The Effects of Age and Body Fat Content on Post-Downhill Run Recovery Following Whole Body Cryotherapy
Abstract
:1. Introduction
- To assess the overall impact of a single WBC treatment on recovery following a downhill run.
- To assess the impact of age and body fat content on recovery response to a single WBC treatment post-exercise.
2. Materials and Methods
2.1. Participants
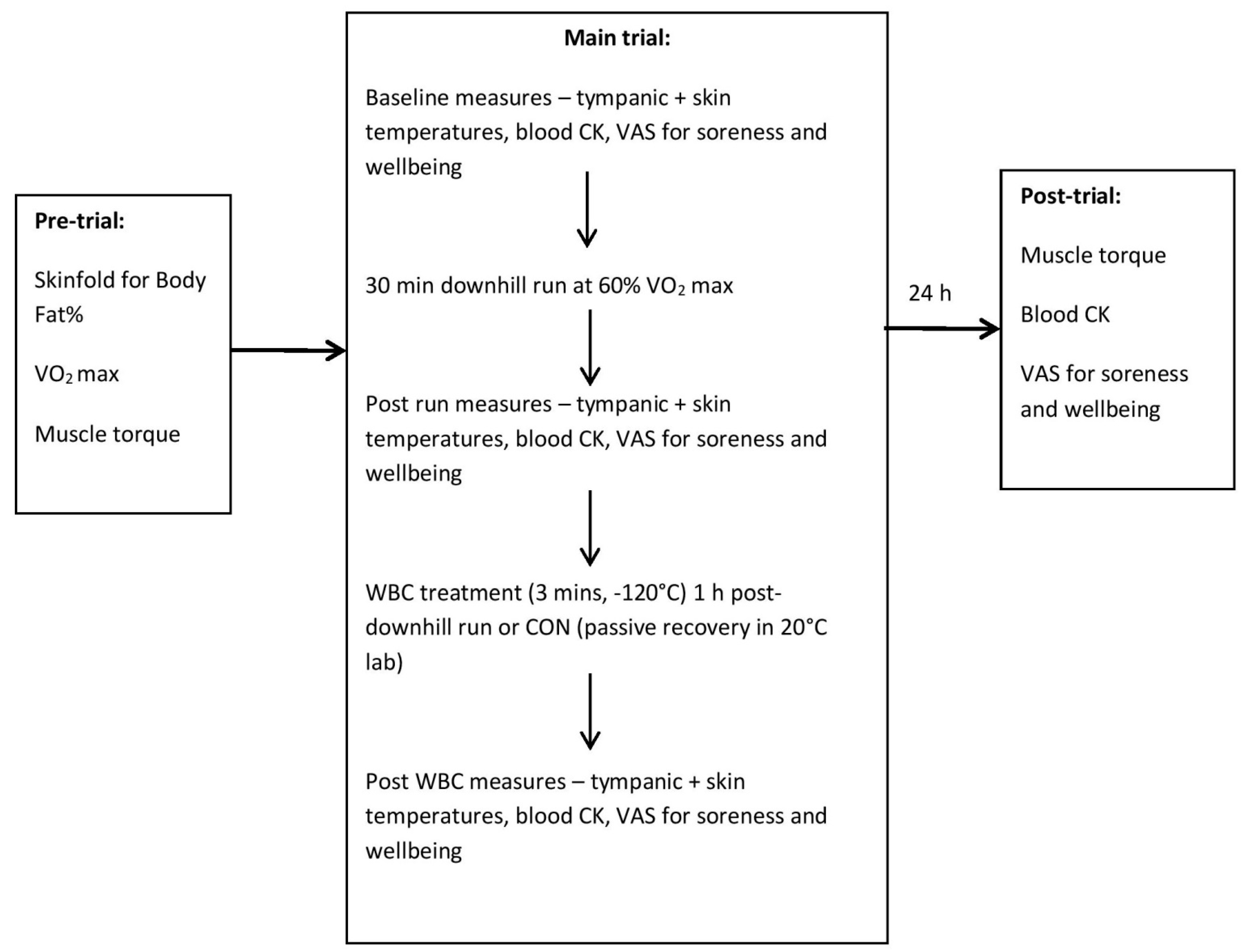
2.2. Initial Trial
2.3. Second Trial
2.4. Third Trial
2.5. Assessment of Muscle Torque
2.6. Whole Body Cryotherapy Treatment
2.7. Statistical Analyses
3. Results
3.1. WBC vs. CON
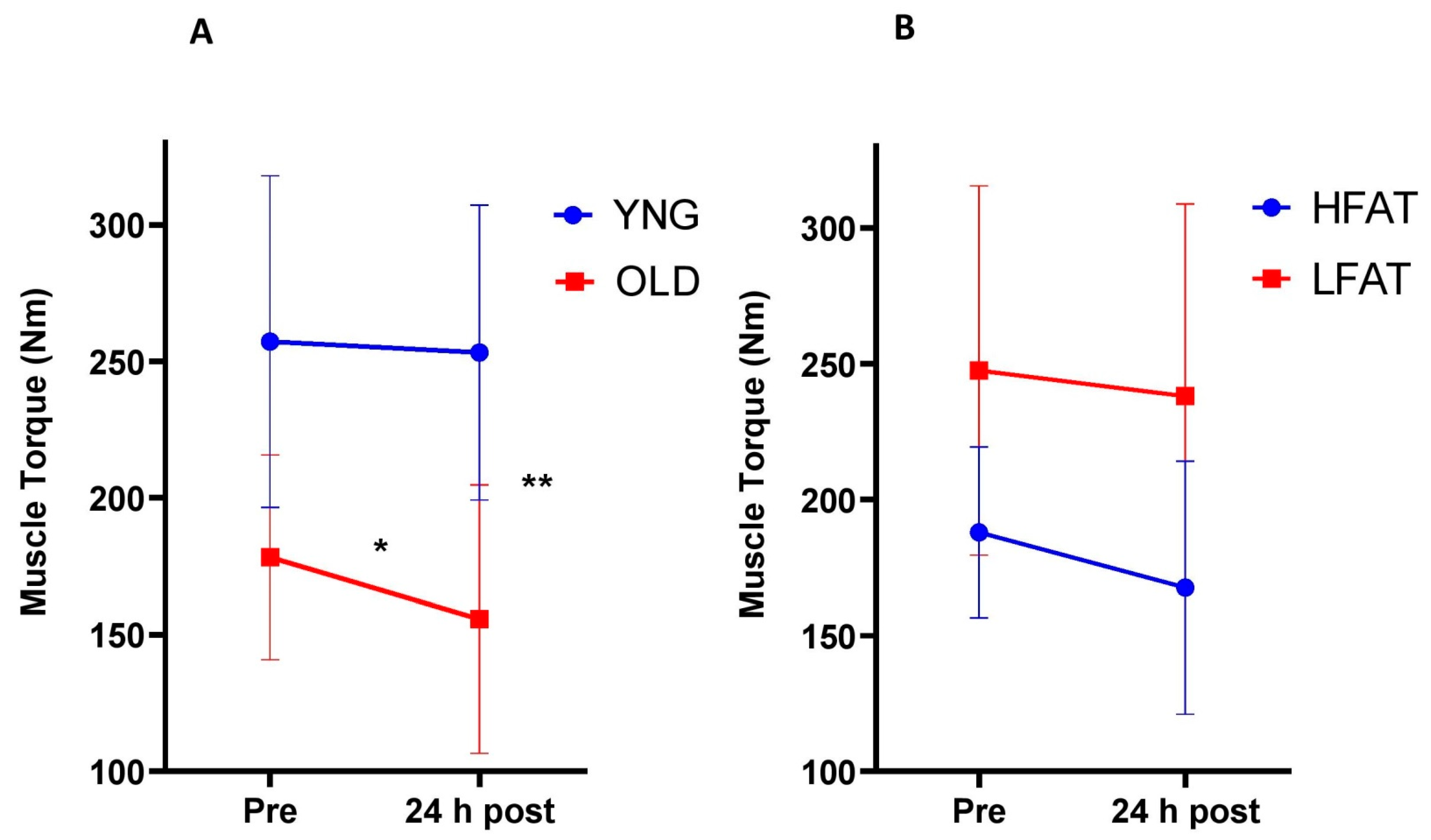
3.1.1. Muscle Torque
3.1.2. Muscle Soreness
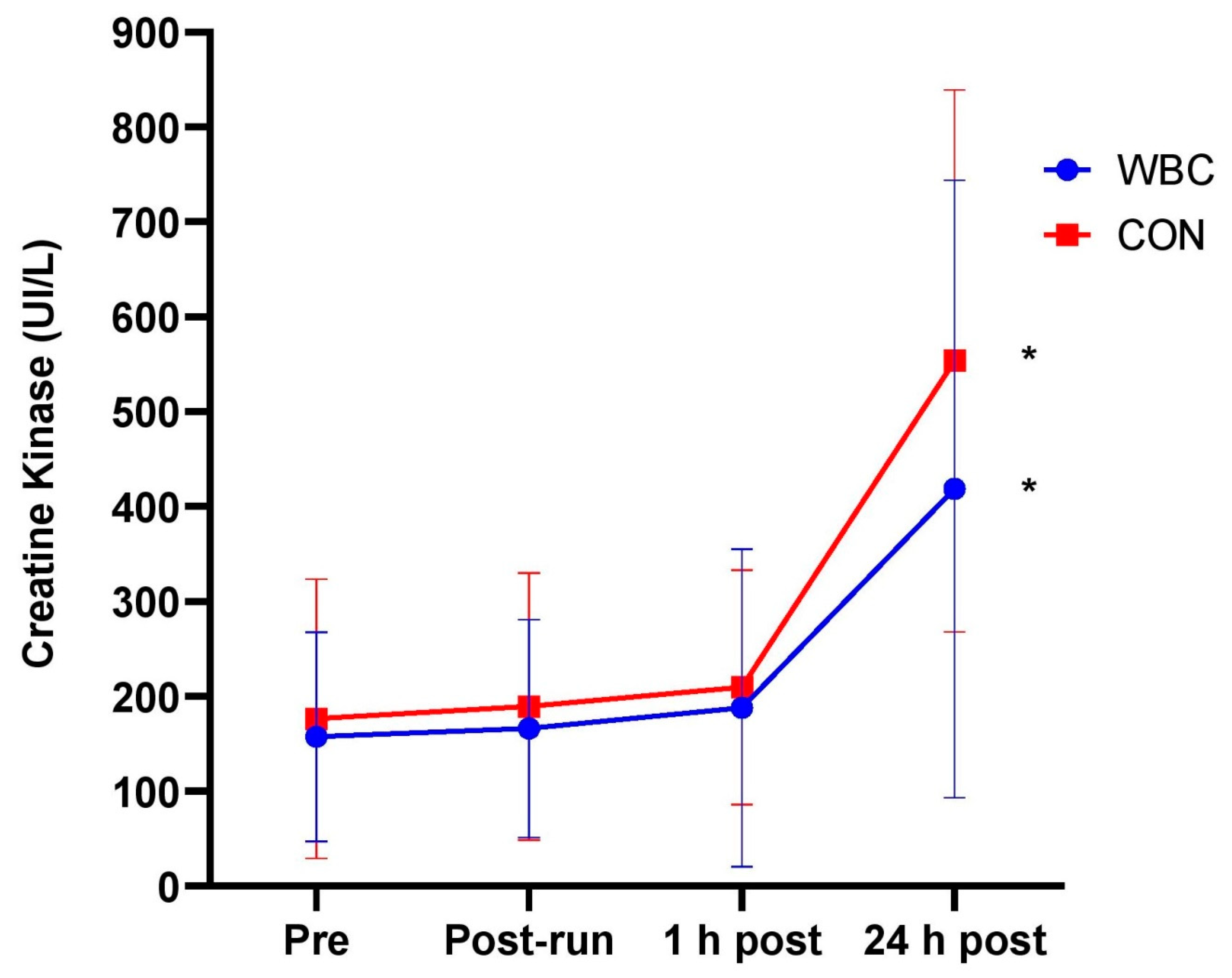
3.1.3. Creatine Kinase
3.1.4. Tympanic Temperature
3.1.5. Skin Temperature
3.1.6. VAS Wellbeing
3.2. Effect of Age and Body Fat
