Oyster Shell Powder, Zeolite and Red Mud as Binders for Immobilising Toxic Metals in Fine Granular Contaminated Soils (from Industrial Zones in South Korea)
Abstract
1. Introduction
2. Materials and Methods
2.1. Contaminated Soil Samples
2.2. Binders
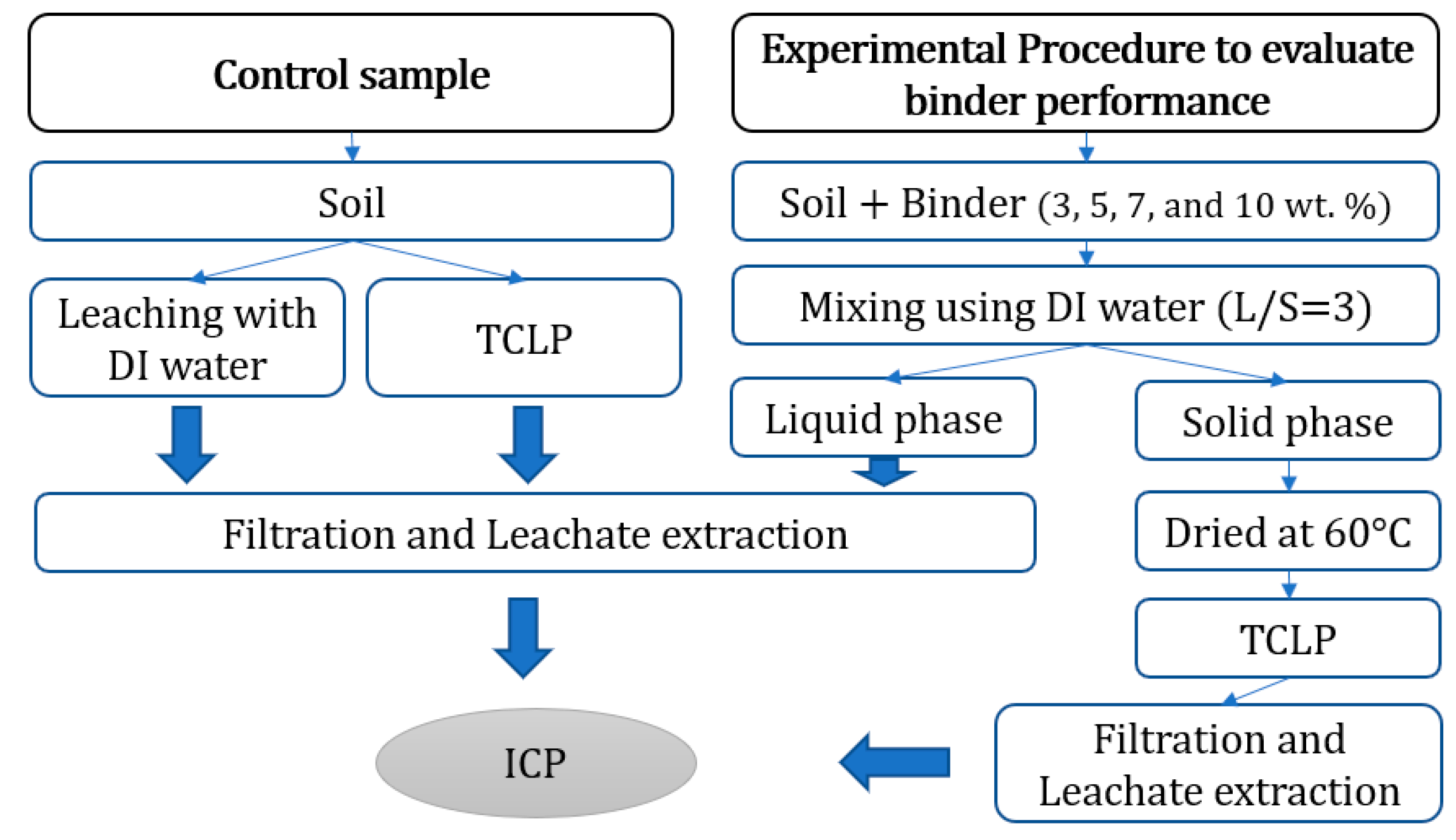
2.3. Binder Performance Evaluation Method
- The test was initiated by taking samples of 50 g of air-dried soil as the control and measuring the initial toxic metal concentrations (Pb, Cu, Zn, Cd and Ni). Then, 50 g of stabilised soil under different binder dosages per total weight (1, 3, 5, 7 and 10 wt%) was placed in a 250 mL glass flask and agitated for 2 h at 150 rpm with DI water at an L/S of 3.
