A Model-Based Meta-Analysis of Willingness to Participate in Cancer Screening
Abstract
:1. Introduction
2. Literature Review
2.1. Health Belief Model (HBM) and Its Extensions
2.2. Theory of Reasoned Action (TRA) and Theory of Planned Behavior (TPB)
2.3. Research Questions
- What are the magnitudes of effects of perceived severity, perceived barriers, perceived benefits, health literacy, cues to action, perceived susceptibility, and perceived behavioral control on cancer-screening intentions and behaviors?
- What kinds of significant relationships among the predictors mentioned in the first research question and outcomes (cancer-screening intentions and behaviors) are present? Which theories underpin these relationships?
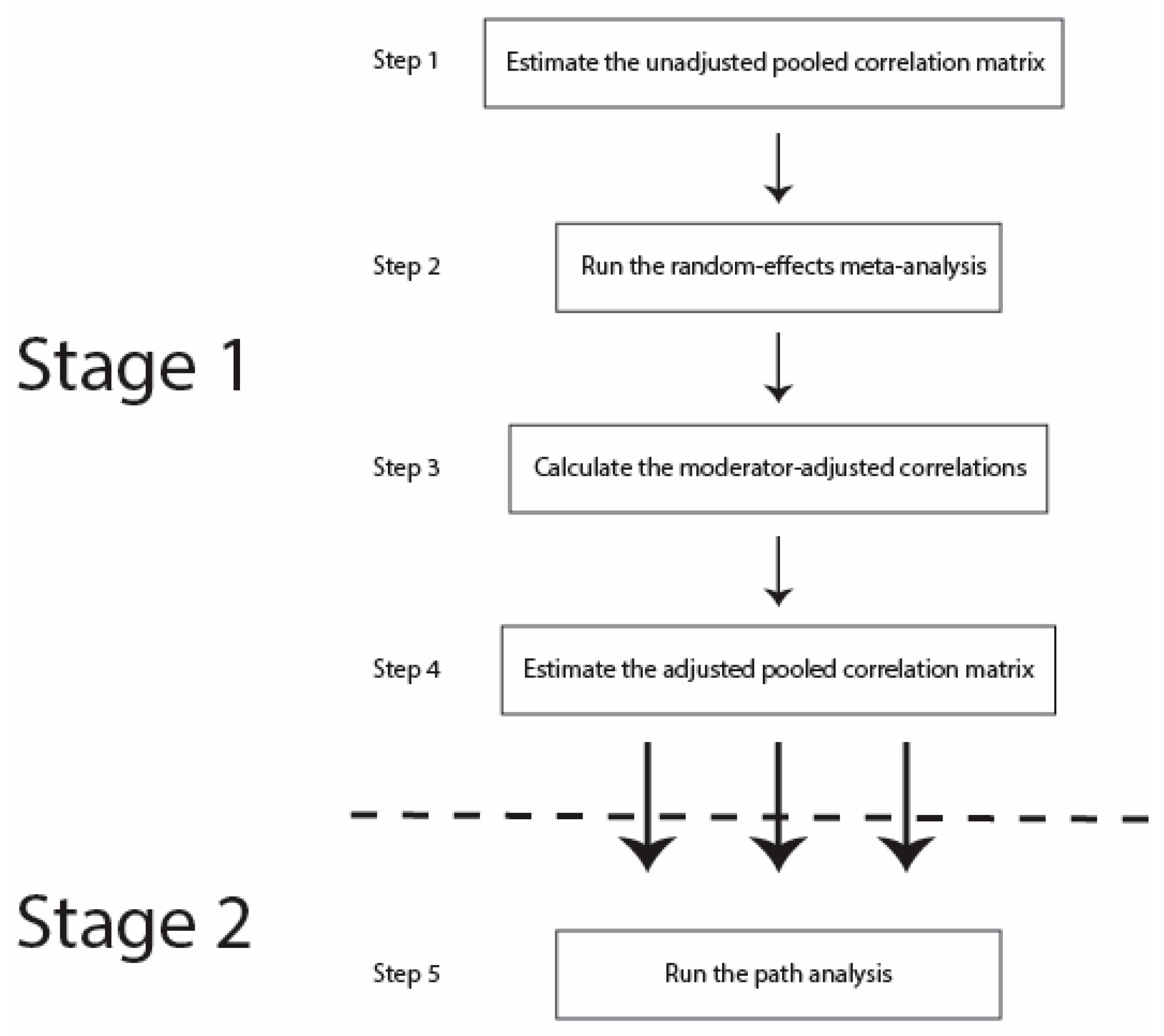
3. Methods
3.1. Classification of Constructs
3.2. Selection Criteria
3.3. Unit of Analysis
3.4. Coding Categories of the Moderators
3.5. Procedures
4. Results

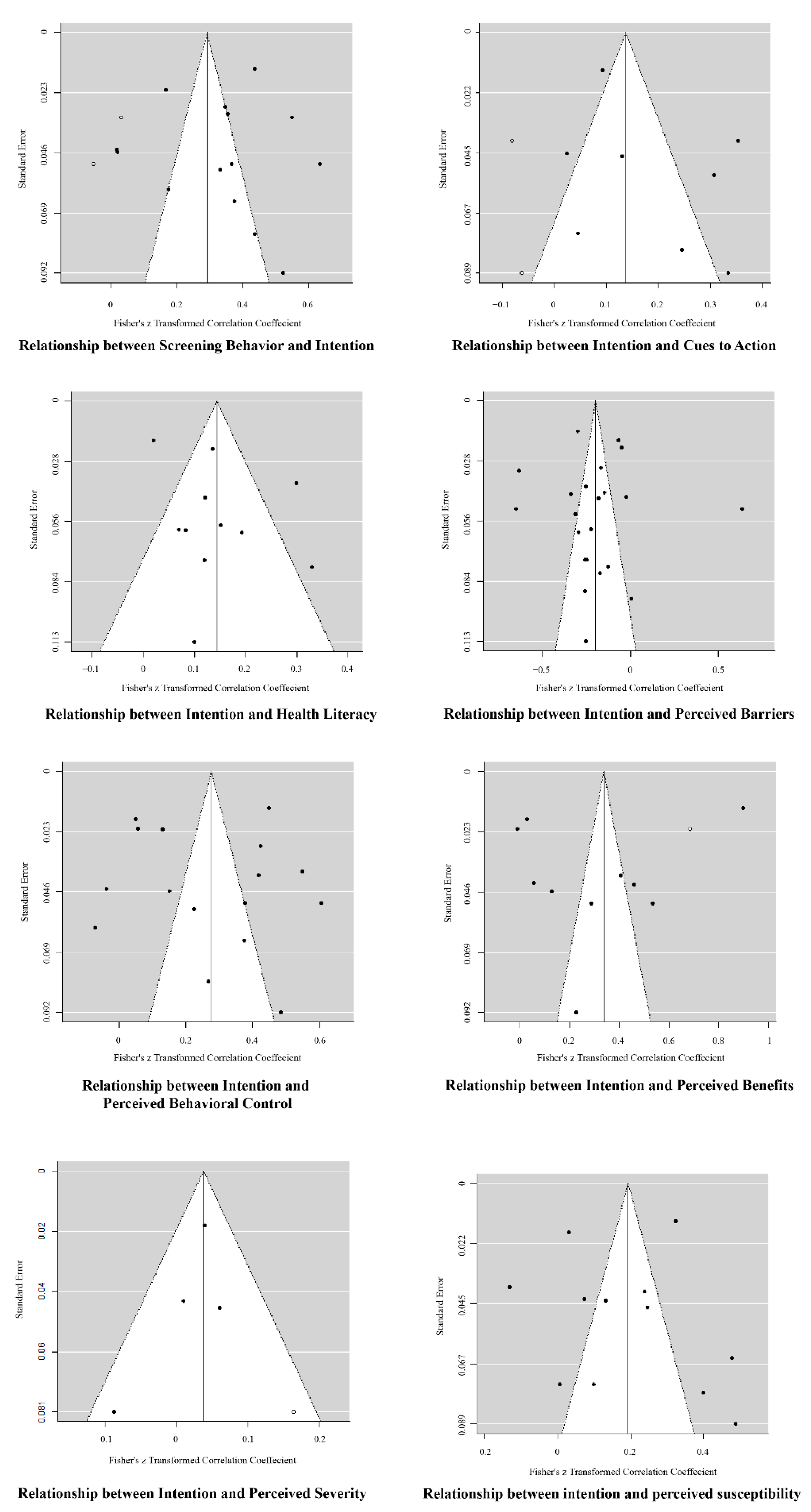
5. Discussion
5.1. Discussion of Estimation Results
5.2. Gap between Health Intentions and Health Behaviors
6. Implications
6.1. Theoretical Implications
6.2. Practical and Methodological Implications
7. Limitations and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- WHO. WHO|Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 21 February 2021).
- American Cancer Society. Cancer Facts & Figures 2021. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/cancer-facts-and-figures-2021.pdf (accessed on 21 February 2021).
- Ogden, J. Health Psychology; McGraw-Hill: London, UK, 2012. [Google Scholar]
- Rex, D.K.; Johnson, D.A.; Lieberman, D.A.; Burt, R.W.; Sonnenberg, A. Colorectal cancer prevention 2000: Screening recommendations of the American College of Gastroenterology. Am. J. Gastroenterol. 2000, 95, 868. [Google Scholar] [CrossRef]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Faivre, J.; Dancourt, V.; Lejeune, C.; Tazi, M.A.; Lamour, J.; Gerard, D.; Dassonville, F.; Bonithon-Kopp, C. Reduction in colorectal cancer mortality by fecal occult blood screening in a French controlled study. Gastroenterology 2004, 126, 1674–1680. [Google Scholar] [CrossRef]
- USPSTF. Published Recommendations—US Preventive Services Task Force. Available online: https://www.uspreventiveservicestaskforce.org/BrowseRec/Index/browse-recommendations (accessed on 21 February 2021).
- National Cancer Institute. Cancer Screening. Available online: https://www.cancer.gov/about-cancer/screening (accessed on 21 February 2021).
- Wakefield, M.A.; Loken, B.; Hornik, R.C. Use of mass media campaigns to change health behaviour. Lancet 2010, 376, 1261–1271. [Google Scholar] [CrossRef] [Green Version]
- Sung, J.J.Y.; Lau, J.Y.W.; Young, G.P.; Sano, Y.; Chiu, H.M.; Byeon, J.S.; Yeoh, K.G.; Goh, K.L.; Sollano, J.; Rerknimitr, R.; et al. Asia Pacific consensus recommendations for colorectal cancer screening. Gut 2008, 57, 1166. [Google Scholar] [CrossRef] [Green Version]
- Smith Robert, A.; Brooks, D.; Cokkinides, V.; Saslow, D.; Brawley Otis, W. Cancer screening in the United States, 2013. CA Cancer J. Clin. 2013, 63, 87–105. [Google Scholar] [CrossRef]
- Vernon, S.W.; Laville, E.A.; Jackson, G.L. Participation in breast screening programs: A review. Soc. Sci. Med. 1990, 30, 1107–1118. [Google Scholar] [CrossRef]
- Gracie, K.; Kennedy, M.; Robson, J.; Callister, M. S131 What proportion of the uk population would be eligible for ct screening for lung cancer according to various proposed inclusion criteria? Thorax 2016, 71, A78. [Google Scholar] [CrossRef] [Green Version]
