Abstract
An association between fiber intake and allergic diseases in children has been reported; however, many studies have not been conducted to assess this association in adults. We aimed to evaluate the association between dietary fiber intake and allergic diseases (asthma, allergic rhinitis, and atopic dermatitis) among 10,479 adults using data from the Korean National Health and Nutrition Examination Survey (2010–2011). As dietary fiber intake increased, the prevalence of asthma (Q4 adjusted odds ratio (OR): 0.656; 95% confidence interval (CI): 0.48–0.91, p for trend < 0.0001) and atopic dermatitis (Q3 crude OR: 0.746; 95% CI: 0.57–0.98; Q4 adjusted OR: 0.712; 95% CI: 0.50–1.01, p for trend < 0.0001) decreased. The prevalence of allergic rhinitis (Q2 adjusted OR: 0.840; 95% CI: 0.70–1.00, p for trend < 0.0001) tended to decrease, especially in males. Subgroup analysis revealed that fiber intake reduced allergic rhinitis symptoms, including watery rhinorrhea (Q3 adjusted OR: 0.734; 95% CI: 0.55–0.97; Q4 adjusted OR: 0.722; 95% CI: 0.54–0.97) and dog allergen sensitization (Q3 adjusted OR: 0.319; 95% CI: 0.13–0.82; Q4 adjusted OR: 0.338; 95% CI: 0.13–0.86), exclusively in males. Thus, dietary fiber intake influences allergic diseases in adults, especially males.
1. Introduction
Dietary fibers can be defined as a group of carbohydrates found in plant-origin foods that are not digested or absorbed by the human body [1]. More specifically, dietary fibers include polysaccharides, oligosaccharides, lignin, and resistant starch [2]. Epidemiological studies have widely reported associations of dietary fiber intake with reduced risks of various diseases, including cardiovascular [3,4], metabolic [5,6], gastrointestinal [7,8], and pulmonary diseases [9] and malignancies [10,11]. Furthermore, increased dietary fiber intake influences the composition of the intestinal microbiota [12], thereby reducing chronic inflammation [13] and improving immune function [14].
Allergy is a damaging immune response by the body against a substance that the body has become hypersensitive toward [15]. As the prevalence of allergic diseases, including asthma, allergic rhinitis, and atopic dermatitis, increases up to 40% in the global population [16], the importance of the association between dietary fiber intake and allergic diseases increases. Many studies evaluated the associations between dietary fiber intake and allergic diseases, including asthma, allergic rhinitis, and atopic disease, especially in the prenatal period and during infancy and childhood [17,18,19]. Prenatal fiber treatment viva maternal dietary fiber intake showed a reduction in the occurrence of infant eczema [20] and protection against infant wheezing [21]. Previous studies have shown no effect of dietary fibers on food allergies among children [22], with some conflicting results regarding eczema, allergic rhinitis, and asthma. Large trials on fiber treatment in children with eczema failed to show beneficial effects [23]; however, most recent studies reported improved symptoms of eczema in the fiber consumption group compared with the placebo group [24]. Several double-blind, placebo-controlled studies showed no beneficial effects in children with asthma and allergic rhinitis in the fiber-treated group [25]; however, a recent study among school-aged children with asthma and allergic rhinitis showed significantly reduced clinical symptoms and improved pulmonary functions with increased dietary fiber intake [26]. However, there is not much information about dietary fiber intake and allergic diseases in adults.
Herein, we assessed the association of dietary fiber intake with allergic diseases, including asthma, allergic rhinitis, and atopic dermatitis, in adults based on data from a large nationwide survey in South Korea.
2. Materials and Methods
2.1. Study Population
This cross-sectional study obtained data from the Korean National Health and Nutrition Examination Survey (KNHANES) conducted from 2010 to 2011. KNHANES is a nationwide survey that represents the nonindustrialized South Korean population by extracting samples using a stratified multistage-clustered probability sampling design. The survey consisted of a health interview, nutritional survey, and physical examination. The fifth KNHANES, with the participation of the Korean Society of Otorhinolaryngology-Head and Neck, detailed medical interviews, and endoscopic otorhinolaryngological examination, was conducted by residents of the otorhinolaryngology department.
