Sensory Input Modulates Microsaccades during Heading Perception
Abstract
1. Introduction
2. Materials and Methods
2.1. Optic Flow Stimuli
2.2. Eye Movements and Eye Position Recordings
2.3. Data Analysis
3. Results
3.1. Main Sequence
3.2. Microsaccades Duration
3.3. Microsaccades Peak Velocity
3.4. Microsaccades Rate
3.5. Microsaccades Directions
4. Discussion
4.1. The Effect of Optic Flow Stimuli on Microsaccades
4.2. The Role of the Proprioceptive Input on Microsaccades Characteristics and Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibson, J.J. The Perception of the Visual World; Houghton Mifflin: Boston, MA, USA, 1950. [Google Scholar]
- Gibson, J.J. The visual perception of objective motion and subjective movement. Psychol. Rev. 1954, 61, 304–314. [Google Scholar] [CrossRef]
- Gibson, J.J. Visually controlled locomotion and visual orientation in animals. Br. J. Psychol. 1958, 49, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Duffy, C.J.; Wurtz, R.H. Sensitivity of MST neurons to optic flow stimuli. I. A continuum of response selectivity to large field stimuli. J. Neurophysiol. 1991, 65, 1329–1345. [Google Scholar] [CrossRef]
- Duffy, C.J.; Wurtz, R.H. Sensitivity of MST neurons to optic flow stimuli. II. Mechanism of response selectivity revealed by small-field stimuli. J. Neurophysiol. 1991, 65, 1346–1359. [Google Scholar] [CrossRef]
- Raffi, M.; Maioli, M.G.; Squatrito, S. Optic flow direction coding in area PEc of the behaving monkey. Neuroscience 2011, 194, 136–149. [Google Scholar] [CrossRef]
- Raffi, M.; Piras, A.; Calzavara, R.; Squatrito, S. Area PEc neurons use a multiphasic pattern of activity to signal the spatial properties of optic flow. BioMed Res. Int. 2017, 2017, 6495872. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.M.; Read, H.L. Analysis of optic flow in the monkey parietal area 7a. Cereb. Cortex 1997, 7, 327–346. [Google Scholar] [CrossRef]
