Sleep and Mental Health Disturbances Due to Social Isolation during the COVID-19 Pandemic in Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Recruitment
2.2. Electronic Instruments
2.3. Data Analyses
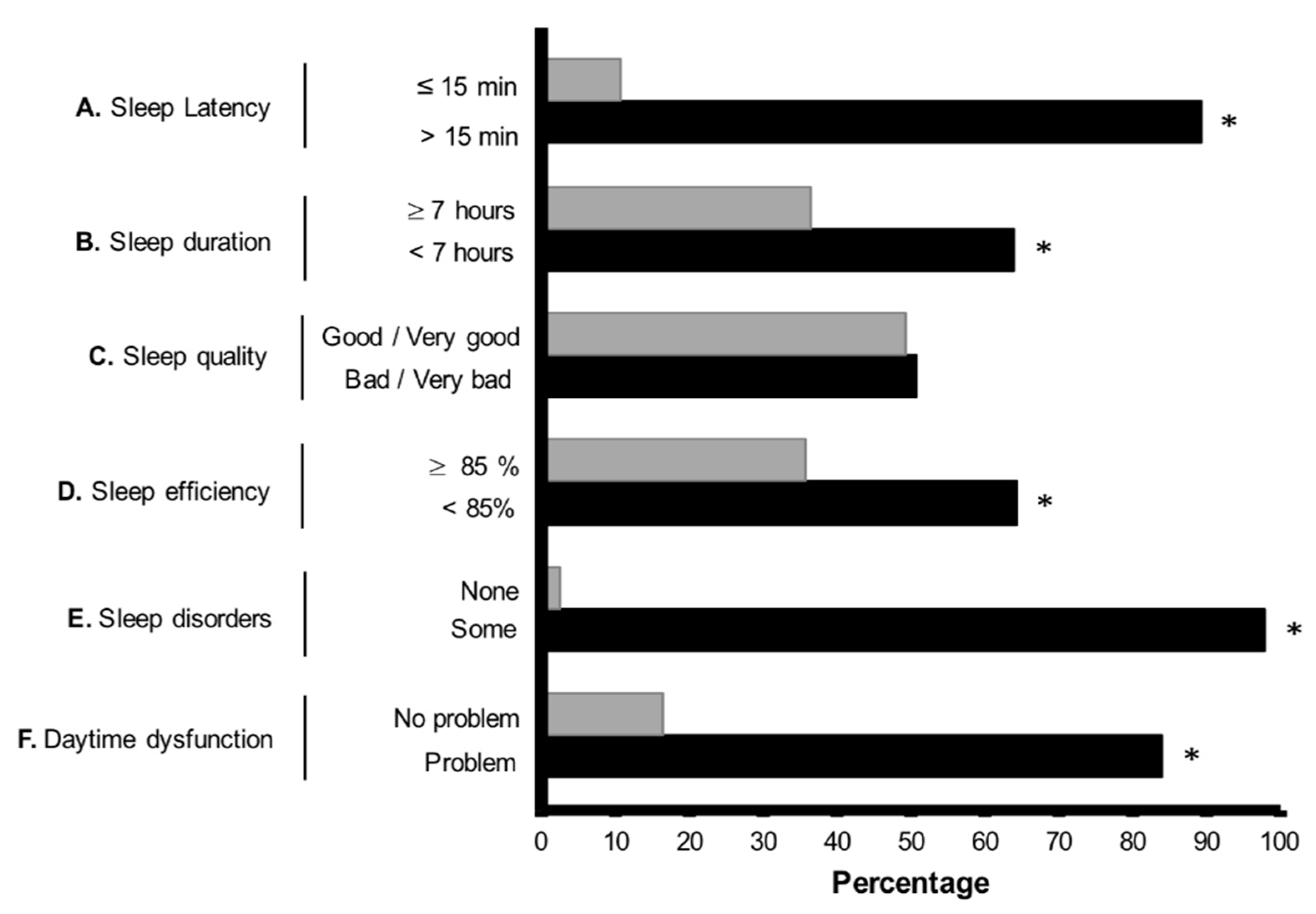
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tsang, H.W.; Scudds, R.J.; Chan, E. Psychosocial impact of SARS. Emerg. Infect. Dis. 2004, 10, 1326–1327. [Google Scholar] [PubMed]
- Vindegaard, N.; Benros, M.E. COVID-19 pandemic and mental health consequences: Systematic review of the current evidences. Brain Behav. Immun. 2020, S0889-1591, 30954–30955. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The psychological impact of quarantine and how to reduce it: Rapid review of the evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhao, N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: A web-based cross-sectional survey. Psychiatry Res. 2020, 288, 112954. [Google Scholar] [CrossRef]
- Grassian, S. Psychopathological effects of solitary confinement. Am. J. Psychiatry 1983, 140, 1450–1454. [Google Scholar]
- Mengin, A.; Allé, M.C.; Rolling, J.; Ligier, F.; Schroder, C.; Lalanne, L.; Berna, F.; Jardri, R.; Vaivaj, G.; Geoffroy, P.A.; et al. Psychological consequences of confinement. L’Encéphale 2020, 46, S43–S52. [Google Scholar] [CrossRef]
- Krystal, A. Psychiatric disorders and sleep. Neurol. Clin. 2012, 30, 1389–1413. [Google Scholar] [CrossRef] [Green Version]
- Van Laethem, M.; Beckers, D.G.J.; Dijksterhuis, A.; Geurts, S.A.E. Stress, fatigue, and sleep quality leading up to and following a stressful life event. Stress Health 2017, 33, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Van Laethem, M.; Beckers, D.G.; van Hooff, M.L.; Dijksterhuis, A.; Geurts, S.A. Day-to-day relations between stress and sleep and the mediating role of perseverative cognition. Sleep Med. 2016, 24, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Van Laethem, M.; Beckers, D.G.; Kompier, M.A.; Kecklund, G.; van den Bossche, S.N.; Geurts, S.A. Bidirectional relations between work-related stress, sleep quality and perseverative cognition. J. Psychosom Res. 2015, 79, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Lowe, C.J.; Safati, A.; Hall, P.A. The neurocognitive consequences of sleep restriction: A meta-analytic review. Neuroscie Biobehav. Rev. 2017, 80, 586–604. [Google Scholar] [CrossRef] [PubMed]
- Pires, G.N.; Bezerra, A.G.; Tufik, S.; Andersen, M.L. Effects of acute sleep deprivation on state anxiety levels: A systematic review and meta-analysis. Sleep Med. 2016, 24, 109–118. [Google Scholar] [CrossRef] [PubMed]
- McHill, A.W.; Hull, J.T.; Cohen, D.A.; Wang, W.; Czeisler, C.A.; Klerman, E.B. Chronic sleep restriction greatly magnifies performance decrements immediately after awakening. Sleep 2019, 42, zsz032. [Google Scholar] [CrossRef] [PubMed]