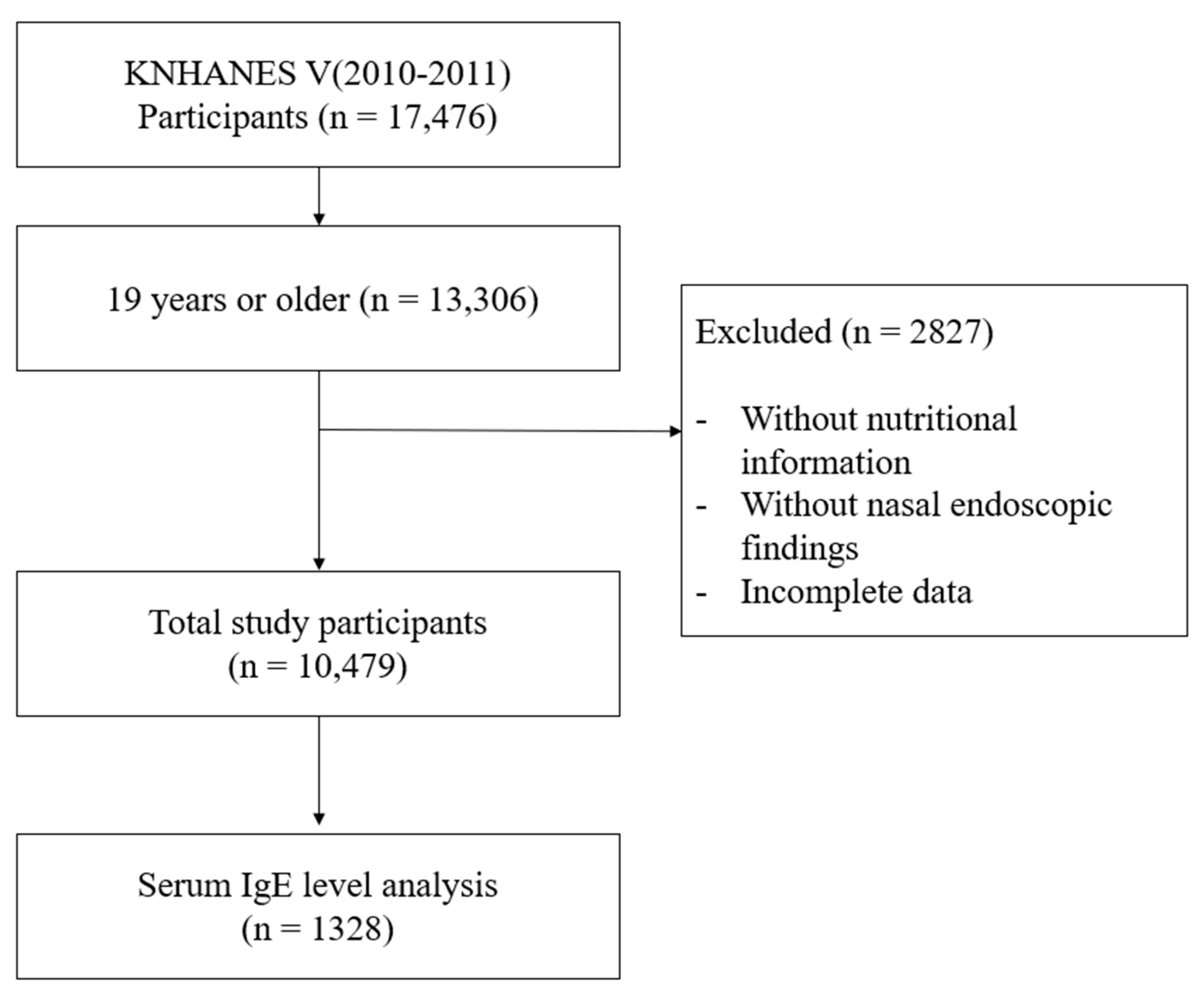
Among 17,476 participants, those aged under 19 years, without nutritional intake information, without nasal endoscopic examination, or with incomplete data, were excluded. Finally, a total of 10,479 participants were enrolled in this study (Figure 1). The baseline characteristics of participants are shown in Supplementary Table S1.
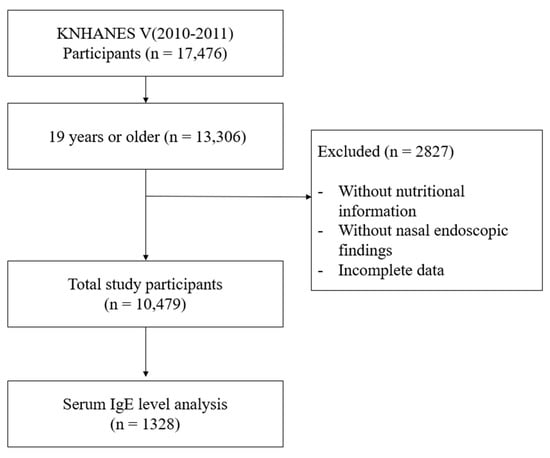
Figure 1.
Flow chart for study population selection. KNHANES: Korea National Health and Nutrition Examination Survey.