- Merchant, H.; Battaglia-Mayer, A.; Georgopoulos, A.P. Effects of optic flow in motor cortex and area 7a. J. Neurophysiol. 2001, 86, 1937–1954. [Google Scholar] [CrossRef]
- Raffi, M.; Siegel, R.M. A functional architecture of optic flow in the inferior parietal lobule of the behaving monkey. PLoS ONE 2007, 2, e200. [Google Scholar] [CrossRef]
- Lee, B.; Pesaran, B.; Andersen, R.A. Area MSTd neurons encode visual stimuli in eye coordinates during fixation and pursuit. J. Neurophysiol. 2011, 105, 60–68. [Google Scholar] [CrossRef]
- Raffi, M.; Carrozzini, C.; Maioli, M.G.; Squatrito, S. Multimodal representation of optic flow in area PEc of macaque monkey. Neuroscience 2010, 171, 1241–1255. [Google Scholar] [CrossRef]
- Chen, X.; DeAngelis, G.C.; Angelaki, D.E. Eye-centered representation of optic flow tuning in the ventral intraparietal area. J. Neurosci. 2013, 33, 18574–18582. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raffi, M.; Persiani, M.; Piras, A.; Squatrito, S. Optic flow neurons in area PEc integrate eye and head position signals. Neurosci. Lett. 2014, 568, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Avila, E.; Lakshminarasimhan, K.J.; DeAngelis, G.C.; Angelaki, D.E. Visual and Vestibular Selectivity for Self-Motion in Macaque Posterior Parietal Area 7a. Cereb. Cortex 2019, 29, 3932–3947. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; DeAngeli, S.G.C.; Angelaki, D.E. Convergence of vestibular and visual self-motion signals in an area of the posterior sylvian fissure. J. Neurosci. 2011, 31, 11617–11627. [Google Scholar] [CrossRef] [PubMed]