- Hart, A.R.; Barone, T.L.; Mayberry, J.F. Increasing compliance with colorectal cancer screening: The development of effective health education. Health Educ. Res. 1997, 12, 171–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elobaid, Y.E.; Aw, T.C.; Grivna, M.; Nagelkerke, N. Breast Cancer Screening Awareness, Knowledge, and Practice among Arab Women in the United Arab Emirates: A Cross-Sectional Survey. PLoS ONE 2014, 9, e105783. [Google Scholar] [CrossRef] [Green Version]
- Azubuike, S.O.; Okwuokei, S.O. Knowledge, Attitude and Practices of Women Towards Breast Cancer in Benin City, Nigeria. Ann. Med. Health Sci. Res. 2013, 3, 155–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Malley, M.S.; Earp, J.A.; Hawley, S.T.; Schell, M.J.; Mathews, H.F.; Mitchell, J. The association of race/ethnicity, socioeconomic status, and physician recommendation for mammography: Who gets the message about breast cancer screening? Am. J. Public Health 2001, 91, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, M.A.; Ward, L.M.; Pérez-Stable, E.J. Breast and Cervical Cancer Screening: Impact of Health Insurance Status, Ethnicity, and Nativity of Latinas. Ann. Fam. Med. 2005, 3, 235–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maheswaran, R.; Pearson, T.; Jordan, H.; Black, D. Socioeconomic deprivation, travel distance, location of service, and uptake of breast cancer screening in North Derbyshire, UK. J. Epidemiol. Community Health 2006, 60, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, J.N.; Ojo, A.A. Barriers to utilisation of cervical cancer screening in Sub Sahara Africa: A systematic review. Eur. J. Cancer Care 2017, 26, e12444. [Google Scholar] [CrossRef] [PubMed]
- Klabunde, C.N.; Vernon, S.W.; Nadel, M.R.; Breen, N.; Seeff, L.C.; Brown, M.L. Barriers to colorectal cancer screening: A comparison of reports from primary care physicians and average-risk adults. Med. Care 2005, 43, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Prentice-Dunn, S.; Rogers, R.W. Protection motivation theory and preventive health: Beyond the health belief model. Health Educ. Res. 1986, 1, 153–161. [Google Scholar] [CrossRef]
- Kelly, J.A.; Murphy, D.A.; Sikkema, K.J.; Kalichman, S.C. Psychological interventions to prevent HIV infection are urgently needed: New priorities for behavioral research in the second decade of AIDS. Am. Psychol. 1993, 48, 1023–1034. [Google Scholar] [CrossRef]
- Michie, S.; Prestwich, A. Are interventions theory-based? Development of a theory coding scheme. Health Psychol. 2010, 29, 1–8. [Google Scholar] [CrossRef]
- Kuhn, T.S. The Structure of Scientific Revolutions; University of Chicago Press: Chicago, IL, USA, 1970. [Google Scholar]
- Rosenstock, I.M. Why People Use Health Services. Milbank Meml. Fund Q. 1966, 44, 94–127. [Google Scholar] [CrossRef] [Green Version]
- Rosenstock, I.M. Public response to cancer screening and detection programs. Determinants of health behavior. J. Chronic Dis. 1963, 16, 407–418. [Google Scholar] [CrossRef] [Green Version]
- Ajzen, I.; Fishbein, M. Understanding Attitudes and Predicting Social Behavior; Prentice-Hall: Englewood Cliffs, NJ, USA, 1980. [Google Scholar]
- Fishbein, M.; Ajzen, I. Belief, Attitude, Intention, and Behavior: An Introduction to Theory and Research; Addison-Wesley Pub. Co.: Reading, MA, USA, 1975. [Google Scholar]
- Ajzen, I. From Intentions to Actions: A Theory of Planned Behavior. In Action Control: From Cognition to Behavior; Kuhl, J., Beckmann, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1985; pp. 11–39. [Google Scholar]
- Hochbaum, G.; Rosenstock, I.; Kegels, S. Health Belief Model; United States Public Health Services: Washington, DC, USA, 1952.