2.2. Diagnosis of Allergic Diseases and Assessment of Related Conditions
Allergic diseases, including asthma, allergic rhinitis, and atopic dermatitis, were defined based on whether the participants had ever been diagnosed with asthma/allergic rhinitis/atopic dermatitis by a physician.
Asthma-related conditions were determined based on the following question: “Have you experienced wheezing or whistling sounds during breathing in the last one year?” and “Have you experienced wheezing or whistling sounds when you breathe while or after exercising in the last one year?” Allergic rhinitis-related conditions were determined based on the question “Have you ever experienced rhinitis symptoms, including rhinorrhea, itching sensation of nose, and sneezing, in the last one year?” and based on nasal endoscopic findings of pale mucosa or watery rhinorrhea before mucosal shrinkage.
In 2010, serum total immunoglobulin E (IgE) and specific IgE levels for three common indoor allergens (house dust mite, dog, and cockroach) were measured in 10% of the total participants via ImmunoCAP. The cut-off values for total IgE and specific IgEs were defined as 100 kU/L and 0.35 kU/L, respectively.
2.3. Assessment of Daily Nutritional Intake
Daily intake of nutrients, including energy, carbohydrate, protein, fat, fiber, and water, was investigated based on questionnaires about the participants’ consumption frequency of 63 common Korean foods in the previous year. The daily amount of each nutrient, including dietary fiber, was quantified using the nutrient database provided by the Korea Health and Industry of Development Institute (Ministry of Health and Welfare, 2010). Participants were classified into quartiles (Q1–Q4) according to the amount of dietary fiber intake.
2.4. Assessment of Other Variables
Characteristics of participants, including age, sex, household income, residency, alcohol consumption, smoking status, body mass index, and physical activity, were assessed. Household income was categorized into the four following groups with respect to quartiles: <25%, 25–50%, 51–75%, and ≥75%. Residency was classified as urban and rural areas according to the official addresses of the participants. Participants were classified into two groups according to their alcohol consumption ≥1 time a month. Regarding smoking habits, participants were divided into two groups: current smokers and ex-smokers/nonsmokers. Additionally, participants were classified into two groups depending on whether they performed moderate physical activity for more than 20 min more than 5 days a week.
2.5. Statistical Analysis
Statistical analysis was conducted using the Statistical Analysis System (SAS) version 9.4 (SAS Institute, Inc., Cary, NC, USA). Univariate and multivariate logistic regression analyses were conducted to evaluate the association between dietary fiber intake and each allergic disease or related condition. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. For multivariate logistic regression analysis, confounding factors, including age, sex, residency, household income, smoking status, alcohol consumption, physical activity, BMI, and amount of nutrients (energy, carbohydrate, protein, fat, and water intake), were used. p values < 0.05 were considered statistically significant. Furthermore, subgroup analysis according to sex was performed, and the p-value for interaction was calculated to investigate the effect of sex on the relationship between dietary fiber and allergy.
3. Results
3.1. Association between Fiber Intake and Allergic Diseases
Table 1 shows the daily amount of nutritional intake according to the presence of each allergic disease. The allergic rhinitis group had a greater energy intake (1973.80 ± 866.38) than the population without allergic rhinitis (2034.46 ± 829.07) (p = 0.013), whereas participants with asthma (1881.07 ± 759.46) had a lesser amount of energy intake compared to participants without asthma (1986.63 ± 866.53) (p = 0.003). Participants with either allergic rhinitis or atopic dermatitis showed greater intake of protein, fat, and water compared to those without disease. In contrast, subjects with asthma had a lesser intake of protein, fat, and water than those without asthma. The amount of fiber intake was lesser in participants with either allergic rhinitis (p = 0.045), atopic dermatitis (p = 0.011) or asthma (p = 0.009) compared to the population without disease.

Table 1.
Comparison of daily nutritional intake according to the presence of allergic rhinitis, atopic dermatitis, and asthma with nutrients.