- The supernatant fluid from the previous step was extracted 8 h after Step 1. For silty sand soil, however, additional extractions were performed at 12, 24 and 36 h after the first extraction to evaluate the effect of contact time with the binder on soil. After the supernatant fluid was extracted, it was filtered using a 0.45 μm membrane filter and then collocated in a 14 mL tube for toxic metal (Pb, Cu, Zn, Cd and Ni) concentration measurement via inductively coupled plasma optical emission spectroscopy (ICP-OES). Furthermore, pH was measured using a Thermo Scientific Orion 5-Star Plus Portable pH/ORP/ISE/Conductivity/DO Multiparameter Meter Model Number: PH3642-2(Beverly, MA, USA) as presented in Table S2.
- The control samples (without binder) and stabilised soil (after Step 2, solid phase) were placed in an oven and dried at 60 °C for 24 h.
- TCLP test was conducted on all the soil samples obtained after Step 3.
- 5.
- A 2 g sample (from Step 3) was placed in small tubes that contained 40 mL of the extract solution (L/S = 20). Extract solution type depends on the pH of the medium (previously measured in Step 2).
- 6.
- After mixing thoroughly using a rotary tumbler at 30 ± 2 rpm for 18 h, the samples were allowed to settle for 12 h. Then, the supernatant fluid was extracted and sieved using a 0.45 μm membrane filter and collocated in tubes with a 14 mL capacity to measure toxic metal concentration via ICP-OES. For the HCS treated with oyster shell, additional extractions were performed after 1 day and 10 days of mixing to evaluate the effect of contact time.
- 7.
2.4. Measurement of Initial Toxic Metal Concentrations
- C1: metal concentration of the analytical specimens obtained from the calibration curves (mg/L),
- C0: metal concentration of the blank solution obtained from the calibration curve (mg/L),
- f: dilution rate,
- V: volume of the specimen container and
- Wd: dry weight of the soil specimen