- Rosenstock, I.M. Historical Origins of the Health Belief Model. Health Educ. Monogr. 1974, 2, 328–335. [Google Scholar] [CrossRef]
- Rosenstock, I.M. The Health Belief Model and Preventive Health Behavior. Health Educ. Monogr. 1974, 2, 354–386. [Google Scholar] [CrossRef]
- Aiken, L.S.; West, S.G.; Woodward, C.K.; Reno, R.R. Health beliefs and compliance with mammography-screening recommendations in asymptomatic women. Health Psychol. 1994, 13, 122. [Google Scholar] [CrossRef]
- Katapodi, M.C.; Northouse, L.L.; Schafenacker, A.M.; Duquette, D.; Duffy, S.A.; Ronis, D.L.; Anderson, B.; Janz, N.K.; McLosky, J.; Milliron, K.J.; et al. Using a state cancer registry to recruit young breast cancer survivors and high-risk relatives: Protocol of a randomized trial testing the efficacy of a targeted versus a tailored intervention to increase breast cancer screening. BMC Cancer 2013, 13, 97. [Google Scholar] [CrossRef] [Green Version]
- Ronis, D.L. Conditional health threats: Health beliefs, decisions, and behaviors among adults. Health Psychol. 1992, 11, 127. [Google Scholar] [CrossRef]
- Bandura, A. Self-Efficacy: Toward a Unifying Theory of Behavioral Change. Psychol. Rev. 1977, 84, 191–215. [Google Scholar] [CrossRef]
- Rosenstock, I.M.; Strecher, V.J.; Becker, M.H. Social learning theory and the Health Belief Model. Health Educ. Q. 1988, 15, 175–183. [Google Scholar] [CrossRef]
- Montaño, D.E.; Thompson, B.; Taylor, V.M.; Mahloch, J. Understanding mammography intention and utilization among women in an inner city public hospital clinic. Prev. Med. 1997, 26, 817–824. [Google Scholar] [CrossRef]
- Ogunsanya, M.E.; Brown, C.M.; Odedina, F.T.; Barner, J.C.; Corbell, B.; Adedipe, T.B. Beliefs Regarding Prostate Cancer Screening Among Black Males Aged 18 to 40 Years. Am. J. Men’s Health 2017, 11, 41–53. [Google Scholar] [CrossRef]
- Mary, G. The Influence of Spirituality and Religiosity on Breast Cancer Screening Delay in African American Women: Application of the Theory of Reasoned Action and Planned Behavior (TRA/TPB). J. Assoc. Black Nurs. Fac. High. Educ. 2006, 17, 89. [Google Scholar]
- Montano, D.E.; Taplin, S.H. A test of an expanded theory of reasoned action to predict mammography participation. Soc. Sci. Med. 1991, 32, 733–741. [Google Scholar] [CrossRef]
- Sieverding, M.; Matterne, U.; Ciccarello, L. What Role Do Social Norms Play in the Context of Men’s Cancer Screening Intention and Behavior? Application of an Extended Theory of Planned Behavior. Health Psychol. 2010, 29, 72–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roncancio, A.M.; Ward, K.K.; Sanchez, I.A.; Cano, M.A.; Byrd, T.L.; Vernon, S.W. Using the Theory of Planned Behavior to Understand Cervical Cancer Screening Among Latinas. Health Educ. Behav. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, S.C.; Bowling, J.M.; Brewer, N.T.; Lipkus, I.M.; Skinner, C.S.; Strigo, T.S.; Rimer, B.K. Intentions to maintain adherence to mammography. J. Women’s Health 2008, 17, 1133–1141. [Google Scholar] [CrossRef] [Green Version]
- Steadman, L.; Rutter, D.R. Belief importance and the theory of planned behaviour: Comparing modal and ranked modal beliefs in predicting attendance at breast screening. Br. J. Health Psychol. 2004, 9, 447–463. [Google Scholar] [CrossRef]
- Gaston, G.; Gerjo, K. The Theory of Planned Behavior: A Review of its Applications to Health-Related Behaviors. Am. J. Health Promot. 1996, 11, 87–98. [Google Scholar] [CrossRef]
- Conner, M.; Armitage, C.J. Extending the Theory of Planned Behavior: A Review and Avenues for Further Research. J. Appl. Soc. Psychol. 1998, 28, 1429–1464. [Google Scholar] [CrossRef]
- Ajzen, I. The theory of planned behavior. Organ. Behav. Hum. Decis. Process. 1991, 50, 179–211. [Google Scholar] [CrossRef]
- Fisher, J.D.; Fisher, W.A.; Bryan, A.D.; Misovich, S.J. Information-motivation-behavioral skills model-based HIV risk behavior change intervention for inner-city high school youth. Health Psychol. 2002, 21, 177. [Google Scholar] [CrossRef]
- Glass, G.V.; Smith, M.L.; McGaw, B. Meta-Analysis in Social Research; Sage Publications, Incorporated: Thousand Oaks, CA, USA, 1981. [Google Scholar]
- Hunter, J.E.; Schmidt, F.L.; Jackson, G.B. Meta-Analysis: Cumulating Research Findings Across Studies; Sage Publications, Inc.: Thousand Oaks, CA, USA, 1982; Volume 4. [Google Scholar]
- Rosenthal, R. Meta-Analytic Procedures for Social Research; Sage: Newbury Park, CA, USA, 1991; Volume 6. [Google Scholar]
- Harrison, J.A.; Mullen, P.D.; Green, L.W. A meta-analysis of studies of the Health Belief Model with adults. Health Educ. Res. 1992, 7, 107–116. [Google Scholar] [CrossRef]
- Carpenter, C.J. A Meta-Analysis of the Effectiveness of Health Belief Model Variables in Predicting Behavior. Health Commun. 2010, 25, 661–669. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, R.S.; Vernberg, D. Models of preventive health behavior: Comparison, critique, and meta-analysis. Adv. Med. Sociol. 1994, 4, 45–67. [Google Scholar]