- Killgore, W.D. Effects of sleep deprivation on cognition. Prog. Brain Res. 2010, 185, 105–129. [Google Scholar] [PubMed]
- Short, M.A.; Chee, M.W.L. Adolescent sleep restriction effects on cognition and mood. Prog. Brain Res. 2019, 246, 55–71. [Google Scholar]
- Vriend, J.; Davidson, F.; Rusak, B.; Corkum, P. Emotional and Cognitive Impact of Sleep Restriction in Children. Sleep Med. Clin. 2015, 10, 107–115. [Google Scholar] [CrossRef]
- Basner, M.; Dinges, D.F.; Shea, J.A.; Small, D.S.; Zhu, J.; Norton, L.; Ecker, A.J.; Novak, C.; Bellini, L.M.; Volpp, K.G. Sleep and Alertness in Medical Interns and Residents: An Observational Study on the Role of Extended Shifts. Sleep 2017, 40, zsx027. [Google Scholar] [CrossRef] [PubMed]
- Shea, J.A.; Bellini, L.M.; Dinges, D.F.; Curtis, M.L.; Tao, Y.; Zhu, J.; Small, D.S.; Basner, M.; Norton, L.; Novak, C.; et al. Impact of protected sleep period for internal medicine interns on overnight call on depression, burnout, and empathy. Grad Med. Educ. 2014, 6, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Matsumori, K.; Nishimura, K.; Nishimura, Y.; Ikeda, Y.; Eto, T.; Higuchi, S. Melatonin suppression and sleepiness in children exposed to blue-enriched white LED lighting at night. Physiol. Rep. 2018, 6, e13942. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Genchi, A.; Monteverde-Maldonado, E.; Nenclares-Portocarrero, A.; Esquivel-Adame, G.; Vega-Pacheco, A. Confiabilidad y análisis factorial de la versión en español del índice de calidad de sueño de Pittsburgh en pacientes psiquiátricos. Gac Med. Mex. 2008, 144, 491–496. [Google Scholar]
- García-Campayo, J.; Zamorano, E.; Ruiz, M.A.; Pardo, A.; Perez-Paramo, M.; Lopez-Gomez, V.; Freire, O.; Rejas, J. Cultural adaptation into Spanish of the generalized anxiety disorder-7 (GAD-7) scale as a screening tool. Health Qual. Life Outcomes 2010, 8, 8. [Google Scholar] [CrossRef] [Green Version]
- Padrós-Blázquez, F.; Hurtado-Izguerra, D.; Martínez-Medina, M.P. Propiedades psicométricas de la Escala Generalized Anxiety Disorder Inventory (GADI) para la evaluación del trastorno de ansiedad generalizada en México. Ansiedad y Estrés 2019, 25, 85–90. [Google Scholar] [CrossRef]
- Arrieta, J.; Aguerrebere, M.; Raviola, G.; Flores, H.; Elliott, P.; Espinosa, A.; Reyes, A.; Ortiz-Panozo, E.; Rodriguez-Gutierrez, E.G.; Mukherjee, J.; et al. Validity and utility of the patient health questionnaire (PHQ)-2 and PHQ-9 for screening and diagnosis of depression in rural Chiapas, Mexico: A cross-sectional study. J. Clin. Psychol. 2017, 73, 1076–1090. [Google Scholar] [CrossRef] [PubMed]
- Forte, G.; Favieri, F.; Tambelli, R.; Casagrande, M. COVID-19 pandemic in the Italian population: Validation of a post-traumatic stress disorder questionnaire and prevalence of PSTD symptomatology. Int. J. Environ. Res. Public Health 2020, 17, 4151. [Google Scholar] [CrossRef]