- Leeder, T.; Fallahtafti, F.; Schieber, M.; Myers, S.A.; Blaskewicz Boron, J.; Yentes, J.M. Optic flow improves step width and length in older adults while performing dual task. Aging Clin. Exp. Res. 2019, 31, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Pechtl, K.S.; Jennings, J.R.; Redfern, M.S. Optic flow and attention alter locomotion differently in the young and old. Gait Posture 2020, 76, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Salinas, M.M.; Wilken, J.M.; Dingwell, J.B. How humans use visual optic flow to regulate stepping during walking. Gait Posture 2017, 57, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Zadra, J.R.; Proffitt, D.R. Optic flow is calibrated to walking effort. Psychon. Bull. Rev. 2016, 23, 1491–1496. [Google Scholar] [CrossRef]
- Nougier, V.; Bard, C.; Fleury, M.; Teasdale, N. Contribution of central and peripheral vision to the regulation of stance. Gait Posture 1997, 5, 34–41. [Google Scholar] [CrossRef]
- Persiani, M.; Piras, A.; Squatrito, S.; Raffi, M. Laterality of stance during optic flow stimulation in male and female young adults. BioMed Res. Int. 2015, 2015, 542645. [Google Scholar] [CrossRef]
- Piras, A.; Raffi, M.; Perazzolo, M.; Squatrito, S. Influence of heading perception in the control of posture. J. Electromyogr. Kinesiol. 2018, 39, 89–94. [Google Scholar] [CrossRef]
- Raffi, M.; Piras, A.; Persiani, M.; Perazzolo, M.; Squatrito, S. Angle of gaze and optic flow direction modulate body sway. J. Electromyogr. Kinesiol. 2017, 35, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Raffi, M.; Piras, A.; Persiani, M.; Squatrito, S. Importance of optic flow for postural stability of male and female young adults. Eur. J. Appl. Physiol. 2014, 114, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Conde, S.; Otero-Millan, J.; Macknik, S.L. The impact of microsaccades on vision: Towards a unified theory of saccadic function. Nat. Rev. Neurosci. 2013, 14, 83–96. [Google Scholar] [CrossRef]