- Janz, N.K.; Becker, M.H. The health belief model: A decade later. Health Educ. Q. 1984, 11, 1–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milne, S.; Sheeran, P.; Orbell, S. Prediction and Intervention in Health-Related Behavior: A Meta-Analytic Review of Protection Motivation Theory. J. Appl. Soc. Psychol. 2000, 30, 106–143. [Google Scholar] [CrossRef]
- Wilson, S.J.; Polanin, J.R.; Lipsey, M.W. Fitting Meta-Analytic Structural Equation Models with Complex Datasets. Res. Synth. Methods 2016, 7, 121–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, B.J. Model-based meta-analysis. In The Handbook of Research Synthesis and Meta-Analysis; Cooper, H., Hedges, L.V., Eds.; Russell Sage Foundation: New York, NY, USA, 2009; Volume 2, pp. 377–395. [Google Scholar]
- McMillan, B.; Conner, M. Health cognition assessment. In Cambridge Handbook of Psychology, Health and Medicine; Ayers, A.B.S., McManus, C., Newman, S., Wallston, K., Weinman, J., West, R., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 260–266. [Google Scholar]
- Cheung, M.W.L.; Hong, R.Y. Applications of meta-analytic structural equation modelling in health psychology: Examples, issues, and recommendations. Health Psychol. Rev. 2017, 11, 265–279. [Google Scholar] [CrossRef]
- Johnson, B.T.; Mullen, B.; Salas, E. Comparison of three major meta-analytic approaches. J. Appl. Psychol. 1995, 80, 94–106. [Google Scholar] [CrossRef]
- Hofstede, G. The cultural relativity of the quality of life concept. Acad. Manag. Rev. 1984, 9, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Cheung, M.W.-L.; Chan, W. A Two-Stage Approach to Synthesizing Covariance Matrices in Meta-Analytic Structural Equation Modeling. Struct. Equ. Model. Multidiscip. J. 2009, 16, 28–53. [Google Scholar] [CrossRef]
- Jak, S.; Cheung, M.W.L. Accounting for Missing Correlation Coefficients in Fixed-Effects MASEM. Multivar. Behav. Res. 2018, 53, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Hedges, L.V. Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Stat. 1981, 6, 107–128. [Google Scholar] [CrossRef]
- Brockwell, S.E.; Gordon, I.R. A comparison of statistical methods for meta-analysis. Stat. Med. 2001, 20, 825–840. [Google Scholar] [CrossRef]
- Schmid, E.J.; Koch, G.G.; LaVange, L.M. An overview of statistical issues and methods of meta-analysis. J. Biopharm. Stat. 1991, 1, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Jak, S. Meta-Analytic Structural Equation Modelling; Springer: Cham, Switherlands, 2015. [Google Scholar] [CrossRef]
- National Research Council. Combining Information: Statistical Issues and Opportunities for Research; National Academies: Washington, DC, USA, 1992; Volume 1. [Google Scholar]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Moeyaert, M.; Ugille, M.; Natasha Beretvas, S.; Ferron, J.; Bunuan, R.; Van den Noortgate, W. Methods for dealing with multiple outcomes in meta-analysis: A comparison between averaging effect sizes, robust variance estimation and multilevel meta-analysis. Int. J. Soc. Res. Methodol. 2017, 20, 559–572. [Google Scholar] [CrossRef]
- Berkey, C.S.; Hoaglin, D.C.; Antczak-Bouckoms, A.; Mosteller, F.; Colditz, G.A. Meta-analysis of multiple outcomes by regression with random effects. Stat. Med. 1998, 17, 2537–2550. [Google Scholar] [CrossRef]
- Cheung, M.W.L. Meta-Analysis: A Structural Equation Modeling Approach; John Wiley & Sons, Inc.: Chichester, UK, 2015. [Google Scholar]
- Cheung, M.W.-L. metaSEM: An R package for meta-analysis using structural equation modeling. Front. Psychol. 2015, 5, 1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, L.T.; Bentler, P.M. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. Multidiscip. J. 1999, 6, 1–55. [Google Scholar] [CrossRef]
- Abraido-Lanza, A.F.; Martins, M.C.; Shelton, R.C.; Florez, K.R. Breast Cancer Screening Among Dominican Latinas: A Closer Look at Fatalism and Oather Social and Cultural Factors. Health Educ. Behav. 2015, 42, 633–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abuadas, M.H.; Petro-Nustas, W.; Albikawi, Z.F.; Mari, M. Predictors of prostate cancer screening intention among older men in Jordan. Int. J. Urol. Nurs. 2017, 11, 31–41. [Google Scholar] [CrossRef]
- Allahverdipour, H.; Emami, A. Perceptions of cervical cancer threat, benefits, and barriers of Papanicolaou smear screening programs for women in Iran. Women Health 2008, 47, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Almadi, M.A.; Mosli, M.H.; Bohlega, M.S.; Al Essa, M.A.; AlDohan, M.S.; Alabdallatif, T.A.; AlSagri, T.Y.; Algahtani, F.A.; Mandil, A. Effect of Public Knowledge, Attitudes, and Behavior on Willingness to Undergo Colorectal Cancer Screening Using the Health Belief Model. Saudi J. Gastroenterol. 2015, 21, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Amador, J.A.; Flynn, P.M.; Betancourt, H. Cultural beliefs about health professionals and perceived empathy influence continuity of cancer screening following a negative encounter. J. Behav. Med. 2015, 38, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Ayres, C.G.; Atkins, R.; Lee, J.H. Factors Related to Health Practices: Cervical Cancer Screening Among Filipino Women. Res. Theory Nurs. Pract. 2010, 24, 197–208. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, Q.; Chen, X.; Gao, Y.; Gong, H.; Tan, X.; Zhang, M.; Tuo, J.; Zhang, Y.; Xiang, Q.; et al. Protection motivation theory in predicting intention to receive cervical cancer screening in rural Chinese women. Psycho Oncol. 2017, 27, 442–449. [Google Scholar] [CrossRef]