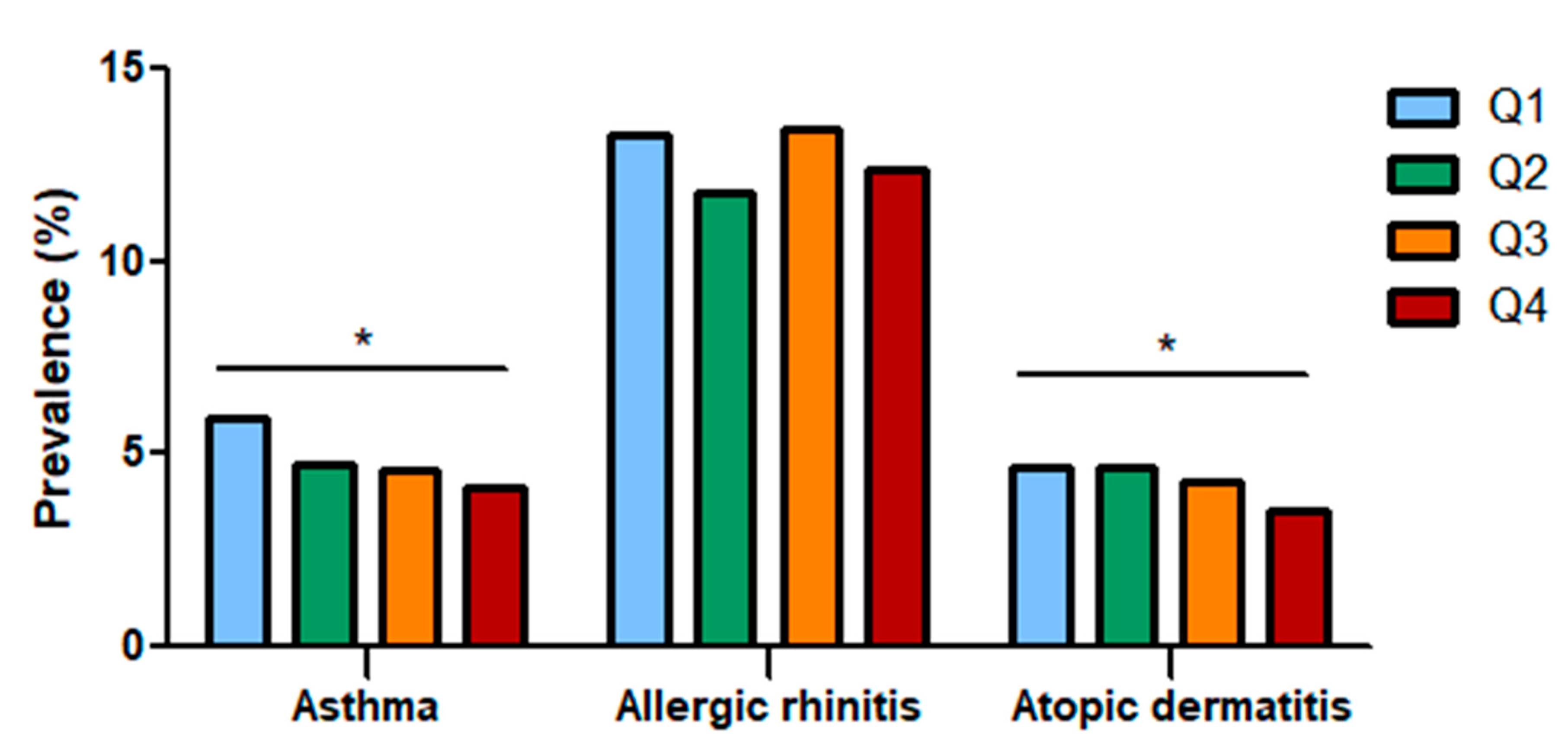
The study population included 10,479 participants who were categorized into quartiles based on dietary fiber intake. The prevalence of asthma, allergic rhinitis, and atopic dermatitis in each group was analyzed (Figure 2). As total fiber intake increased, the prevalence of asthma (Q1: 5.90%, Q2: 4.74%, Q3: 4.55%, Q4: 4.15%) and atopic dermatitis (Q1: 4.65%, Q2: 4.66%, Q3: 4.25%, Q4: 3.51%) decreased, showing that total fiber intake influences allergic diseases; however, the prevalence of allergic rhinitis showed no definite association (Q1: 13.25%, Q2: 11.81%, Q3: 13.47%, Q4: 12.39).
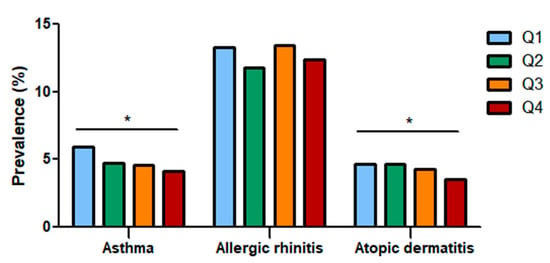
Figure 2.
Prevalence of asthma, allergic rhinitis, and atopic dermatitis according to total fiber intake quartiles. The prevalence of asthma and atopic dermatitis decreased as dietary fiber intake increased. Q: Quartile. *: Statistically significant.