- Martinez-Conde, S.; Macknik, S.L.; Hubel, D.H. The role of fixational eye movements in visual perception. Nat. Rev. Neurosci. 2004, 5, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Conde, S.; Macknik, S.L.; Troncoso, X.G.; Hubel, D.H. Microsaccades: A neurophysiological analysis. Trends Neurosci. 2009, 32, 463–475. [Google Scholar] [CrossRef]
- Engbert, R.; Kliegl, R. Microsaccades uncover the orientation of covert attention. Vis. Res. 2003, 43, 1035–1045. [Google Scholar] [CrossRef]
- Laubrock, J.; Engbert, K.; Kliegl, R. Fixational eye movements predict the perceived direction of ambiguous apparent motion. J. Vis. 2008, 8, 1–17. [Google Scholar] [CrossRef]
- Piras, A.; Raffi, M.; Lanzoni, I.M.; Persiani, M.; Squatrito, S. Microsaccades and prediction of a motor act outcome in a dynamic sport situation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4520–4530. [Google Scholar] [CrossRef]
- Piras, A.; Raffi, M.; Perazzolo, M.; Malagoli Lanzoni, I.; Squatrito, S. Microsaccades and interest areas during free-viewing sport task. J. Sports Sci. 2019, 37, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Meyberg, S.; Sinn, P.; Engbert, R.; Sommer, W. Revising the link between microsaccades and the spatial cueing of voluntary attention. Vis. Res. 2017, 133, 47–60. [Google Scholar] [CrossRef]
- Hafed, Z.M.; Chen, C.Y.; Tian, X. Vision, perception, and attention through the lens of microsaccades: Mechanisms and implications. Front. Syst. Neurosci. 2015, 9, 167. [Google Scholar] [CrossRef] [PubMed]