- Ben-Natan, M.; Adir, O. Screening for cervical cancer among Israeli lesbian women. Int. Nurs. Rev. 2009, 56, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Benjamins, M.R.; Ellison, C.G.; Krause, N.M.; Marcum, J.P. Religion and preventive service use: Do congregational support and religious beliefs explain the relationship between attendance and utilization? J. Behav. Med. 2011, 34, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, H.; Flynn, P.M.; Riggs, M.; Garberoglio, C. A cultural research approach to instrument development: The case of breast and cervical cancer screening among Latino and Anglo women. Health Educ. Res. 2010, 25, 991–1007. [Google Scholar] [CrossRef]
- Bleiker, E.M.; van der Ploeg, H.M.; Mook, J.; Kleijn, W.C. Anxiety, anger, and depression in elderly women. Psychol. Rep. 1993, 72, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Boonyasiriwat, W.; Hung, M.; Hon, S.D.; Tang, P.; Pappas, L.M.; Burt, R.W.; Schwartz, M.D.; Stroup, A.M.; Kinney, A.Y. Intention to Undergo Colonoscopy Screening Among Relatives of Colorectal Cancer Cases: A Theory-Based Model. Ann. Behav. Med. 2014, 47, 280–291. [Google Scholar] [CrossRef]
- Boyer, B.A.; Cantor, R.K. Posttraumatic stress among women with breast cancer and their daughters: Relationship with daughters’ breast cancer screening. Am. J. Fam. Ther. 2005, 33, 443–460. [Google Scholar] [CrossRef]
- Brandt, H.M.; Dolinger, H.R.; Sharpe, P.A.; Hardin, J.W.; Berger, F.G. Relationship of colorectal cancer awareness and knowledge with colorectal cancer screening. Colorectal Cancer 2012, 1, 383–396. [Google Scholar] [CrossRef] [Green Version]
- Brittain, K.; Murphy, V.P. Sociocultural and health correlates related to colorectal cancer screening adherence among urban African Americans. Cancer Nurs. 2015, 38, 118. [Google Scholar] [CrossRef] [Green Version]
- Brown, W.M.; Consedine, N.S.; Magai, C. Time spent in the United States and breast cancer screening behaviors among ethnically diverse immigrant women: Evidence for acculturation? J. Immigr. Minority Health 2006, 8, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Consedine, N.S.; Magai, C.; Neugut, A.I. The contribution of emotional characteristics to breast cancer screening among women from six ethnic groups. Prev. Med. 2004, 38, 64–77. [Google Scholar] [CrossRef]
- Consedine, N.S.; Morgenstern, A.H.; Kudadjie-Gyamfi, E.; Magai, C.; Neugut, A.I. Prostate cancer screening behavior in men from seven ethnic groups: The fear factor. Cancer Epidemiol. Biomark. Prev. 2006, 15, 228–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, L.T.; Subramanian, S.V.; Williams, D.R.; Armstrong, K.; Charles, C.Z.; Kawachi, I. Getting Black Men to Undergo Prostate Cancer Screening: The Role of Social Capital. Am. J. Mens Health 2015, 9, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Devellis, B.M.; Blalock, S.J.; Sandler, R.S. Predicting Participation in Cancer Screening: The Role of Perceived Behavioral Control. J. Appl. Soc. Psychol. 1990, 20, 639–660. [Google Scholar] [CrossRef]
- Dillard, A.J.; Ferrer, R.A.; Ubel, P.A.; Fagerlin, A. Risk Perception Measures’ Associations with Behavior Intentions, Affect, and Cognition Following Colon Cancer Screening Messages. Health Psychol. 2012, 31, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Zhang, J.-H.; Li, X.-H. Investigation and Analysis on Current Situation of Community Residents’ Knowledge of Colorectal Cancer. J. China Med Univ. 2013, 42, 1039–1042. [Google Scholar]
- Fernandez, M.E.; Diamond, P.M.; Rakowski, W.; Gonzales, A.; Tortolero-Luna, G.; Williams, J.; Morales-Campos, D.Y. Development and Validation of a Cervical Cancer Screening Self-Efficacy Scale for Low-income Mexican American Women. Cancer Epidemiol. Biomark. Prev. 2009, 18, 866–875. [Google Scholar] [CrossRef] [Green Version]
- Flynn, P.M.; Betancourt, H.; Ormseth, S.R. Culture, Emotion, and Cancer Screening: An Integrative Framework for Investigating Health Behavior. Ann. Behav. Med. 2011, 42, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharoro, E.P.; Ikeanyi, E.N. An appraisal of the level of awareness and utilization of the Pap smear as a cervical cancer screening test among female health workers in a tertiary health institution. Int. J. Gynecol. Cancer 2006, 16, 1063–1068. [Google Scholar] [CrossRef]
- Hart, S.L.; Bowen, D.J. Sexual Orientation and Intentions to Obtain Breast Cancer Screening. J. Womens Health 2009, 18, 177–185. [Google Scholar] [CrossRef]