Logistic regression analysis revealed that the prevalence of asthma decreased in Q3 (crude OR: 0.761; 95% CI: 0.60–0.97) and Q4 (crude OR: 0.691; 95% CI: 0.54–0.89). The prevalence of atopic dermatitis decreased in Q4 (crude OR: 0.746; 95% CI: 0.57–0.98) (Table 2). After adjusting for confounding factors, the prevalence of asthma decreased (Q4 adjusted OR: 0.656, 95% CI: 0.48–0.91, p for trend < 0.0001) and the prevalence of allergic rhinitis (Q2 adjusted OR: 0.840; 95% CI: 0.70–1.00, p for trend < 0.0001) and atopic dermatitis (Q4 adjusted OR: 0.712; 95% CI: 0.70–1.01, p for trend < 0.0001) tended to decrease as fiber intake increased.

Table 2.
Logistic regression analysis of the association between fiber intake and the prevalence of allergic diseases.
3.2. Association between Fiber Intake and Allergic Diseases with Respect to Sex
Since sex might influence the association between fiber intake and allergic diseases, the association between fiber intake and allergic diseases was analyzed according to participants’ sex (Table 3). In males, after adjusting for confounding factors, fiber intake showed significant negative associations with allergic rhinitis (Q2 adjusted OR: 0.704; 95% CI: 0.51–0.97), asthma (Q3 adjusted OR: 0.589; 95% CI: 0.39–0.90; Q4 adjusted OR: 0.523; 95% CI: 0.39–0.90), and atopic dermatitis (Q4 adjusted OR: 0.568; 95% CI: 0.35–0.91). In females, no significant association was identified.

Table 3.
Multivariate logistic regression analysis of the association between fiber intake and the prevalence of allergic diseases according to sex.
3.3. Association between Fiber Intake and Asthma-Related Conditions
The association between fiber intake and asthma-related conditions was analyzed (Table 4). Wheezing sounds while breathing and exercise-induced exacerbation showed no significant relationships with fiber intake in multivariate logistic regression analysis according to the participants’ sex.

Table 4.
Multivariate logistic regression analysis of the association between fiber intake and asthma-related conditions.
3.4. Association between Fiber Intake and Allergic Rhinitis-Related Conditions
Multivariate logistic regression analysis of the association between fiber intake and allergic rhinitis symptoms and endoscopic findings showed the tendency for an inverse association between fiber intake and watery rhinorrhea (Table 5). Logistic regression analysis according to participants’ sex showed a significant inverse association in fiber intake and watery rhinorrhea in males (Q3 adjusted OR: 0.734; 95% CI: 0.55–0.97; Q4 adjusted OR: 0.722; 95% CI: 0.54–0.97).

Table 5.
Multivariate logistic regression analysis of the association between fiber intake and allergic rhinitis-related conditions.
3.5. Association between Fiber Intake and Serum IgE Level
The association between fiber intake and serum IgE levels was analyzed (Table 6). No association was found between fiber intake and total IgE levels in both sexes; however, the association between dog allergen-specific IgEs and fiber intake was significant in males (Q3 adjusted OR: 0.319; 95% CI: 0.13–0.82; Q4 adjusted OR: 0.338; 95% CI: 0.13–0.86).

Table 6.
Logistic regression analysis of the association between fiber intake and serum immunoglobulin E (IgE) levels.
4. Discussion
In the past decades, the prevalence of allergic diseases has increased considerably, and it has been suggested that industrialization and lifestyle changes, including dietary habits, could be relevant. This study used a nationwide survey to evaluate the association of allergic diseases and related conditions with dietary fiber intake and analyzed sex differences in their association. In the present study, higher dietary fiber intake was associated with a lower prevalence of allergic rhinitis, asthma, and atopic dermatitis, especially in the male population. Among disease-related conditions, the occurrence of allergic diseases has increased, especially in watery rhinorrhea based on nasal endoscopic examination in the male population, while a wheezing sound had no association with dietary fiber intake. Additionally, among the three indoor allergens investigated, only dog allergens showed associations with fiber consumption.
The prevalence of allergic diseases has increased over the last decades, especially in developed countries. Thus, it is suggested that the shift to western dietary habits with an increased consumption of fat-rich food and a decreased consumption of fruits and vegetables could affect allergic immune responses [27]. In several experimental studies, the importance of a fiber-rich diet has recently been emphasized owing to its contribution to the diversity and function of gastrointestinal microbiota and the production of short-chain fatty acids (SCFAs), which have important roles in recruiting immune cells and regulating inflammatory responses [28]. Previous experimental studies have reported that a high intake of dietary fiber, followed by elevated SCFA levels, could lower airway allergic inflammation by inhibiting inflammatory pathways in dendritic cells and macrophages, inducing regulatory T-cell development, and maintaining epithelial barrier integrity [29,30]. Regarding allergic rhinitis, a dietary fiber intake allergic rhinitis murine model showed less eosinophil infiltration, less goblet cell metaplasia in the nasal mucosa and the lungs, and decreased Th2 cytokines compared with the low-fiber intake model, and the attenuated inflammatory response was accompanied by a significant modulation of the gut microbiota composition [31]. Epidemiologic studies on the association between fiber intake and allergic diseases have focused on the preventive effects of dietary fiber against asthma and atopic dermatitis among children; however, many studies have not been conducted to assess those effects in adults [32]. Recently, a French study of 35,380 participants reported that the highest quantile of total dietary fiber was associated with fewer asthma symptoms and greater disease control in adults [33]. Similarly, in our study, participants with allergic diseases had a lesser intake of dietary fiber despite a higher intake of total energy or other nutrients. Furthermore, the inverse association between fiber intake and the prevalence of allergic diseases in adults was consistent with that reported previously.