- Yuval-Greenberg, S.; Merriam, E.P.; Heeger, D.J. Spontaneous microsaccades reflect shifts in covert attention. J. Neurosci. 2014, 34, 13693–13700. [Google Scholar] [CrossRef] [PubMed]
- Hafed, Z.M. Alteration of visual perception prior to microsaccades. Neuron 2013, 77, 775–786. [Google Scholar] [CrossRef]
- Piras, A.; Raffi, M.; Persiani, M.; Perazzolo, M.; Squatrito, S. Effect of heading perception on microsaccade dynamics. Behav. Brain Res. 2016, 312, 246–252. [Google Scholar] [CrossRef]
- Wang, S.; Fukuchi, M.; Koch, C.; Tsuchiya, N. Spatial attention is attracted in a sustained fashion toward singular points in the optic flow. PLoS ONE 2012, 7, e41040. [Google Scholar] [CrossRef]
- McCamy, M.B.; Otero-Millan, J.; Di Stasi, L.L.; Macknik, S.L.; Martinez-Conde, S. Highly informative natural scene regions increase microsaccade production during visual scanning. J. Neurosci. 2014, 34, 2956–2966. [Google Scholar] [CrossRef]
- Meredith, M.A.; Stein, B.E. Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. J. Neurophysiol. 1986, 56, 640–662. [Google Scholar] [CrossRef]
- Costa, M.; Piché, M.; Lepore, F.; Guillemota, J.P. Age-related audiovisual interactions in the superior colliculus of the rat. Neuroscience 2016, 320, 19–29. [Google Scholar] [CrossRef]
- Nagy, B.; Corneil, B.D. Representation of horizontal head-on-body position in the primate superior colliculus. J. Neurophysiol. 2010, 103, 858–874. [Google Scholar] [CrossRef]
- Waleszczyk, W.J.; Wang, C.; Benedek, G.; Burke, W.; Dreher, B. Motion sensitivity in cat′s superior colliculus: Contribution of different visual processing channels to response properties of collicular neurons. Acta Neurobiol. Exp. 2004, 64, 209–228. [Google Scholar]
- Elias, L.J.; Bryden, M.P.; Bulman-Fleming, M.B. Footedness is a better predictor than is handedness of emotional lateralization. Neuropsychologia 1998, 36, 37–43. [Google Scholar] [CrossRef]