- Hay, J.L.; Ford, J.S.; Klein, D.; Primavera, L.H.; Buckley, T.R.; Stein, T.R.; Shike, M.; Ostroff, J.S. Adherence to colorectal cancer screening in mammography-adherent older women. J. Behav. Med. 2003, 26, 553–576. [Google Scholar] [CrossRef]
- Hay, J.L.; Ramos, M.; Li, Y.; Holland, S.; Brennessel, D.; Kemeny, M.M. Deliberative and intuitive risk perceptions as predictors of colorectal cancer screening over time. J. Behav. Med. 2016, 39, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, L.L.; Hu, J.; Leak, A. Immigrant Women’s Cancer Screening Behaviors. J. Community Health Nurs. 2010, 27, 32–45. [Google Scholar] [CrossRef]
- Jacobsen, P.B.; Lamonde, L.A.; Honour, M.; Kash, K.; Hudson, P.B.; Pow-Sang, J. Relation of family history of prostate cancer to perceived vulnerability and screening behavior. Psycho Oncol. 2004, 13, 80–85. [Google Scholar] [CrossRef]
- Juon, H.-S.; Guo, J.; Kim, J.; Lee, S. Predictors of Colorectal Cancer Knowledge and Screening Among Asian Americans Aged 50–75 years old. J. Racial Ethn. Health Disparities 2017, 5, 545–552. [Google Scholar] [CrossRef]
- Katapodi, M.C.; Dodd, M.J.; Lee, K.A.; Facione, N.C. Underestimation of Breast Cancer Risk: Influence on Screening Behavior. Oncol. Nurs. Forum 2009, 36, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Kawar, L.N. Jordanian and Palestinian Immigrant Women’s Knowledge, Affect, Cultural Attitudes, Health Habits, and Participation in Breast Cancer Screening. Health Care Women Int. 2009, 30, 768–782. [Google Scholar] [CrossRef]
- Kim, B.K.; Jo, H.S.; Lee, H.J. Study on the Factors Related with Intention of Cancer Screening Among Korean Residents: Application of Information-Motivation-Behavioral Skills Model. Asia-Pac. J. Public Health 2015, 27, NP2133–NP2143. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R.M.; Brown, K.J. Psychosocial Barriers to Cervical Cancer Screening: Concerns with Self-presentation and Social Evaluation. J. Appl. Soc. Psychol. 1994, 24, 941–958. [Google Scholar] [CrossRef]
- Kudadjie-Gyamfi, E.K.; Magai, C.; Consedine, N.S. The obscuring object of race: Clinical breast exams and coping styles in ethnic subpopulations of women. Br. J. Health Psychol. 2010, 15, 289–305. [Google Scholar] [CrossRef]
- Lechner, L.; de Vries, H.; Offermans, N. Participation in a breast cancer screening program: Influence of past behavior and determinants on future screening participation. Prev. Med. 1997, 26, 473–482. [Google Scholar] [CrossRef]
- Lee, E.E.; Fogg, L.; Menon, U. Knowledge and Beliefs Related to Cervical Cancer and Screening Among Korean American Women. West. J. Nurs. Res. 2008, 30, 960–974. [Google Scholar] [CrossRef] [Green Version]
- Lee, F.-H.; Wang, H.-H.; Yang, Y.-M.; Huang, J.-J.; Tsai, H.-M. Influencing Factors of Intention to Receive Pap Tests in Vietnamese Women who Immigrated to Taiwan for Marriage. Asian Nurs. Res. 2016, 10, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Lindberg, N.M.; Wellisch, D. Anxiety and compliance among women at high risk for breast cancer. Ann. Behav. Med. 2001, 23, 298–303. [Google Scholar] [CrossRef]
- Lostao, L.; Joiner, T.E.; Pettit, J.W.; Chorot, P.; Sandin, B. Health beliefs and illness attitudes as predictors of breast cancer screening attendance. Eur. J. Public Health 2001, 11, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Manne, S.; Markowitz, A.; Winawer, S.; Guillem, J.; Meropol, N.J.; Haller, D.; Jandorf, L.; Rakowski, W.; Babb, J.; Duncan, T. Understanding intention to undergo colonoscopy among intermediate-risk siblings of colorectal cancer patients: A test of a mediational model. Prev. Med. 2003, 36, 71–84. [Google Scholar] [CrossRef]
- McGregor, L.M.; von Wagner, C.; Vart, G.; Yuen, W.C.; Raine, R.; Wardle, J.; Robb, K.A. The impact of supplementary narrative-based information on colorectal cancer screening beliefs and intention. BMC Cancer 2015, 15, 162. [Google Scholar] [CrossRef] [Green Version]
- McQueen, A.; Vernon, S.W.; Rothman, A.J.; Norman, G.J.; Myers, R.E.; Tilley, B.C. Examining the Role of Perceived Susceptibility on Colorectal Cancer Screening Intention and Behavior. Ann. Behav. Med. 2010, 40, 205–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miles, A.; Rainbow, S.; von Wagner, C. Cancer Fatalism and Poor Self-Rated Health Mediate the Association between Socioeconomic Status and Uptake of Colorectal Cancer Screening in England. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2132–2140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaei-Alavijeh, M.; Heydari, S.T.; Ahmadi-Jouybari, T.; Jalilian, F.; Gharibnavaz, H.; Mahboubi, M. Socio-Demographic and Cognitive Determinants of Breast Cancer Screening. Int. J. Adv. Biotechnol. Res. 2017, 8, 539–545. [Google Scholar] [CrossRef]