In the present study, among various allergic disease-related conditions, only watery rhinorrhea (based on nasal endoscopic examination) had a significant inverse association with dietary fiber intake. Watery rhinorrhea is a representative symptom of allergic rhinitis caused by histamine released from mast cells. Dietary fiber inhibits mast cell activation by reducing calcium entry, attenuating JKN/p38 phosphorylation, and reducing histone deacetylase activity, thus resulting in a decreased release of inflammatory mediators such as histamine [34,35]. It is presumed that the greater effect of fiber intake on watery rhinorrhea compared with that on other factors such as wheezing or nasal congestion could be because of its effect on mast cells. Our investigation of the association between dietary fiber intake and aeroallergen sensitization revealed that higher fiber intake was associated with a lower chance of dog allergen sensitization; however, no association was identified with specific IgEs for other allergens or total IgEs. As mentioned above, previous studies reported that dietary fiber intake contributes to diversity in gastrointestinal microbiota, which eventually regulates inflammatory responses. Although microbial diversity is associated with a lower chance of house dust mite sensitization, no previous studies have evaluated the association between allergen sensitization and fiber intake according to allergen type. Further research is needed on the mechanism of different effects of dietary fiber intake depending on the allergen type [36].
In our study, the association between dietary fiber consumption and allergic diseases was prominent in the male population with respect to several aspects, including the prevalence of diseases, watery rhinorrhea, and dog allergen sensitization. The presence of sex differences in the association between fiber intake and immune or health statuses has been previously reported [37]. Unless the underlying mechanism is not clearly understood, it is suggested that differences in immune functions between sexes as well as sex hormones could affect microbial composition, thus resulting in different dietary effects [38,39]. Furthermore, consumption of fibers from different sources, mainly grains for men and fruits for women, could contribute to this difference, in that different health benefits of cereals, fruits, and vegetables-originated fibers have been reported [40].
The strength of this study is that the data source was a nationwide survey, and the association between dietary fiber intake and allergic disease was analyzed based on detailed interviews and objective methods, including endoscopic examination. This study had several limitations. First, owing to the nature of the cross-sectional design, causal associations could not be assessed. Nevertheless, because endoscopic examination findings were obtained at the time of the survey and responses to the food intake questionnaire were obtained through recalls for the past year, it could be considered that fiber intake affected watery rhinorrhea. Second, we could not investigate atopic dermatitis-related symptoms/signs. Furthermore, there was no objective assessment of asthma because of the lack of detailed questionnaires or physical examinations.
5. Conclusions
In conclusion, the study findings suggest that high fiber intake was associated with a decreased prevalence of allergic diseases in adults, especially in the male population. Furthermore, in allergic patients with watery rhinorrhea and dog allergen sensitization, dietary intervention to increase fiber intake could be beneficial for disease control.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-4601/18/6/2889/s1, Table S1. Baseline characteristics of participants according to the presence of allergic rhinitis, atopic dermatitis and asthma.