| Source | USCS 1 | pH | Extraction Method | Extract Fluid 2 | Initial Concentrations | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pb | Cu | Zn | Cd | Ni | |||||
| DI (mg L−1) | DI | 0.015 | 0.110 | 0.045 | 0.002 | - | |||
| Case I: Mine area | SM | 8.1 | TCLP (mg L−1) | I | 0.639 | 3.954 | 102.784 | 0.316 | 0.432 |
| TCLP (mg Kg−1) | 12.780 | 79.080 | 2055.680 | 6.320 | 8.640 | ||||
| DI (mg L−1) | DI | 0.550 | 0.210 | 0.450 | 0.003 | - | |||
| Case II: Military area | SW | 6.7 | TCLP (mg L−1) | I | 0.079 | 2.235 | 10.053 | 0.046 | - |
| TCLP (mg Kg−1) | 1.580 | 44.700 | 201.060 | 0.920 | - | ||||
| DI (mg L−1) | DI | 301.657 | 0.440 | - | - | ||||
| Case III: HCS | SW | 4.9 | TCLP (mg L−1) | II | 159.802 | 0.444 | - | - | - |
| TCLP (mg kg−1) | 3196.04 | 8.880 | - | - | - | ||||
3. Results
3.1. Effects of Binder and Dosage
3.1.1. Case I: Silty Sand Soil from an Abandoned Metal Mine Site
3.1.2. Case II: Sandy Soil from a Military Service Area
3.1.3. Case III: Handmade Contaminated Soil (HCS)
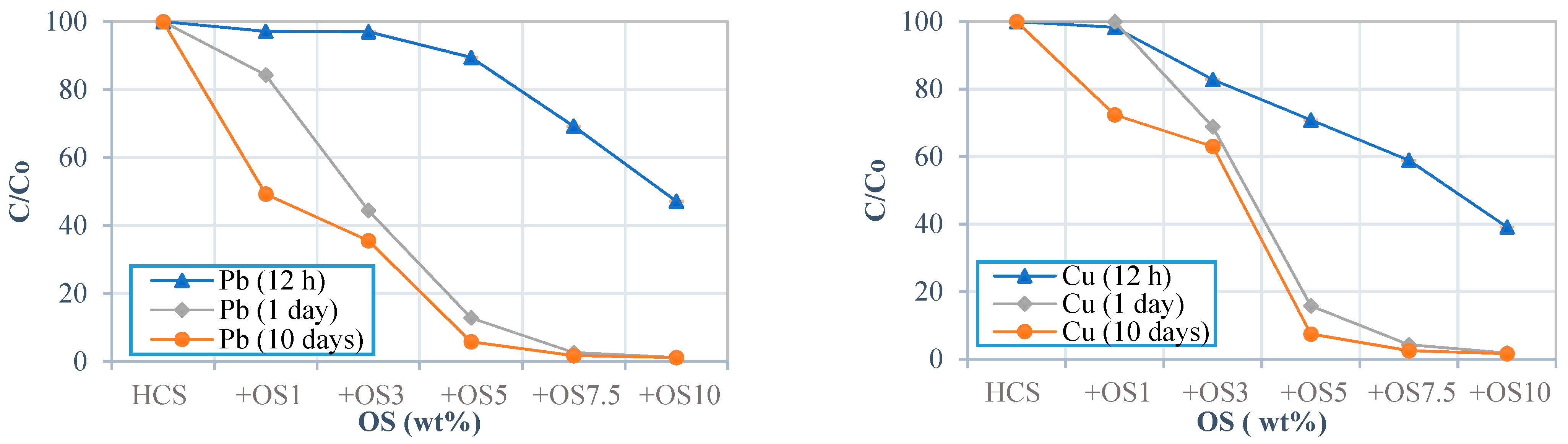
3.2. Effect of Contact Time
4. Discussion
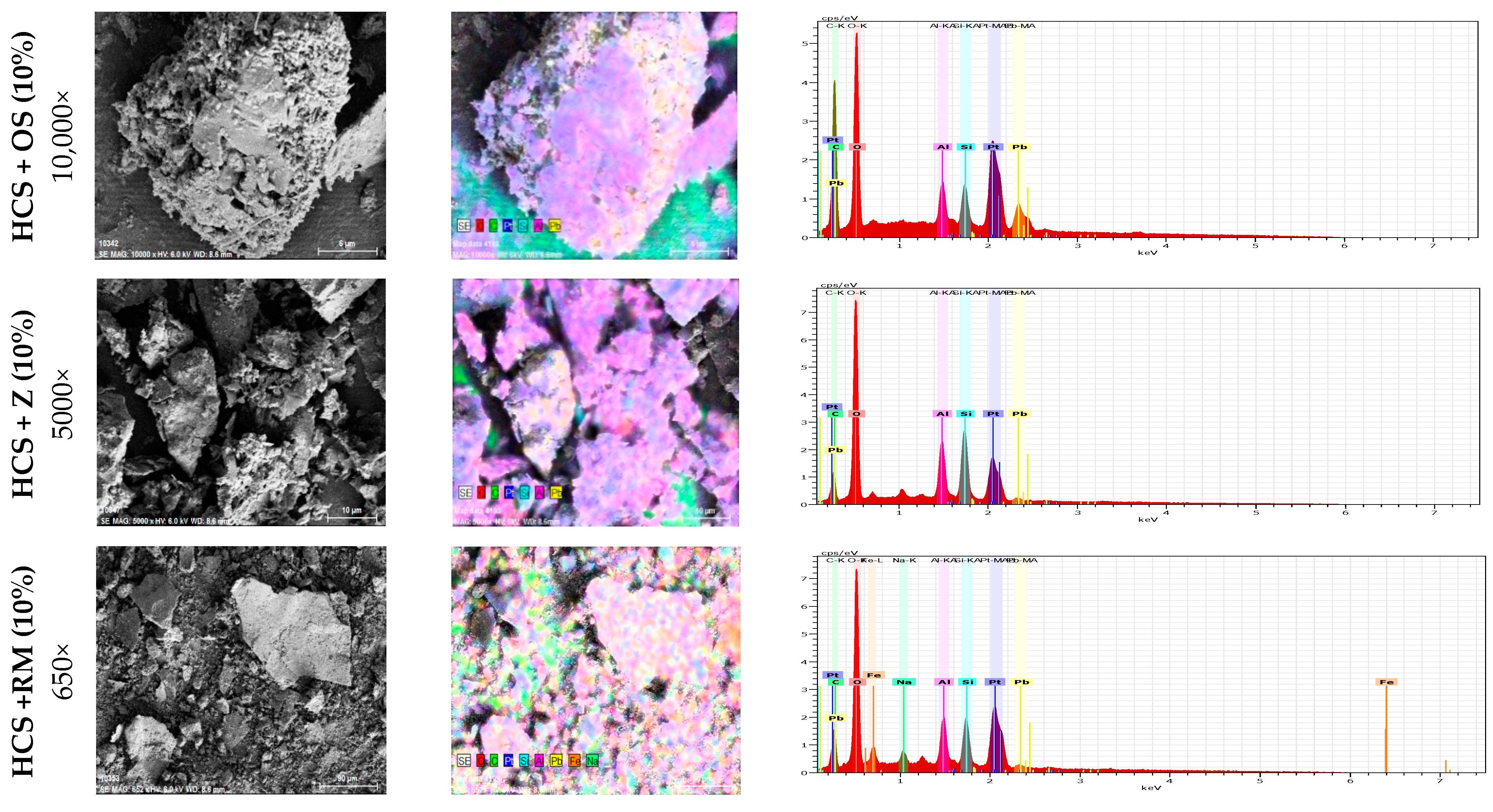
4.1. Oyster Shell Powder
4.2. Zeolite
4.3. Red Mud
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Liu, X.; Bai, J.; Shih, K.; Zeng, E.Y.; Cheng, H. Assessing heavy metal pollution in the surface soils of a region that had undergone three decades of intense industrialization and urbanization. Environ. Sci. Pollut. Res. 2013, 20, 6150–6159. [Google Scholar] [CrossRef] [PubMed]
- Selvi, A.; Rajasekar, A.; Theerthagiri, J.; Ananthaselvam, A.; Sathishkumar, K.; Madhavan, J.; Rahman, P.K.S.M. Integrated Remediation Processes Toward Heavy Metal Removal/Recovery from Various Environments-A Review. Front. Environ. Sci. 2019, 7. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Ma, J.; Chen, Y.; Antoniadis, V.; Wang, K.; Huang, Y.; Tian, H. Assessment of heavy metal(loid)s contamination risk and grain nutritional quality in organic waste-amended soil. J. Hazard. Mater. 