- Levin, H.S.; High, W.M.; Williams, D.H.; Eisenberg, H.M.; Amparo, E.G.; Guinto, F.C.; Ewert, J. Dichotic listening and manual performance in relation to magnetic resonance imaging after closed head injury. J. Neurol. Neurosurg. Psychiatry 1989, 52, 1162–1169. [Google Scholar] [CrossRef]
- Regan, D.; Beverley, K.I. How do we avoid confounding the direction we are looking and the direction we are moving? Science 1982, 215, 194–196. [Google Scholar] [CrossRef]
- Zuber, B.L.; Semmlow, J.L.; Stark, L. Frequency characteristics of the saccadic eye movement. Biophys. J. 1968, 8, 1288–1298. [Google Scholar] [CrossRef]
- Otero-Millan, J.; Castro, J.L.; Macknik, S.L.; Martinez-Conde, S. Unsupervised clustering method to detect microsaccades. J. Vis. 2014, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, X.G.; Macknik, S.L.; Martinez-Conde, S. Microsaccades counteract perceptual filling-in. J. Vis. 2008, 8, 15–19. [Google Scholar] [CrossRef]
- Putnam, N.M.; Hofer, H.J.; Doble, N.; Chen, L.; Carroll, J.; Williams, D.R. The locus of fixation and the foveal cone mosaic. J. Vis. 2005, 5, 632–639. [Google Scholar] [CrossRef]
- Ko, H.K.; Poletti, M.; Rucci, M. Microsaccades precisely relocate gaze in a high visual acuity task. Nat. Neurosci. 2010, 13, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
- Poletti, M.; Listorti, C.; Rucci, M. Microscopic eye movements compensate for nonhomogeneous vision within the fovea. Curr. Biol. 2013, 23, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Hafed, Z.M.; Krauzlis, R.J. Microsaccadic suppression of visual bursts in the primate superior colliculus. J. Neurosci. 2010, 30, 9542–9547. [Google Scholar] [CrossRef]