Author Contributions
H.L. and K.L. planned and designed the work, obtained, analyzed, and interpreted the data, and wrote and revised the manuscript; S.S. obtained and analyzed the data and contributed to the discussion; Y.-C.K. analyzed data and contributed to the discussion; J.W.K. and H.G.K. obtained and interpreted the data; S.H.L. designed the work, reviewed and supervised the manuscript; T.H.K. planned and designed the work, obtained and interpreted the data, and supervised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basic Science Research Program, National Research Foundation of Korea, funded by the Ministry of Science and Technology and the Ministry of Science, ICT and Future Planning (2017R1A2B2003575, NRF-2020R1A2C1006398), the Ministry of Science and ICT (2020R1C1C1012288), Korea, under the ICT Creative Consilience program (IITP-2021-001819) supervised by the IITP (Institute for Information and Communications Technology Planning and Evaluation), and the Korea Health Technology R&D Project (HI17C0387), Korea Health Industry Development Institute (KHIDI), and the Ministry of Health and Welfare. This research was also supported by a Korea University grant and by the statistical support project for writing medical papers using the Korea National Health and Nutrition Examination Survey (KNHANES), Korea University Medical Center and Anam Hospital, Seoul, Republic of Korea.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of The Institutional Review Board of the Korea Centers for Disease Control and Prevention (protocol code 2010-02CON-21-C, 2011-02CON-06-C, and 2012-01EXP-01-2C).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hipsley, E.H. Dietary “fibre” and pregnancy toxaemia. Br. Med. J. 1953, 2, 420–422. [Google Scholar] [CrossRef]
- Chutkan, R.; Fahey, G.; Wright, W.L.; McRorie, J. Viscous versus nonviscous soluble fiber supplements: Mechanisms and evidence for fiber-specific health benefits. J. Am. Acad. Nurse. Pract. 2012, 24, 476–487. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Rangarajan, S.; Sheridan, P.; Mohan, V.; Iqbal, R.; Gupta, R.; Lear, S.; Wentzl-Viljoen, E.; Avezum, A.; et al. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): A prospective cohort study. Lancet 2018, 392, 2288–2297. [Google Scholar] [CrossRef]
- Sun, J.; Buys, N. Effects of probiotics consumption on lowering lipids and CVD risk factors: A systematic review and meta-analysis of randomized controlled trials. Ann. Med. 2015, 47, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Weickert, M.O.; Pfeiffer, A.F.H. Impact of dietary fiber consumption on insulin resistance and the prevention of type 2 diabetes. J. Nutr. 2018, 148, 7–12. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Ystad, S.O.; Mazzawi, T.; Gundersen, D. Dietary fiber in irritable bowel syndrome (Review). Int. J. Mol. Med. 2017, 40, 607–613. [Google Scholar] [CrossRef]
- Ramkumar, D.; Rao, S.S. Efficacy and safety of traditional medical therapies for chronic constipation: Systematic review. Am. J. Gastroenterol. 2005, 100, 936–971. [Google Scholar] [CrossRef]
- Kaluza, J.; Harris, H.; Wallin, A.; Linden, A.; Wolk, A. Dietary fiber intake and risk of chronic obstructive pulmonary disease: A prospective cohort study of men. Epidemiology 2018, 29, 254–260. [Google Scholar] [CrossRef]
- Aune, D.; Chan, D.S.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef]
- Yang, J.J.; Yu, D.; Xiang, Y.B.; Blot, W.; White, E.; Robien, K.; Sinha, R.; Park, Y.; Takata, Y.; Lazovich, D.; et al. Association of dietary fiber and yogurt consumption with lung cancer risk: A pooled analysis. JAMA Oncol. 2020, 6, e194107. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Buyken, A.E.; Goletzke, J.; Joslowski, G.; Felbick, A.; Cheng, G.; Herder, C.; Brand-Miller, J.C. Association between carbohydrate quality and inflammatory markers: Systematic review of observational and interventional studies. Am. J. Clin. Nutr. 2014, 99, 813–833. [Google Scholar] [CrossRef]
- Watzl, B.; Girrbach, S.; Roller, M. Inulin, oligofructose and immunomodulation. Br. J. Nutr. 2005, 93, S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Long, A.; McFadden, C.; DeVine, D.; Chew, P.; Kupelnick, B.; Lau, J. Management of allergic and nonallergic rhinitis. Evid. Rep. Technol. Assess. 2002, 54, 1–6. [Google Scholar]
- Pretorius, R.A.; Bodinier, M.; Prescott, S.L.; Palmer, D.J. Maternal Fiber Dietary Intakes during Pregnancy and Infant Allergic Disease. Nutrients 2019, 11, 1767. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.H.; Licciardi, P.V.; Tang, M.L. Probiotic effects in allergic disease. J. Paediatr. Child Health 2013, 49, 709–715. [Google Scholar] [CrossRef]