2020, 399, 123095. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Guan, Q.; Tian, J.; Lin, J.; Yang, Y.; Yang, L.; Pan, N. Contamination characteristics, source apportionment, and health risk assessment of heavy metals in agricultural soil in the Hexi Corridor. Catena 2020, 191. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, S.; Yadav, K.K.; Shrivastava, M.; Gupta, N.; Nagar, S.; Bach, Q.-V.; Kamyab, H.; Khan, S.A.; Yadav, S.; et al. Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches—A review. Environ. Res. 2019, 179, 108792. [Google Scholar] [CrossRef]
- U.S. EPA. General Principles for Performing Aggregate Exposure and Risk Assessments; U.S. Environmental Protection Agency: Washington, DC, USA, 2001.
- ATSDR. Draft Toxicological Profile for Lead; ATSDR: Atlanta, GA, USA, 2005.
- Jung, M.C. Contamination by Cd, Cu, Pb, and Zn in mine wastes from abandoned metal mines classified as mineralization types in Korea. Environ. Geochem. Health 2008, 30, 205–217. [Google Scholar] [CrossRef]
- NJDEP. Technical Guidance on the capping of sites undergoing remediation. In Site Remediation Program; New Jersey Department of Environmental Protection (NJDEP): Trenton, NJ, USA, 2014. [Google Scholar]
- Khan, F.I.; Husain, T.; Hejazi, R. An overview and analysis of site remediation technologies. J. Environ. Manag. 2004, 71, 95–122. [Google Scholar] [CrossRef] [PubMed]
- Kutuniva, J.; Mäkinen, J.; Kauppila, T.; Karppinen, A.; Hellsten, S.; Luukkonen, T.; Lassi, U. Geopolymers as active capping materials for in situ remediation of metal(loid)-contaminated lake sediments. J. Environ. Chem. Eng. 2019, 7, 102852. [Google Scholar] [CrossRef]
- FHWA. INDOT Guidance Document for In-Situ Soil Flushing; Joint Transportation Research Program, Indiana Department of Transportation and Purdue University: West Lafayette, IN, USA, 2007.