- Forte, G.; Favieri, F.; Tambelli, R.; Casagrande, M. The enemy which sealed the world: Effects of COVID-19 diffusion on the psychological state of the Italian population. J. Clin. Med. 2020, 9, 1802. [Google Scholar] [CrossRef]
- Gulati, G.; Kelly, B.D. Domestic violence against women and the COVID-19 pandemic: What is the role of psychiatry? Int. J. Law Psychiatry 2020, 71, 101594. [Google Scholar] [CrossRef]
- Goel, N.; Basner, M.; Rao, H.; Dinges, D. Circadian rhythms, sleep deprivation and human performance. Prog. Mol. Biol. Transl. Sci. 2013, 119, 155–190. [Google Scholar]
- Goldstein, A.; Walker, M. The role of sleep in emotional brain function. Annu. Rev. Clin. Psychol. 2014, 10, 679–708. [Google Scholar] [CrossRef] [Green Version]
- Papp, K.K.; Miller, C.M.; Kingman, P.S. Clinical Review: Graduate medical training, learning, relationship and sleep loss. Sleep Med. Rev. 2016, 10, 339–345. [Google Scholar] [CrossRef]
- Neil, J. Domestic violence and COVID-19: Our hidden epidemic. Aust. J. Gen. Pract. 2020, 49. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Bolaños, R.; Cartujano-Barrera, F.; Cartujano, B.; Flores, Y.N.; Cupertino, A.P.; Gallegos-Carrillo, K. The urgent need to address violence against health workers during the COVID-19 Pandemic. Med. Care 2020, 58, 663. [Google Scholar] [CrossRef]
- Perrault, A.A.; Bayer, L.; Peuvrier, M.; Afyouni, A.; Ghisletta, P.; Brockman, C.; Spiridon, M.; Hulo Vesely, S.; Haller, D.M.; Pichon, S.; et al. Reducing the use of screen electronic devices in the evening is associated with improved sleep and daytime vigilance in adolescents. Sleep 2019, 42, zsz125. [Google Scholar] [CrossRef]
- Harbard, E.; Allen, N.; Trinder, J.; Bei, B. What’s keeping teenagers up? Prebedtime behaviors and actigraphy-assessed sleep over school and vacation. J. Adolesc. Health 2016, 58, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.; Guan, S. Screen time and sleep among school-aged children and adolescents: A systematic literature review. Sleep Med. Rev. 2015, 21, 50–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Buxton, O.; Lee, S.; Chang, A.M.; Berger, L.; Hale, L. Sleep mediates the association between adolescent screen time and depressive symptoms. Sleep Med. 2019, 57, 51–60. [Google Scholar] [CrossRef]