- Hafed, Z.M.; Lovejoy, L.P.; Krauzlis, R.J. Modulation of microsaccades in monkey during a covert visual attention task. J. Neurosci. 2011, 31, 15219–15230. [Google Scholar] [CrossRef] [PubMed]
- Herrington, T.M.; Masse, N.Y.; Hachmeh, K.J.; Smith, J.E.; Assad, J.A.; Cook, E.P. The effect of microsaccades on the correlation between neural activity and behavior in middle temporal, ventral intraparietal, and lateral intraparietal areas. J. Neurosci. 2009, 29, 5793–5805. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Matsuo, Y.; Zha, L.; Macaskill, M.R.; Kobayashi, Y. Fixational saccades alter the gap effect. Eur. J. Neurosci. 2014, 39, 2098–2106. [Google Scholar] [CrossRef] [PubMed]
- Hafed, Z.M.; Clark, J. Microsaccades as an overt measure of covert attention shifts. Vis. Res. 2002, 42, 2533–2545. [Google Scholar] [CrossRef]
- Rolfs, M.; Kliegl, R.; Engbert, K. Toward a model of microsaccade generation: The case of microsaccadic inhibition. J. Vis. 2008, 8, 1–23. [Google Scholar] [CrossRef]
- Cui, J.; Wilke, M.; Logothetis, N.K.; Leopold, D.A.; Liang, H. Visibility states modulate microsaccade rate and direction. Vis. Res. 2009, 49, 228–236. [Google Scholar] [CrossRef]
- Dalmaso, M.; Castelli, L.; Scatturin, P.; Galfano, G. Working memory load modulates microsaccadic rate. J. Vis. 2017, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yan, H.; Sun, H.J. Modulation of microsaccade rate by task difficulty revealed through between- and within-trial comparisons. J. Vis. 2015, 15, 1–15. [Google Scholar] [CrossRef]
- Xue, L.; Huang, D.; Wang, T.; Hu, Q.; Chai, X.; Li, L.; Chen, Y. Dynamic modulation of the perceptual load on microsaccades during a selective spatial attention task. Sci. Rep. 2017, 7, 16496. [Google Scholar] [CrossRef] [PubMed]
