0.68–1.11) | 0.259 | Fixed | 0.0 | 0.411 |
| Source of controls | ||||||
| Clinical medical center | 2 | 0.87 (0.59–1.28) | 0.480 | Fixed | 47.1 | 0.169 |
| General population | 3 | 1.07 (0.55–2.10) | 0.836 | Random | 83.9 | 0.002 |
| Subgroups | No. of Studies | Meta-Analyses | Model | Heterogeneity | ||
|---|---|---|---|---|---|---|
| ORs (95% CIs) | p-Value | I2 | p-Value | |||
| Region | ||||||
| Non-America | 2 | 1.41(0.93–2.12) | 0.102 | Fixed | 0.0 | 0.843 |
| America | 2 | 1.00 (0.75–1.33) | 0.985 | Fixed | 0.0 | 0.468 |
| Source of controls | ||||||
| Clinical medical center | 1 | 1.50 (0.71–3.17) | 0.288 | - | - | - |
| General population | 3 | 1.08 (0.84–1.39) | 0.533 | Fixed | 0.0 | 0.423 |
| Subgroups | No. of Studies | Meta-Analyses | Model | Heterogeneity | ||
|---|---|---|---|---|---|---|
| ORs (95% CIs) | p-Value | I2 | p-Value | |||
| Region | ||||||
| Non-America | 2 | 1.62 (0.84–3.11) | 0.150 | Random | 52.9 | 0.145 |
| America | 2 | 0.82 (0.60–1.12) | 0.214 | Fixed | 0.0 | 0.686 |
| Source of controls | ||||||
| Clinical medical center | 1 | 2.43 (1.12–5.26) | 0.024 | - | - | - |
| General population | 3 | 0.92 (0.71–1.20) | 0.551 | Fixed | 1.0 | 0.364 |
| Subgroups | No. of Studies | Meta-Analyses | Model | Heterogeneity | ||
|---|---|---|---|---|---|---|
| ORs (95% CIs) | p-Value | I2 | p-Value | |||
| Region | ||||||
| Non-America | 2 | 0.92 (0.61–1.38) | 0.686 | Fixed | 0.0 | 0.491 |
| America | 1 | 1.19 (0.71–1.99) | 0.508 | - | - | - |
| Source of controls | ||||||
| Clinical medical center | 1 | 1.15 (0.54–2.44) | 0.716 | - | - | - |
| General population | 2 | 0.99 (0.70–1.40) | 0.945 | Fixed | 0.0 | 0.333 |
| All studies | 3 | 1.01 (0.74–1.40) | 0.927 | Fixed | 0.0 | 0.587 |
| Subgroups | No. of Studies | Meta-Analyses | Model | Heterogeneity | ||
|---|---|---|---|---|---|---|
| ORs (95% CIs) | p-Value | I2 | p-Value | |||
| Region | ||||||
| Non-America | 1 | 0.44 (0.24–0.80) | 0.008 | - | - | - |
| America | 2 | 0.89 (0.63–1.25) | 0.496 | Random | 70.9 | 0. 064 |
| Source of controls | ||||||
| Clinical medical center | 1 | 1.02 (0.90–1.16) | 0.760 | - | - | - |
| General population | 2 | 0.63 (0.46–0.85) | 0.025 | Fixed | 43.9 | 0.182 |
| All studies | 3 | 0.74 (0.48–1.14) | 0.173 | Random | 83.8 | 0.002 |
| Subgroups | No. of Studies | Meta-Analyses | Model | Heterogeneity | ||
|---|---|---|---|---|---|---|
| ORs (95% CIs) | p-Value | I2 | p-Value | |||
| Region | ||||||
| Non-America | 2 | 0.77 (0.48–1.22) | 0.266 | Fixed | 0.0 | 0.602 |
| America | 1 | 0.85 (0.47–1.64) | 0.593 | - | - | - |
| Source of controls | ||||||
| Clinical medical center | 1 | 0.66 (0.32–1.38) | 0.267 | - | - | - |
| General population | 2 | 0.85 (0.56–1.30) | 0.453 | Fixed | 0.0 | 1.000 |
| All studies | 3 | 0.80 (0.55–1.55) | 0.228 | Fixed | 0.0 | 0.843 |
| Subgroups | No. of Studies | Meta-Analyses | Model | Heterogeneity | ||
|---|---|---|---|---|---|---|
| ORs (95% CIs) | p-Value | I2 | p-Value | |||
| Region | ||||||
| Non-America | 1 | 0.77 (0.48–1.22) | 0.244 | - | - | - |
| America | 2 | 0.76 (0.57–1.03) | 0.073 | Fixed | 0.0 | 0.740 |
| All studies | 3 | 0.75 (0.58–0.98) | 0.033 | Fixed | 0.0 | 0.937 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Cai, W.; Liu, H.; Jiang, H.; Bi, Y.; Wang, H. The Association of Bisphenol A and Phthalates with Risk of Breast Cancer: A Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 2375. https://doi.org/10.3390/ijerph18052375
Liu G, Cai W, Liu H, Jiang H, Bi Y, Wang H. The Association of Bisphenol A and Phthalates with Risk of Breast Cancer: A Meta-Analysis. International Journal of Environmental Research and Public Health. 2021; 18(5):2375. https://doi.org/10.3390/ijerph18052375
Chicago/Turabian StyleLiu, Ge, Wei Cai, Huan Liu, Haihong Jiang, Yongyi Bi, and Hong Wang. 2021. "The Association of Bisphenol A and Phthalates with Risk of Breast Cancer: A Meta-Analysis" International Journal of Environmental Research and Public Health 18, no. 5: 2375. https://doi.org/10.3390/ijerph18052375
APA StyleLiu, G., Cai, W., Liu, H., Jiang, H., Bi, Y., & Wang, H. (2021). The Association of Bisphenol A and Phthalates with Risk of Breast Cancer: A Meta-Analysis. International Journal of Environmental Research and Public Health, 18(5), 2375. https://doi.org/10.3390/ijerph18052375