- Dermont, G.; Bergeron, M.; Mercier, G.; Richer-Laflèche, M. Soil washing for metal removal: A review of physical/chemical technologies and field applications. J. Hazard. Mater. 2008, 152, 1–31. [Google Scholar] [CrossRef]
- Andreozzi, R.; Fabbricino, M.; Ferraro, A.; Lerza, S.; Marotta, R.; Pirozzi, F.; Race, M. Simultaneous removal of Cr(III) from high contaminated soil and recovery of lactic acid from the spent solution. J. Environ. Manag. 2020, 268, 110584. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Kim, S.O.; Kim, K.W. Enhanced electrokinetic extraction of heavy metals from soils assisted by ion exchange membranes. J. Hazard. Mater. 2005, 118, 93–102. [Google Scholar] [CrossRef]
- Tang, J.; He, J.; Tang, H.; Wang, H.; Sima, W.; Liang, C.; Qiu, Z. Heavy metal removal effectiveness, flow direction and speciation variations in the sludge during the biosurfactant-enhanced electrokinetic remediation. Sep. Purif. Technol. 2020, 246. [Google Scholar] [CrossRef]
- U.S. EPA. Resource Guide for Electrokinetics Laboratory and Field Processes Applicable to Radioactive and Hazardous Mixed Wastes in Soil and Groundwater from 1992 to 1997; U.S. Environmental Protection Agency: Washington, DC, USA, 1997; p. 83.
- Hunce, S.Y.; Akgul, D.; Demir, G.; Mertoglu, B. Solidification/stabilization of landfill leachate concentrate using different aggregate materials. Waste Manag. 2012, 32, 1394–1400. [Google Scholar] [CrossRef]
- Xia, W.-Y.; Du, Y.-J.; Li, F.-S.; Li, C.-P.; Yan, X.-L.; Arulrajah, A.; Wang, F.; Song, D.-J. In-situ solidification/stabilization of heavy metals contaminated site soil using a dry jet mixing method and new hydroxyapatite based binder. J. Hazard. Mater. 2019, 369, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.-J.; Liu, S.-Y.; Liu, Z.-B.; Chen, L.; Zhang, F.; Jin, F. An Overview of Stabilization/Solidification Technique for Heavy Metals Contaminated Soils. In Advances in Environmental Geotechnics; Chen, Y., Zhan, L., Tang, X., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 760–766. [Google Scholar]
- Meegoda Jay, N.; Ezeldin, A.S.; Fang, H.-Y.; Inyang Hilary, I. Waste Immobilization Technologies. Pract. Period. Hazard. Toxic Radioact. Waste Manag. 2003, 7, 46–58. [Google Scholar] [CrossRef]
- Shu, X.; Li, Y.; Huang, W.; Chen, S.; Xu, C.; Zhang, S.; Li, B.; Wang, X.; Qing, Q.; Lu, X. Rapid vitrification of uranium-contaminated soil: Effect and mechanism. Environ. Pollut. 2020, 263, 114539. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Dubey, N.K.; Kumar, V. Phytoremediation of Environmental Pollutants; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Babel, S.; Kurniawan, T.A. Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 633, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Paria, S.; Yuet, P.K. Solidification–stabilization of organic and inorganic contaminants using Portland cement: A literature review. Environ. Rev. 2006, 14, 217–255. [Google Scholar] [CrossRef]
- Yakubu, Y.; Zhou, J.; Ping, D.; Shu, Z.; Chen, Y. Effects of pH dynamics on solidification/stabilization of municipal solid waste incineration fly ash. J. Environ. Manag. 2018, 207, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Liu, Y.; Tang, Y. Oyster shell powder for Pb(II) immobilization in both aquatic and sediment environments. Environ. Geochem. Health 2020. [Google Scholar] [CrossRef]
- Zheng, S.-a.; Zheng, X.; Chen, C. Leaching Behavior of Heavy Metals and Transformation of Their Speciation in Polluted Soil Receiving Simulated Acid Rain. PLoS ONE 2012, 7, e49664. [Google Scholar] [CrossRef]