- Reed-Jones, R.; Reed-Jones, J.; Vallis, L.A.; Hollands, M. The effects of constraining eye movements on visually evoked steering responses during walking in a virtual environment. Exp. Brain Res. 2009, 197, 357–367. [Google Scholar] [CrossRef] [PubMed]
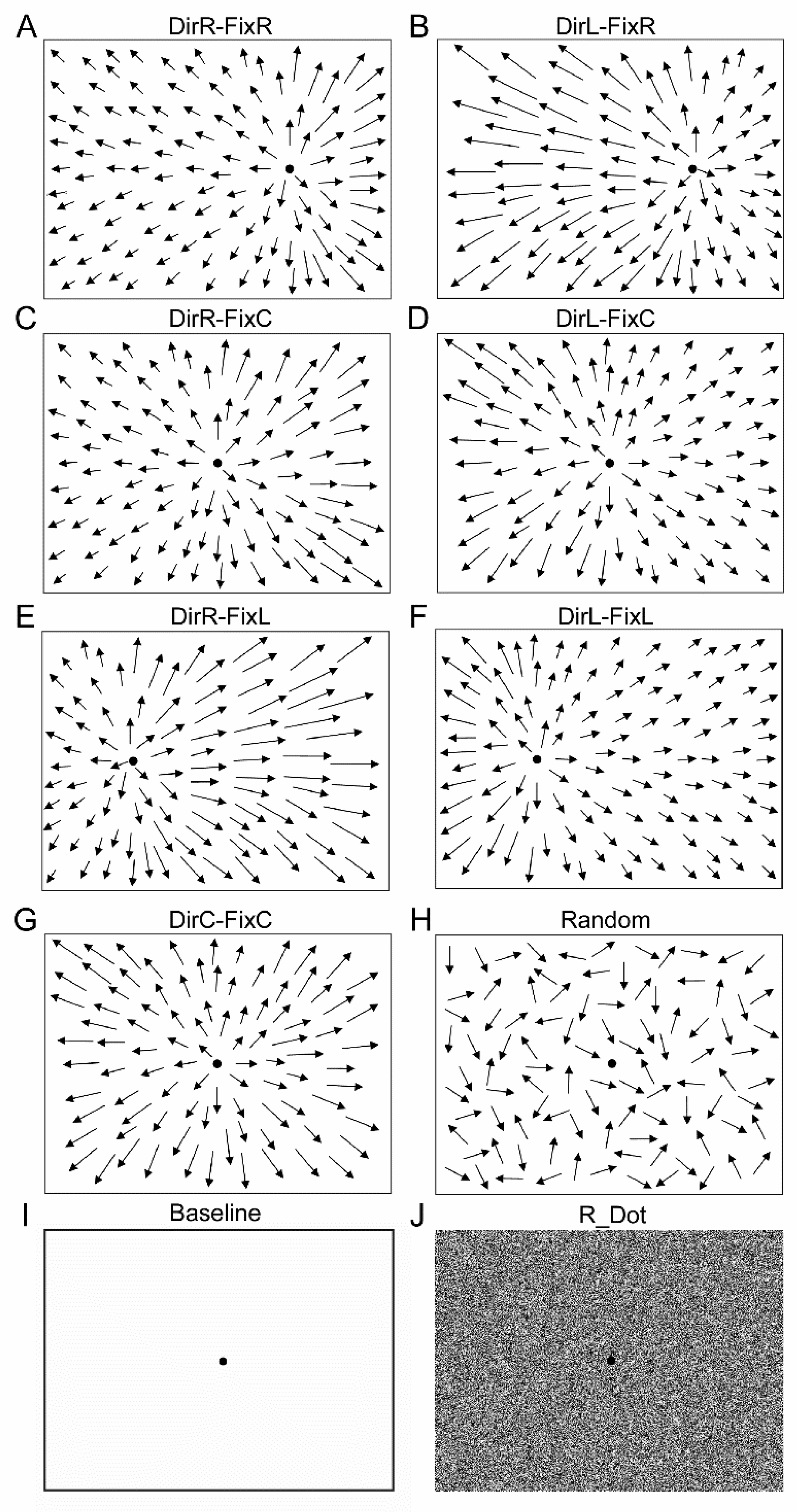

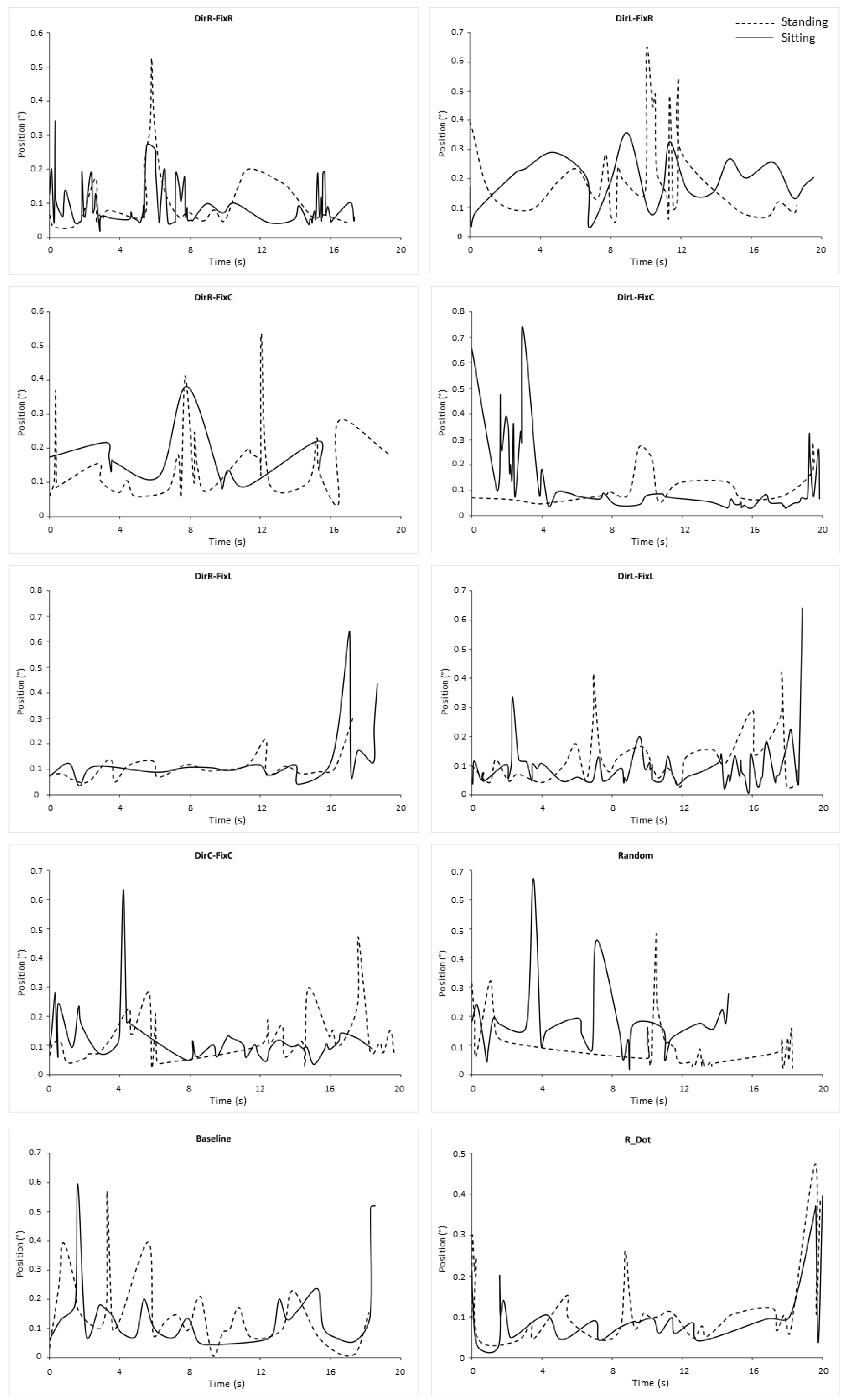
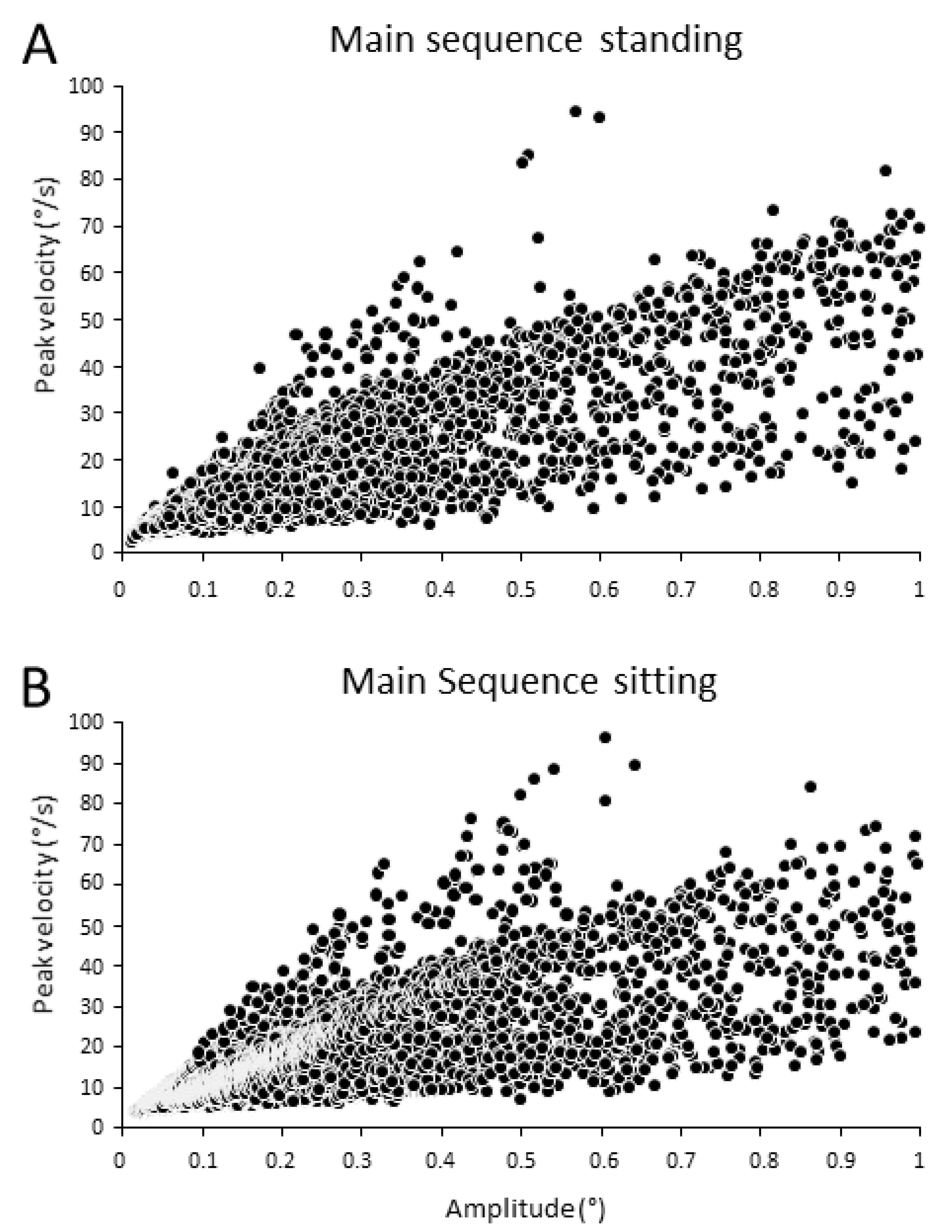
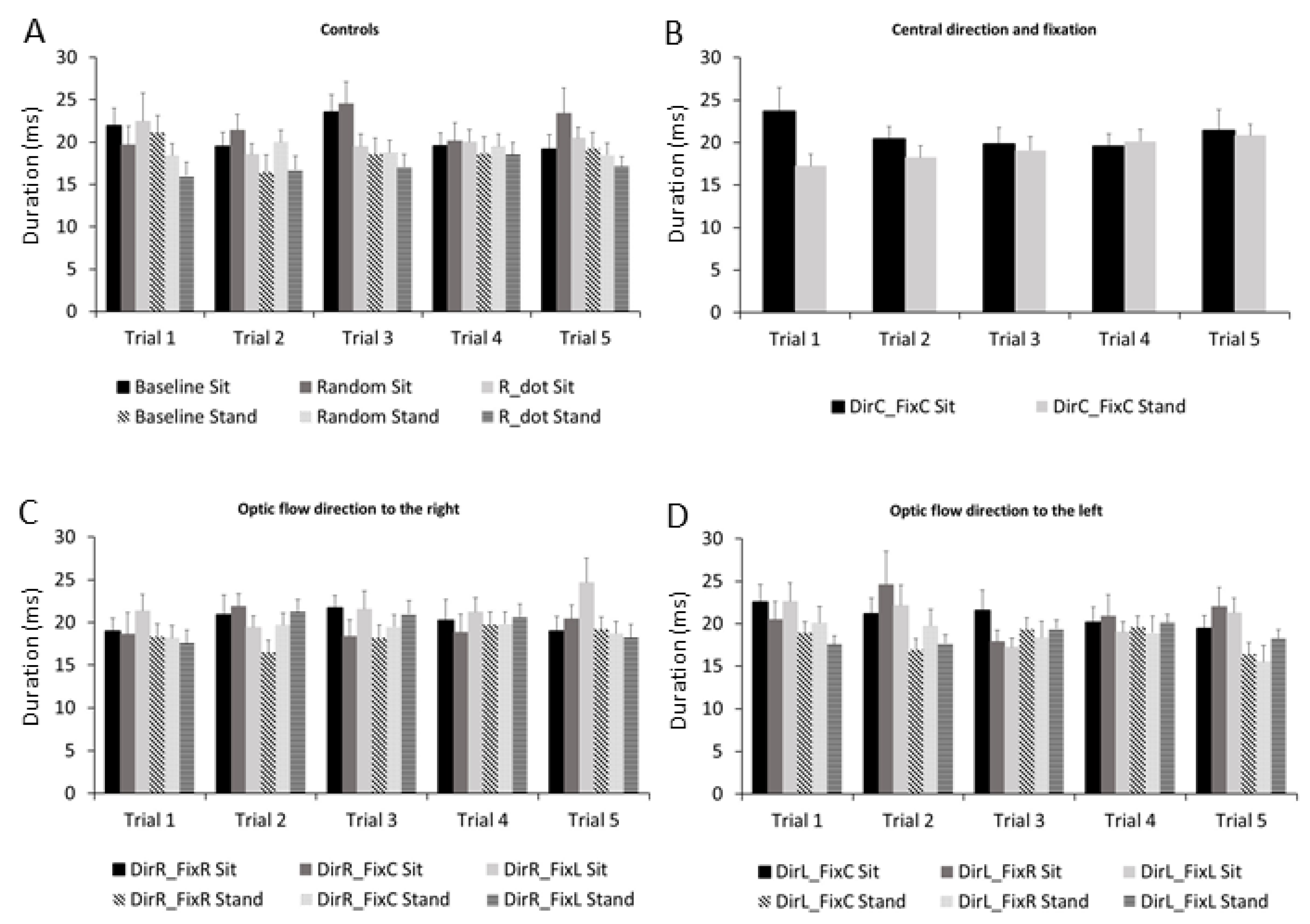

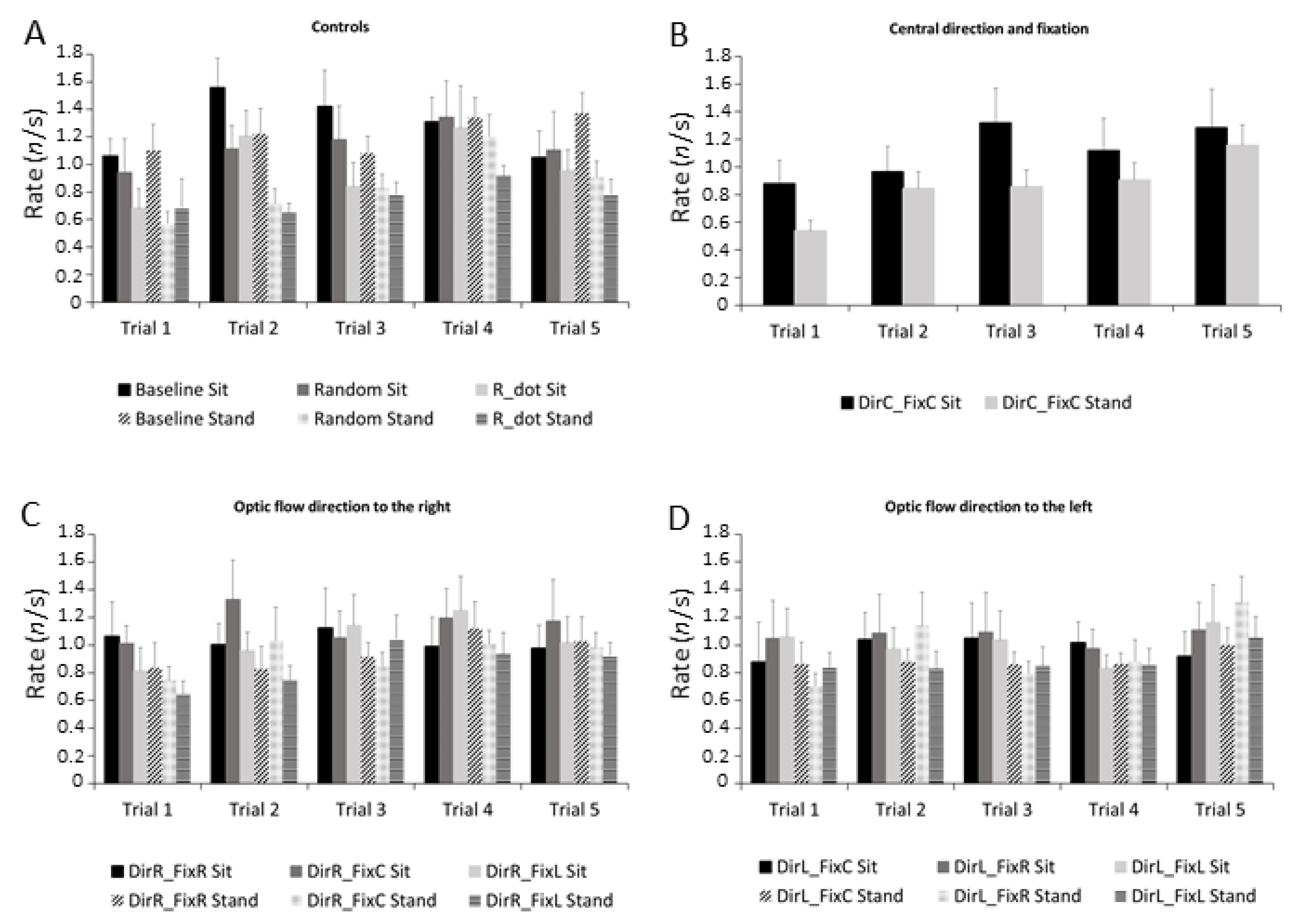
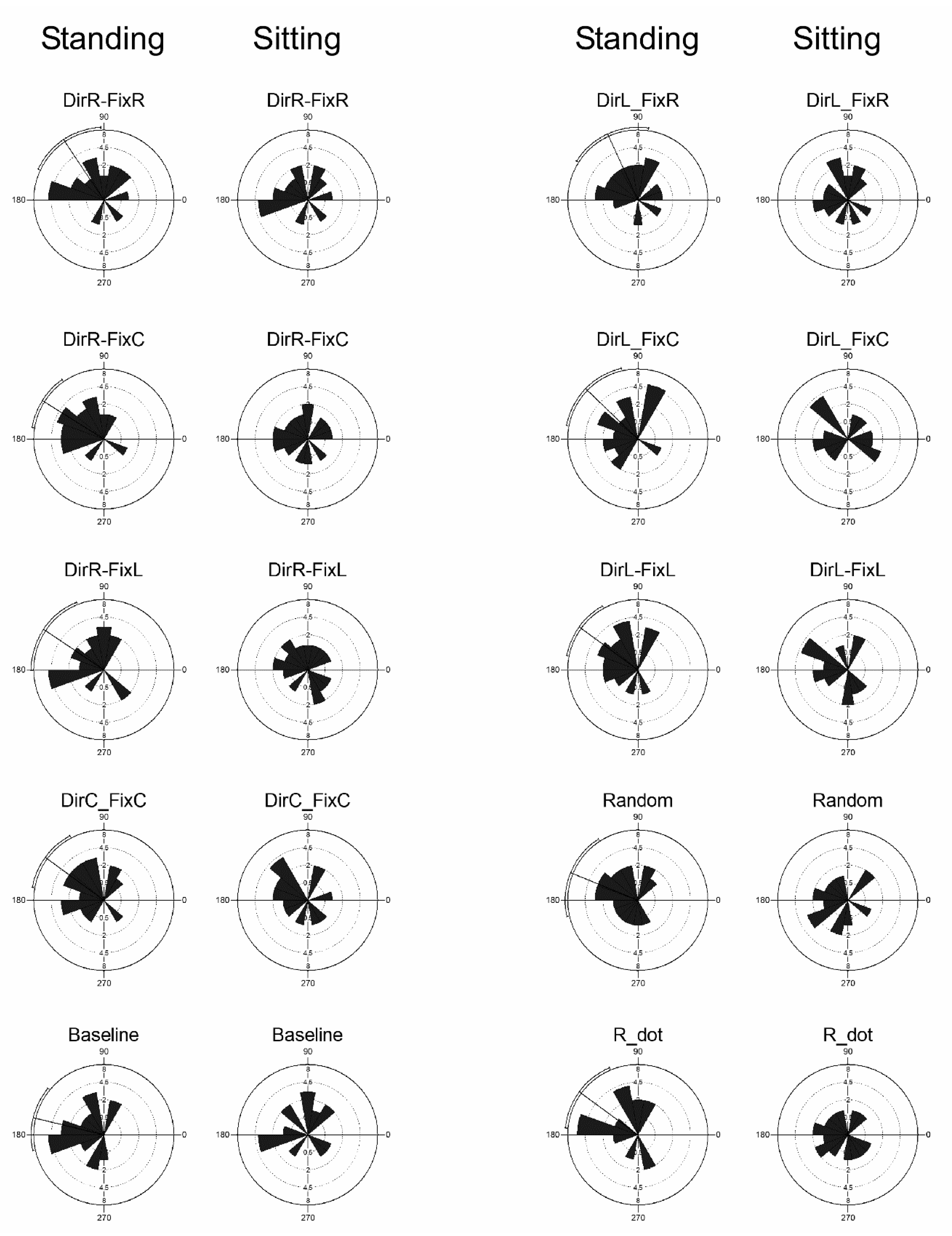
| Stimulus Pairwise Comparison | p-Value |
|---|---|
| Baseline vs. R_dot | p = 0.005 |
| DirR-FixC vs. R_dot | p = 0.003 |
| DirR-FixL vs. R_dot | p = 0.001 |
| DirL-FixC vs. R_dot | p = 0.002 |
| DirL-FixL vs. R_dot | p = 0.001 |
| Random vs. R_dot | p = 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raffi, M.; Trofè, A.; Perazzolo, M.; Meoni, A.; Piras, A. Sensory Input Modulates Microsaccades during Heading Perception. Int. J. Environ. Res. Public Health 2021, 18, 2865. https://doi.org/10.3390/ijerph18062865
Raffi M, Trofè A, Perazzolo M, Meoni A, Piras A. Sensory Input Modulates Microsaccades during Heading Perception. International Journal of Environmental Research and Public Health. 2021; 18(6):2865. https://doi.org/10.3390/ijerph18062865
Chicago/Turabian StyleRaffi, Milena, Aurelio Trofè, Monica Perazzolo, Andrea Meoni, and Alessandro Piras. 2021. "Sensory Input Modulates Microsaccades during Heading Perception" International Journal of Environmental Research and Public Health 18, no. 6: 2865. https://doi.org/10.3390/ijerph18062865
APA StyleRaffi, M., Trofè, A., Perazzolo, M., Meoni, A., & Piras, A. (2021). Sensory Input Modulates Microsaccades during Heading Perception. International Journal of Environmental Research and Public Health, 18(6), 2865. https://doi.org/10.3390/ijerph18062865