- Mitchell, J.A.; Watkins, D.C.; Modlin, C.S., Jr. Social Determinants Associated with Colorectal Cancer Screening in an Urban Community Sample of African-American Men. J. Mens. Health 2013, 10, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Muliira, J.K.; Al-Saidi, H.S.; Al-Yahyai, A.N. Determinants of behavioral intentions to screen for prostate cancer in Omani men. Asia-Pac. J. Oncol. Nurs. 2017, 4, 348–355. [Google Scholar] [CrossRef]
- Murray, M.; McMillan, C. Health beliefs, locus of control, emotional control and women’s cancer screening behaviour. Br. J. Clin. Psychol. 1993, 32 Pt 1, 87–100. [Google Scholar] [CrossRef]
- Nguyen, A.B.; Clark, T.T.; Belgrave, F.Z. Gender Roles and Acculturation: Relationships With Cancer Screening Among Vietnamese American Women. Cult. Divers. Ethn. Minority Psychol. 2014, 20, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, S.; Goldstein, B.; DiMatteo, M.R.; Fox, S.A.; John, C.R.; Obrzut, J.E. Adherence to Mammography and Colorectal Cancer Screening in Women 50–80 Years of Age: The Role of Psychological Distress. Womens Health Issues 2010, 20, 343–349. [Google Scholar] [CrossRef]
- Ogunsanya, M.E.; Brown, C.M.; Odedina, F.T.; Barner, J.C.; Adedipe, T. Determinants of prostate cancer screening intentions of young black men aged 18 to 40 years. J. Racial Ethn. Health Disparities 2017, 4, 1009–1020. [Google Scholar] [CrossRef]
- Orbell, S.; Crombie, I.; Johnston, G. Social cognition and social structure in the prediction of cervical screening uptake. Br. J. Health Psychol. 1996, 1, 35–50. [Google Scholar] [CrossRef]
- Rahaei, Z.; Ghofranipour, F.; Morowatisharifabad, M.A.; Mohammadi, E. Determinants of cancer early detection behaviors: Application of protection motivation theory. Health Promot. Perspect. 2015, 5, 138. [Google Scholar] [CrossRef] [PubMed]
- Rutter, D.R. Attendance and reattendance for breast cancer screening: A prospective 3-year test of the Theory of Planned Behaviour. Br. J. Health Psychol. 2000, 5, 1–13. [Google Scholar] [CrossRef]
- Savage, S.A.; Clarke, V.A. Factors associated with screening mammography and breast self-examination intentions. Health Educ. Res. 1996, 11, 409–421. [Google Scholar] [CrossRef]
- Sen, C.K.N.; Baruh, L.; Kumkale, G.T. Beyond a Paycheck: The Influence of Workforce Participation on Women’s Cancer Screening in Turkey. Sex Roles 2016, 75, 599–611. [Google Scholar] [CrossRef]
- Shelton, R.C.; Winkel, G.; Davis, S.N.; Roberts, N.; Valdimarsdottir, H.; Hall, S.J.; Thompson, H.S. Validation of the Group-Based Medical Mistrust Scale Among Urban Black Men. J. Gen. Intern. Med. 2010, 25, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subramanian, P.; Oranye, N.O.; Masri, A.M.; Taib, N.A.; Ahmad, N. Breast Cancer Knowledge and Screening Behaviour among Women with a Positive Family History: A Cross Sectional Study. Asian Pac. J. Cancer Prev. 2013, 14, 6783–6790. [Google Scholar] [CrossRef] [Green Version]
- Talley, C.H.; Yang, L.; Williams, K.P. Breast Cancer Screening Paved with Good Intentions: Application of the Information–Motivation–Behavioral Skills Model to Racial/Ethnic Minority Women. J. Immigr. Minority Health 2017, 19, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Tanjasiri, S.P.; Mouttapa, M.; Sablan-Santos, L.; Quitugua, L.F. What Promotes Cervical Cancer Screening Among Chamorro Women in California? J. Cancer Educ. 2012, 27, 725–730. [Google Scholar] [CrossRef] [Green Version]
- Thompson, V.L.S.; Harris, J.; Clark, E.M.; Purnell, J.; Deshpande, A.D. Broadening the examination of sociocultural constructs relevant to African-American colorectal cancer screening. Psychol. Health Med. 2015, 20, 47–58. [Google Scholar] [CrossRef] [Green Version]
- VanDyke, S.D.; Shell, M.D. Health Beliefs and Breast Cancer Screening in Rural Appalachia: An Evaluation of the Health Belief Model. J. Rural Health 2017, 33, 350–360. [Google Scholar] [CrossRef]
- Yeh, V.M.; Schnur, J.B.; Margolies, L.; Montgomery, G.H. Dense Breast Tissue Notification: Impact on Women’s Perceived Risk, Anxiety, and Intentions for Future Breast Cancer Screening. J. Am. Coll. Radiol. 2015, 12, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.-Y.; Wu, T.-Y.; Mood, D.W. Cultural affiliation and mammography screening of Chinese women in an urban county of Michigan. J. Transcult. Nurs. Off. J. Transcult. Nurs. Soc. 2005, 16, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, M.; Cappella, J.N. The Role of Theory in Developing Effective Health Communications. J. Commun. 2006, 56, S1–S17. [Google Scholar] [CrossRef]
- Webb, T.L.; Sheeran, P. Does changing behavioral intentions engender behavior change? A meta-analysis of the experimental evidence. Psychol. Bull. 2006, 132, 249–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bish, A.; Sutton, S.; Golombok, S. Predicting uptake of a routine cervical smear test: A comparison of the health belief model and the theory of planned behaviour. Psychol. Health 2000, 15, 35–50. [Google Scholar] [CrossRef]
- Fishbein, M. The role of theory in HIV prevention. Aids Care 2000, 12, 273–278. [Google Scholar] [CrossRef]