- Xia, W.-Y.; Feng, Y.-S.; Jin, F.; Zhang, L.-M.; Du, Y.-J. Stabilization and solidification of a heavy metal contaminated site soil using a hydroxyapatite based binder. Constr. Build. Mater. 2017, 156, 199–207. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The removal of heavy metal cations by natural zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef]
- Kargar, M.; Clark, O.G.; Hendershot, W.H.; Jutras, P.; Prasher, S.O. Immobilization of Trace Metals in Contaminated Urban Soil Amended with Compost and Biochar. Water Air Soil Pollut. 2015, 226. [Google Scholar] [CrossRef]
- Ma, W.; Chen, D.; Pan, M.; Gu, T.; Zhong, L.; Chen, G.; Yan, B.; Cheng, Z. Performance of chemical chelating agent stabilization and cement solidification on heavy metals in MSWI fly ash: A comparative study. J. Environ. Manag. 2019, 247, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.H.; Mesquita-Guimarães, J.; Henriques, B.; Silva, F.S.; Fredel, M.C. The Potential Use of Oyster Shell Waste in New Value-Added By-Product. Resources 2019, 8, 13. [Google Scholar] [CrossRef]
- Lu, J.; Cong, X.; Li, Y.; Hao, Y.; Wang, C. Scalable recycling of oyster shells into high purity calcite powders by the mechanochemical and hydrothermal treatments. J. Clean. Prod. 2018, 172, 1978–1985. [Google Scholar] [CrossRef]
- Wang, P.; Liu, D.-Y. Physical and Chemical Properties of Sintering Red Mud and Bayer Red Mud and the Implications for Beneficial Utilization. Materials 2012, 5, 1800–1810. [Google Scholar] [CrossRef]
- Han, Y.S.; Ji, S.; Lee, P.K.; Oh, C. Bauxite residue neutralization with simultaneous mineral carbonation using atmospheric CO2. J. Hazard. Mater. 2017, 326, 87–93. [Google Scholar] [CrossRef]
- Shin, W.-S.; Kang, K.; Kim, Y.-K. Adsorption Characteristics of Multi-Metal Ions by Red Mud, Zeolite, Limestone, and Oyster Shell. Environ. Eng. Res. 2014, 19, 15–22. [Google Scholar] [CrossRef]
- Xu, X.; Liu, X.; Oh, M.; Park, J. Oyster Shell as a Low-Cost Adsorbent for Removing Heavy Metal Ions from Wastewater. Pol. J. Environ. Stud. 2019, 28, 2949–2959. [Google Scholar] [CrossRef]
- Jeong, S.H.; Lim, J.E.; Lee, S.S.; Chang, Y.Y.; Moon, D.H.; Ok, Y.S. Evaluation on remediation efficiency on Acid-Spilled soil using Oyster Shell and Biochar. J. Agric. Life Sci. 2013, 25, 10–16. [Google Scholar]
- Moon, D.H.; Park, J.W.; Cheong, K.H.; Hyun, S.; Koutsospyros, A.; Park, J.H.; Ok, Y.S. Stabilization of lead and copper contaminated firing range soil using calcined oyster shells and fly ash. Environ. Geochem. Health 2013, 35, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.E.; Moon, D.-H.; Kim, D.; Kwon, O.-K.; Yang, J.E.; Ok, Y.S. Evaluation of the Feasibility of Oyster-Shell and Eggshell Wastes for Stabilization of Arsenic-Contaminated Soil. J. Korean Soc. Environ. Eng. 2009, 31, 1095–1104. [Google Scholar]
- Ouki, S.K.; Kavannagh, M. Performance of Natural Zeolites for the treatments of mixed metal—Contaminated Effluents. Waste Manag. Res. 1997, 15, 383–394. [Google Scholar] [CrossRef]
- Grant, D.C.; Skriba, M.C.; Saha, A.K. Removal of radiactive contaminants from West Valley Waste Streams using Natual Zeolites. Environ. Prog. 1987, 6. [Google Scholar] [CrossRef]
- Zamzow, M.J.; Eichbaum, B.R.; Sandgren, K.R.; Shanks, D.E. Removal of Heavy Metals and Other Cations from Wastewater Using Zeolites. Sep. Sci. Technol. 1990, 25, 1555–1569. [Google Scholar] [CrossRef]