- Yu, B.; Steptoe, A.; Niu, K.; Ku, P.W.; Chen, L.J. Prospective associations of social isolation and loneliness with por sleep quality in older adults. Qual. Life Res. 2018, 27, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Garbazza, C.; Spitschan, M. Effects of light on human circadian rhythms, sleep and mood. Somnologie 2019, 23, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Gualano, M.R.; Lo Moro, G.; Voglino, G.; Bert, F.; Siliquini, R. Effects of Covid-19 Lockdown on Mental Health and Sleep Disturbances in Italy. Int. J. Environ. Res. Public Health 2020, 17, 4779. [Google Scholar] [CrossRef]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended Amount of Sleep for a Healthy Adult: A Joint ConsensusStatement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep 2015, 38, 843–844. [Google Scholar] [CrossRef]

| Gender | Pittsburgh Sleep Quaility Index Item | Chi-Square (n, p-Value) | |||
|---|---|---|---|---|---|
| Sleep latency | |||||
| 5–15 min | 16–30 min | 31–60 min | >60 min | n = 1154 p < 0.001 | |
| Women | 8.3% | 25.9% | 32.6% | 33.1% | |
| Men | 17.9% | 37.9% | 25.2% | 19.0% | |
| Sample % | 10.7% | 28.9% | 30.8% | 29.5% | |
| Sleep Duration | |||||
| ≥7 h | 6 h | 5 h | <5 h | n = 1154 p < 0.05 | |
| Women | 35.4% | 27.1% | 22.3% | 15.2% | |
| Men | 38.6% | 27.9% | 24.8% | 8.6% | |
| Sample % | 36.2% | 27.3% | 23.0% | 13.5% | |
| Sleep Quality | |||||
| Very good | Good | Bad | Very bad | n = 1154 p < 0.001 | |
| Women | 7.5% | 37.5% | 44.9% | 10.1% | |
| Men | 15.5% | 46.6% | 31.7% | 6.2% | |
| Sample % | 9.5% | 39.8% | 41.6% | 9.1% | |
| Sleep Efficiency | |||||
| ≥85% | 75 a 84% | 65 a 74% | <65% | n = 1005 p < 0.001 | |
| Women | 34.0% | 19.7% | 20.6% | 25.6% | |
| Men | 40.9% | 26.6% | 13.5% | 18.9% | |
| Sample % | 35.8% | 21.5% | 18.8% | 23.9% | |
| Sleep Disorders | |||||
| Not during the past month | Less than once a week | Once or twice a week | Three or more times | n = 1154 p < 0.001 | |
| Women | 2.0% | 64.2% | 32.3% | 1.5% | |
| Men | 3.4% | 77.6% | 17.9% | 1.0% | |
| Sample % | 2.3% | 67.6% | 28.7% | 1.4% | |
| Sleep Medications | |||||
| Not during the past month | Less than one a week | Once or twice a week | Three or more times | n= 1154 p < 0.57 | |
| Women | 78.6% | 7.8% | 5.9% | 7.8% | |
| Men | 82.4% | 6.2% | 4.8% | 6.6% | |
| Sample % | 79.5% | 7.4% | 5.6% | 7.5% | |
| Daytime Dysfunction | |||||
| No problem at all | Very slight problem | Somewhat of a problem | A very big problem | n = 976 p < 0.001 | |
| Women | 13.9% | 39.5% | 42.4% | 4.2% | |
| Men | 22.7% | 44.7% | 29.4% | 3.1% | |
| Sample % | 16.2% | 40.9% | 39.0% | 3.9% | |
| Age | Pittsburgh Sleep Quaility Index Item | Chi-Square (n, p-Value) | |||
|---|---|---|---|---|---|
| Sleep Latency | |||||
| 5–15 min | 16–30 min | 31–60 min | >60 min | n = 1157 p < 0.001 | |
| 18–40 | 7.2% | 24.7% | 34.1% | 34.1% | |
| >40 | 16.7% | 35.6% | 25.2% | 22.5% | |
| Sample % | 10.8% | 28.9% | 30.7% | 29.6% | |
| Sleep Duration | |||||
| ≥7 h | 6 h | 5 h | <5 h | n = 1157 p < 0.001 | |
| 18–40 | 34.2% | 27.3% | 21.5% | 17.0% | |
| >40 | 39.4% | 27.0% | 25.2% | 8.3% | |
| Sample % | 36.2% | 27.2% | 22.9% | 13.7% | |
| Sleep Quality | |||||