- Bisgaard, H.; Li, N.; Bonnelykke, K.; Chawes, B.L.; Skov, T.; Paludan-Müller, G.; Stokholm, J.; Smith, B.; Krogfelt, K.A. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J. Allergy Clin. Immunol. 2011, 128, 646–652. [Google Scholar] [CrossRef]
- Dotterud, C.K.; Storrø, O.; Johnsen, R.; Oien, T. Probiotics in pregnant women to prevent allergic disease: A randomized, double-blind trial. Br. J. Dermatol. 2010, 163, 616–623. [Google Scholar] [CrossRef]
- Thorburn, A.N.; McKenzie, C.I.; Shen, S.; Stanley, D.; Macia, L.; Mason, L.J.; Roberts, L.K.; Wong, C.H.; Shim, R.; Robert, R.; et al. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat. Commun. 2015, 6, 7320. [Google Scholar] [CrossRef]
- Flinterman, A.E.; Knol, E.F.; Dijk, A.G.va.; Timmerman, H.M.; Knulst, A.C.; Bruijnzeel-Koomen, C.A.; Pasmans, S.G.; van Hoffen, E. Probiotics have a different immunomodulatory potential in vitro versus ex vivo upon oral administration in children with food allergy. Int. Arch. Allergy Immunol. 2007, 143, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, M.L.; Wolt-Plompen, S.A.; Dubois, A.E.; van der Heide, S.; Jansen, D.F.; Hoijer, M.A.; Kauffman, H.F.; Duiverman, E.J. No effects of probiotics on atopic dermatitis in infancy: A randomized placebo-controlled trial. Clin. Exp. Allergy. 2006, 36, 899–906. [Google Scholar] [CrossRef]
- Gerasimov, S.V.; Vasjuta, V.V.; Myhovych, O.O.; Bondarchuk, L.I. Probiotic supplement reduces atopic dermatitis in preschool children: A randomized, double-blind, placebo-controlled, clinical trial. Am. J. Clin. Dermatol. 2010, 11, 351–361. [Google Scholar] [CrossRef]
- Giovannini, M.; Agostoni, C.; Riva, E.; Salvini, F.; Ruscitto, A.; Zuccotti, G.V.; Radaelli, G. A randomized prospective double blind controlled trial on effects of long-term consumption of fermented milk containing Lactobacillus casei in pre-school children with allergic asthma and/or rhinitis. Pediatr. Res. 2007, 62, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Jan, R.L.; Lin, Y.L.; Chen, H.H.; Wang, J.Y. Randomized placebo-controlled trial of lactobacillus on asthmatic children with allergic rhinitis. Pediatr. Pulmonol. 2010, 45, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Devereux, G. The increase in the prevalence of asthma and allergy: Food for thought. Nat. Rev. Immunol. 2006, 6, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018, 23, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Huffnagle, G.B. Increase in dietary fiber dampens allergic responses in the lung. Nat. Med. 2014, 20, 120–121. [Google Scholar] [CrossRef]
- Anand, S.; Mande, S.S. Diet, microbiota and gut-lung connection. Front. Microbiol. 2018, 9, 2147. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, L.; Pang, W.; Liu, W.; Li, J.; Wang, H.; Shi, G. Dietary fiber intake regulates intestinal microflora and inhibits ovalbumin-induced allergic airway inflammation in a mouse model. PLoS ONE 2016, 11, e0147778. [Google Scholar] [CrossRef]
- Peroni, D.G.; Bonomo, B.; Casarotto, S.; Boner, A.L.; Piacentini, G.L. How changes in nutrition have influenced the development of allergic diseases in childhood. Ital. J. Pediatr. 2012, 38, 22. [Google Scholar] [CrossRef]
- Andrianasolo, R.M.; Hercberg, S.; Kesse-Guyot, E.; Druesne-Pecollo, N.; Touvier, M.; Galan, P.; Varraso, R. Association between dietary fibre intake and asthma (symptoms and control): Results from the French national e-cohort NutriNet-Santé. Br. J. Nutr. 2019, 122, 1040–1051. [Google Scholar] [CrossRef]
- Folkerts, J.; Stadhouders, R.; Redegeld, F.A.; Tam, S.Y.; Hendriks, R.W.; Galli, S.J.; Maurer, M. Effect of dietary fiber and metabolites on mast cell activation and mast cell-associated diseases. Front. Immunol. 2018, 9, 1067. [Google Scholar] [CrossRef]
- Cildir, G.; Pant, H.; Lopez, A.F.; Tergaonkar, V. The transcriptional program, functional heterogeneity, and clinical targeting of mast cells. J. Exp. Med. 2017, 214, 2491–2506. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Chan, Y.L.; Tsai, Y.S.; Chen, S.A.; Wang, C.J.; Chen, K.F.; Chung, I.F. Airway microbial diversity is inversely associated with mite-sensitized rhinitis and asthma in early childhood. Sci. Rep. 2017, 7, 1820. [Google Scholar] [CrossRef] [PubMed]
- Fernstrand, A.M.; Bury, D.; Garssen, J.; Verster, J.C. Dietary intake of fibers: Differential effects in men and women on perceived general health and immune functioning. Food Nutr. Res. 2017, 61, 1297053. [Google Scholar] [CrossRef]
- Markle, J.G.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Hauner, H.; Bechthold, A.; Boeing, H.; Brönstrup, A.; Buyken, A.; Leschik-Bonnet, E.; Linseisen, J.; Schulze, M.; Strohm, D.; Wolfram, G. Evidence-based guideline of the German Nutrition Society: Carbohydrate intake and prevention of nutrition-related diseases. Ann. Nutr. Metab. 2012, 60, 1–58. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).