- Parades-Aguilar, J.; Reyes-Martinez, V.; Bustamante, G.; Almendariz-Tapia, F.J.; Martinez-Meza, G.; Vilchez-Vargas, R.; Link, A.; Certucha-Barragan, M.T.; Calderon, K. Removal of nickel(II) from wastewater using a zeolite-packed anaerobic bioreactor: Bacterial diversity and community structure shifts. J. Environ. Manag. 2020, 111558. [Google Scholar] [CrossRef]
- Kwon, P.S.; Shahrokhi-shahraki, R.; Park, J.B. Assessment of Zeolite Soil Mixture as Adsorptive Fill Material at Industrial Zones. J. Korean Soc. Civ. Eng. 2019, 39, 203–209. [Google Scholar] [CrossRef]
- Wen, J.; Zeng, G. Chemical and biological assessment of Cd-polluted sediment for land use: The effect of stabilization using chitosan-coated zeolite. J. Environ. Manag. 2018, 212, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Ujaczki, É.; Klebercz, O.; Feigl, V.; Molnár, M.; Magyar, Á.; Uzinger, N.; Gruiz, K. Environmental Toxicity Assessment of the Spilled Ajka Red Mud in Soil Microcosms for Its Potential Utilisation as Soil Ameliorant. Period. Polytech. Chem. Eng. 2015, 59, 253–261. [Google Scholar] [CrossRef]
- KIGAM. JP2014-008-2015 Recycling of Red Mud as a Landfill cover Material and a Stabilizer of Acid Mine Tailing Generated from a Working Mine; Korean Institute of Geoscience and Mineral Resources: Daejeon, Korea, 2015. [Google Scholar]
- Watts, R.J. Hazardous Wastes: Sources, Pathways, Receptors; Wiley: Hoboken, NJ, USA, 1998; p. 764. [Google Scholar]
- Du, Y.J.; Wei, M.L.; Reddy, K.R.; Liu, Z.P.; Jin, F. Effect of acid rain pH on leaching behavior of cement stabilized lead-contaminated soil. J. Hazard. Mater. 2014, 271, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.A.; Shang, J.Q. Monitoring Lead in Soil Water System Using Complex Permittivity. In Proceedings of the 8th International Conference on Environment Science and Engineering (ICESE 2018), Barcelona, Spain, 11–13 March 2018; IOP Publishing Ltd.: Barcelona, Spain, 2018. [Google Scholar]
- MEPC. HJ-557-2010 Solid Waste-Extraction Procedure for Leaching Toxicity-Horizontal Vibration Method; Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2010. [Google Scholar]
- U.S. EPA. METHOD 1311 Toxicity characteristic Leaching Procedure. In Hazardous Waste Test Methods; U.S. Environmental Protection Agency: Washington, DC, USA, 1992. [Google Scholar]
- Lu, C.-C.; Hsu, M.H.; Lin, Y.-P. Evaluation of heavy metal leachability of incinerating recycled aggregate and solidification/stabilization products for construction reuse using TCLP, multi-final pH and EDTA-mediated TCLP leaching tests. J. Hazard. Mater. 2019, 368, 336–344. [Google Scholar] [CrossRef]
- Sahuquillo, A.; Rigol, A.; Rauret, G. Comparison of leaching tests for the study of trace metals remobilization in soils and sediments. J. Environ. Monit. 2002, 4, 1003–1009. [Google Scholar] [CrossRef]
- EHTI. No. 2018-53 Standards for Soil Pollution Process. In ES 07400.2c Metals-Inductively Coupled Plasma-Atomic Emission Spectrometry; Environmental Health Technology Institute: Seoul, Korea, 2018. [Google Scholar]
- Saca, N.; Dimache, A.; Radu, L.R.; Iancu, I. Leaching behavior of some demolition wastes. J. Mater. Cycles Waste Manag. 2017, 19, 623–630. [Google Scholar] [CrossRef]
- Desta, M.B. Batch Sorption Experiments: Langmuir and Freundlich Isotherm Studies for the Adsorption of Textile Metal Ions onto Teff Straw (Eragrostis tef) Agricultural Waste. J. Thermodyn. 2013, 2013, 375830. [Google Scholar] [CrossRef]
- U.S. EPA. METHOD 200.7 Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry; U.S. Environmental Protection Agency: Washington, DC, USA, 1994.
- U.S. EPA. 40 CFR 261.24—Toxicity Characteristic; Environmental Protection Agency: Washington, DC, USA, 2011.