| Very good | Good | Bad | Very bad | n = 1157 p < 0.001 | |
| 18–40 | 4.2% | 37.0% | 48.0% | 10.8% | |
| >40 | 18.2% | 44.4% | 31.1% | 6.3% | |
| Sample % | 9.6% | 39.8% | 41.5% | 9.1% | |
| Sleep Efficiency | |||||
| ≥85% | 75 a 84% | 65 a 74% | <65% | n = 1007 p < 0.003 | |
| 18–40 | 31.7% | 22.1% | 19.2% | 26.9% | |
| >40 | 42.3% | 20.6% | 18.0% | 19.1% | |
| Sample % | 35.7% | 21.5% | 18.8% | 23.9% | |
| Sleep Disorders | |||||
| Not during the past month | Less than once a week | Once or twice a week | Three or more times | n = 1157 p < 0.001 | |
| 18–40 | 1.3% | 66.9% | 31.0% | 0.8% | |
| >40 | 4.1% | 68.9% | 24.8% | 2.3% | |
| Sample % | 2.3% | 67.7% | 28.6% | 1.4% | |
| Sleep Medications | |||||
| Not during the past month | Less than one a week | Once or twice a week | Three or more times | n= 1157 p < 0.003 | |
| 18–40 | 82.5% | 6.9% | 5.3% | 5.3% | |
| >40 | 74.8% | 8.1% | 6.3% | 10.8% | |
| Sample % | 79.5% | 7.3% | 5.7% | 7.4% | |
| Daytime Dysfunction | |||||
| No problem at all | Very slight problem | Somewhat of a problem | A very big problem | n = 978 p < 0.001 | |
| 18–40 | 12.1% | 36.9% | 45.6% | 5.4% | |
| >40 | 22.6% | 46.9% | 29.0% | 1.5% | |
| Sample % | 16.3% | 40.9% | 39.0% | 3.9% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terán-Pérez, G.; Portillo-Vásquez, A.; Arana-Lechuga, Y.; Sánchez-Escandón, O.; Mercadillo-Caballero, R.; González-Robles, R.O.; Velázquez-Moctezuma, J. Sleep and Mental Health Disturbances Due to Social Isolation during the COVID-19 Pandemic in Mexico. Int. J. Environ. Res. Public Health 2021, 18, 2804. https://doi.org/10.3390/ijerph18062804
Terán-Pérez G, Portillo-Vásquez A, Arana-Lechuga Y, Sánchez-Escandón O, Mercadillo-Caballero R, González-Robles RO, Velázquez-Moctezuma J. Sleep and Mental Health Disturbances Due to Social Isolation during the COVID-19 Pandemic in Mexico. International Journal of Environmental Research and Public Health. 2021; 18(6):2804. https://doi.org/10.3390/ijerph18062804
Chicago/Turabian StyleTerán-Pérez, Guadalupe, Angelica Portillo-Vásquez, Yoaly Arana-Lechuga, Oscar Sánchez-Escandón, Roberto Mercadillo-Caballero, Rosa Obdulia González-Robles, and Javier Velázquez-Moctezuma. 2021. "Sleep and Mental Health Disturbances Due to Social Isolation during the COVID-19 Pandemic in Mexico" International Journal of Environmental Research and Public Health 18, no. 6: 2804. https://doi.org/10.3390/ijerph18062804
APA StyleTerán-Pérez, G., Portillo-Vásquez, A., Arana-Lechuga, Y., Sánchez-Escandón, O., Mercadillo-Caballero, R., González-Robles, R. O., & Velázquez-Moctezuma, J. (2021). Sleep and Mental Health Disturbances Due to Social Isolation during the COVID-19 Pandemic in Mexico. International Journal of Environmental Research and Public Health, 18(6), 2804. https://doi.org/10.3390/ijerph18062804