- Gollwitzer, P.M. Goal Achievement: The Role of Intentions. Eur. Rev. Soc. Psychol. 1993, 4, 141–185. [Google Scholar] [CrossRef]
- Gollwitzer, P.M.; Sheeran, P. Implementation Intentions and Goal Achievement: A Meta-analysis of Effects and Processes. In Advances in Experimental Social Psychology; Academic Press: Cambridge, MA, USA, 2006; Volume 38, pp. 69–119. [Google Scholar]
- Bowdy, M.A. The Cues to Behavior Change Model: Integration of the Health Belief Model. and the Transtheoretical Model; University of Kentucky: Lexington, KY, USA, 1998. [Google Scholar]
- Champion, V.; Maraj, M.; Hui, S.; Perkins, A.J.; Tierney, W.; Menon, U.; Skinner, C.S. Comparison of tailored interventions to increase mammography screening in nonadherent older women. Prev. Med. 2003, 36, 150–158. [Google Scholar] [CrossRef]
- Manne, S.; Markowitz, A.; Winawer, S.; Meropol, N.J.; Haller, D.; Rakowski, W.; Babb, J.; Jandorf, L. Correlates of colorectal cancer screening compliance and stage of adoption among siblings of individuals with early onset colorectal cancer. Health Psychol. 2002, 21, 3–15. [Google Scholar] [CrossRef]
- Sniehotta, F.F.; Scholz, U.; Schwarzer, R. Bridging the intention–behaviour gap: Planning, self-efficacy, and action control in the adoption and maintenance of physical exercise. Psychol. Health 2005, 20, 143–160. [Google Scholar] [CrossRef]
- Schwarzer, R. Modeling Health Behavior Change: How to Predict and Modify the Adoption and Maintenance of Health Behaviors. Appl. Psychol. 2008, 57, 1–29. [Google Scholar] [CrossRef]
- Donohue, G.A.; Tichenor, P.J.; Olien, C.N. Mass Media and the Knowledge Gap: A Hypothesis Reconsidered. Commun. Res. 1975, 2, 3–23. [Google Scholar] [CrossRef]
- Scarinci, I.C.; Beech, B.M.; Kovach, K.W.; Bailey, T.L. An Examination of Sociocultural Factors Associated with Cervical Cancer Screening Among Low-Income Latina Immigrants of Reproductive Age. J. Immigr. Health 2003, 5, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Weller, D.P.; Campbell, C. Uptake in cancer screening programmes: A priority in cancer control. Br. J. Cancer 2009, 101, S55–S59. [Google Scholar] [CrossRef] [Green Version]
- Eisend, M. Meta-Analysis in Advertising Research. J. Advert. 2017, 46, 21–35. [Google Scholar] [CrossRef]
- James, M.G. Measuring love in romantic relationships: A meta-analysis. J. Soc. Pers. Relatsh. 2010, 28, 748–771. [Google Scholar] [CrossRef]
- Milhabet, I.; Duprez, C.; Krzeminski, A.; Christophe, V. Cancer risk comparative perception and overscreening behaviours of non-carriers from BRCA1/2 families. Eur. J. Cancer Care 2013, 22, 540–548. [Google Scholar] [CrossRef] [PubMed]

| PBAR | PBEN | PBC | INT | PSEV | PSUS | CTA | CS | LIT | |
|---|---|---|---|---|---|---|---|---|---|
| PBAR | 1 | 51 (53,317) | 73 (64,065) | 86 (62,920) | 31 (19,138) | 45 (41,254) | 44 (33,560) | 129 (67,178) | 65 (38,460) |
| PBEN | −0.123 *** | 1 | 19 (31,881) | 21 (35,175) | 12 (8412) | 20 (25,018) | 12 (15,568) | 20 (18,416) | 19 (9630) |
| PBC | −0.09 *** | 0.279 *** | 1 | 32 (39,986) | 8 (6359) | 15 (20,030) | 14 (17,779) | 42 (30,184) | 20 (16,721) |
| INT | −0.116 *** | 0.267 *** | 0.212 *** | 1 | 10 (5389) | 25 (20,021) | 33 (20,319) | 26 (26,423) | 34 (16,113) |
| PSEV | −0.016 | 0.081 | 0.009 | 0.029 | 1 | 10 (7505) | 9 (3922) | 16 (6106) | 10 (7344) |
| PSUS | −0.043 * | 0.183 *** | 0.038 | 0.181 *** | 0.197 *** | 1 | 9 (9555) | 25 (18,411) | 18 (16,883) |
| CTA | −0.086 *** | 0.103 * | 0.087 # | 0.166 *** | 0.01 | 0.16 * | 1 | 36 (24,323) | 14 (9225) |
| CS | −0.147 *** | 0.196 *** | 0.164 *** | 0.259 *** | 0.031 | 0.071 ** | 0.167 *** | 1 | 50 (28,376) |
| LIT | −0.05 * | 0.175 *** | 0.172 *** | 0.158 *** | 0.008 | 0.045 | 0.072 | 0.145 *** | 1 |
| Estimate | Std. Error | z Value | Pr (>|z|) | |
|---|---|---|---|---|
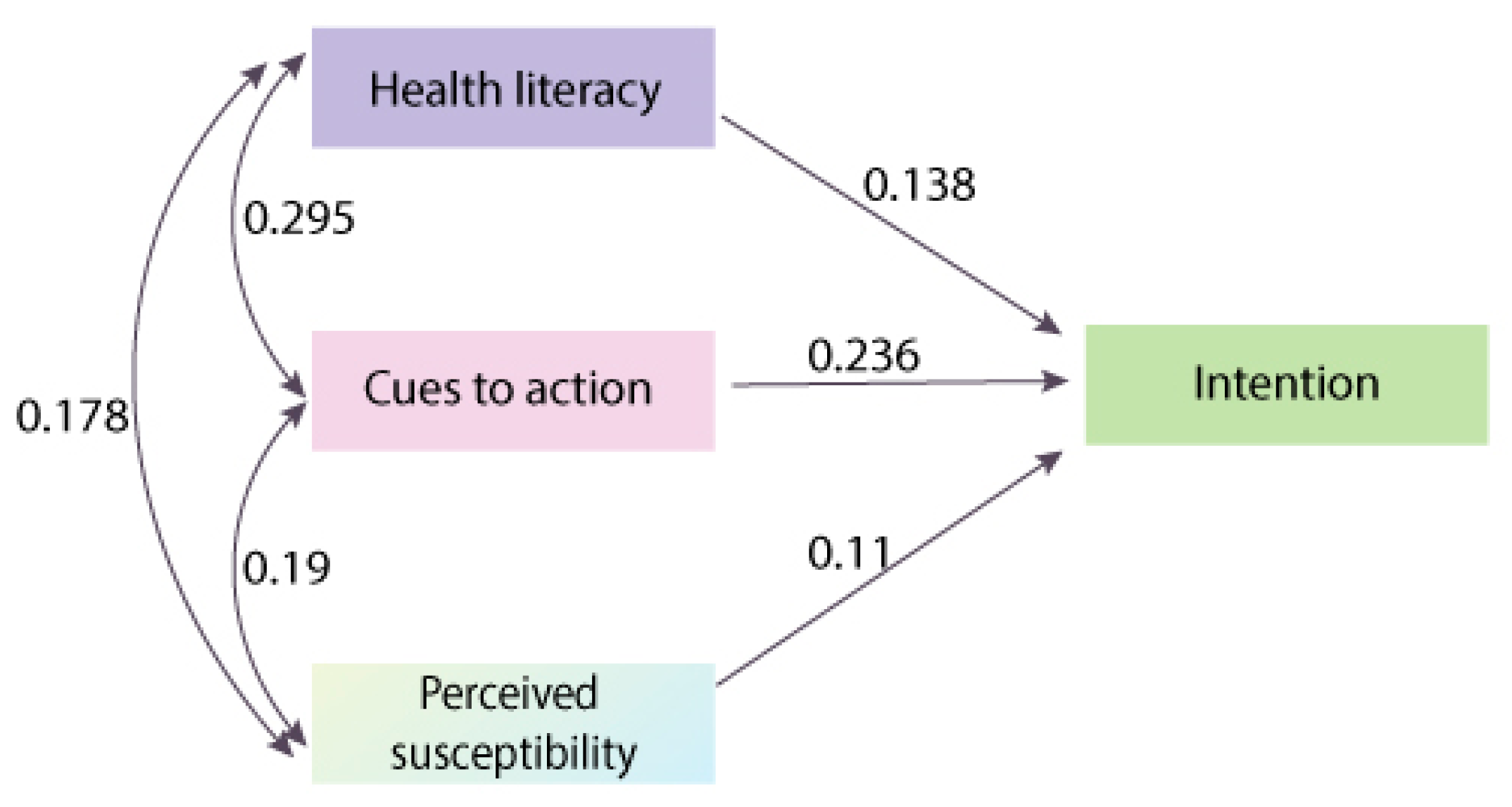
| CS on INT | −0.13 | 0.041 | −3.203 | 0.001 |
| INT on CTA | 0.236 | 0.027 | 8.673 | 0 |
| INT on LIT | 0.138 | 0.035 | 3.981 | 0 |
| INT on PBAR | −0.012 | 0.047 | −0.253 | 0.801 |
| INT on PBC | −0.03 | 0.074 | −0.409 | 0.683 |
| INT on PBEN | 0.039 | 0.044 | 0.903 | 0.367 |
| INT on PSEV | 0.093 | 0.071 | 1.319 | 0.187 |
| INT on PSUS | 0.11 | 0.056 | 1.972 | 0.049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, G.C.; Lin, Z.; Ou, W.; Su, X.; Yan, Q. A Model-Based Meta-Analysis of Willingness to Participate in Cancer Screening. Int. J. Environ. Res. Public Health 2021, 18, 2580. https://doi.org/10.3390/ijerph18052580
Feng GC, Lin Z, Ou W, Su X, Yan Q. A Model-Based Meta-Analysis of Willingness to Participate in Cancer Screening. International Journal of Environmental Research and Public Health. 2021; 18(5):2580. https://doi.org/10.3390/ijerph18052580
Chicago/Turabian StyleFeng, Guangchao Charles, Zhiliang Lin, Wanhua Ou, Xianglin Su, and Qing Yan. 2021. "A Model-Based Meta-Analysis of Willingness to Participate in Cancer Screening" International Journal of Environmental Research and Public Health 18, no. 5: 2580. https://doi.org/10.3390/ijerph18052580