- EHTI. No. 2017-54 Waste Process Test Standard. In ES 06002.a Standards, Labeling Limits and Result Indication of Hazardous Substances Contained in Designated Waste—2014; Environmental Health Technology Institute: Seoul, Korea, 2017. [Google Scholar]
- WHO. Permessible Limits for Heavy Metals in Soils and Plants; World Health Organization (WHO): Geneva, Switzerland, 1996. [Google Scholar]
- Ok, Y.S.; Oh, S.-E.; Ahmad, M.; Hyun, S.; Kim, K.-R.; Moon, D.H.; Lee, S.S.; Lim, K.J.; Jeon, W.-T.; Yang, J.E. Effects of natural and calcined oyster shells on Cd and Pb immobilization in contaminated soils. Environ. Earth Sci. 2010, 61, 1301–1308. [Google Scholar] [CrossRef]
- Bonnard, M.; Boury, B.; Parrot, I. Key Insights, Tools, and Future Prospects on Oyster Shell End-of-Life: A Critical Analysis of Sustainable Solutions. Environ. Sci. Technol. 2020, 54, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Lee, D.K.; Ali, M.A.; Kim, P.J. Effects of oyster shell on soil chemical and biological properties and cabbage productivity as a liming materials. Waste Manag. 2008, 28, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Dong, X.; Zhang, S.; Zhao, Y.; Cen, Q.; Zhang, K. The effect and mechanism of Si/Al ratio on microstructure of zeolite modified ceramsite derived from industrial wastes. Micropor. Mesopor. Mat. 2021, 311. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, J.; Zhang, Y.; Peng, D.; Huang, T.; Sun, C. pH-Dependent Leaching Characteristics of Major and Toxic Elements from Red Mud. Int. J. Environ. Res. Public Health 2019, 16, 2046. [Google Scholar] [CrossRef] [PubMed]
- Mayes, W.M.; Burke, I.T.; Gomes, H.I.; Anton, Á.D.; Molnár, M.; Feigl, V.; Ujaczki, É. Advances in Understanding Environmental Risks of Red Mud After the Ajka Spill, Hungary. J. Sustain. Metall. 2016, 2, 332–343. [Google Scholar] [CrossRef]
- Anton, A.; Rékási, M.; Uzinger, N.; Széplábi, G.; Makó, A. Modelling the Potential Effects of the Hungarian Red Mud Disaster on Soil Properties. Water Air Soil Pollut. 2012, 223, 5175–5188. [Google Scholar] [CrossRef]
- Buchman, M.F. NOAA Screening Quick Reference Tables; NOAA OR&R Report 08-1; Seattle, WA, USA, 2008; p. 34. Available online: https://repository.library.noaa.gov/view/noaa/9327 (accessed on 10 November 2020).
- CCME Canadian Environmental Quality Guideline. Available online: http://st-ts.ccme.ca/en/index.html (accessed on 13 November 2020).
- MEPC. GB 5085.3-2007 Identification Standards for Hazardous Wastes—Identification for Extraction Toxicity; Ministry of Environmental Protection of the People’s Republic of China: Beijing, China, 2007. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Quiroz, C.; Dissanayake, J.; Park, J. Oyster Shell Powder, Zeolite and Red Mud as Binders for Immobilising Toxic Metals in Fine Granular Contaminated Soils (from Industrial Zones in South Korea). Int. J. Environ. Res. Public Health 2021, 18, 2530. https://doi.org/10.3390/ijerph18052530
Torres-Quiroz C, Dissanayake J, Park J. Oyster Shell Powder, Zeolite and Red Mud as Binders for Immobilising Toxic Metals in Fine Granular Contaminated Soils (from Industrial Zones in South Korea). International Journal of Environmental Research and Public Health. 2021; 18(5):2530. https://doi.org/10.3390/ijerph18052530
Chicago/Turabian StyleTorres-Quiroz, Cecilia, Janith Dissanayake, and Junboum Park. 2021. "Oyster Shell Powder, Zeolite and Red Mud as Binders for Immobilising Toxic Metals in Fine Granular Contaminated Soils (from Industrial Zones in South Korea)" International Journal of Environmental Research and Public Health 18, no. 5: 2530. https://doi.org/10.3390/ijerph18052530
APA StyleTorres-Quiroz, C., Dissanayake, J., & Park, J. (2021). Oyster Shell Powder, Zeolite and Red Mud as Binders for Immobilising Toxic Metals in Fine Granular Contaminated Soils (from Industrial Zones in South Korea). International Journal of Environmental Research and Public Health, 18(5), 2530. https://doi.org/10.3390/ijerph18052530