3.2.1. Muscle Torque
3.2.2. Other Variables
4. Discussion
4.1. Effects of Age and Body Fat Content
4.2. Potential Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lubkowska, A. Cryotherapy: Physiological considerations and applications to physical therapy. In Physical Therapy Perspectives in the 21st Century—Challenges and Possibilities; InTechOpen: London, UK, 2012; pp. 155–176. [Google Scholar]
- Straburzyńska-Lupa, A.; Kasprzak, M.P.; Romanowski, M.W.; Kwaśniewska, A.; Romanowski, W.; Iskra, M.; Rutkowski, R. The effect of whole-body cryotherapy at different temperatures on proinflammatory cytokines, oxidative stress parameters, and disease activity in patients with ankylosing spondylitis. Oxid. Med. Cell. Longev. 2018, 2157496. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, G.; Ziemann, E.; Banfi, G. Whole-body cryotherapy in athletes: From therapy to stimulation. An updated review of the literature. Front. Physiol. 2017, 8, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partridge, E.M.; Cooke, J.; McKune, A.; Pyne, D.B. Whole-Body Cryotherapy: Potential to Enhance Athlete Preparation for Competition? Front. Physiol. 2019, 10, 1007. [Google Scholar] [CrossRef] [Green Version]
- Ziemann, E.; Olek, R.A.; Kujach, S.; Grzywacz, T.; Antosiewicz, J.; Garsztka, T.; Laskowski, R. Five-day whole-body cryostimulation, blood inflammatory markers, and performance in high-ranking professional tennis players. J. Athl. Train. 2012, 47, 664–672. [Google Scholar] [CrossRef]
- Fonda, B.; Sarabon, N. Effects of whole-body cryotherapy on recovery after hamstring damaging exercise: A crossover study. Scand. J. Med. Sci. Sports 2013, 23, e270–e278. [Google Scholar] [CrossRef]
- Pournot, H.; Bieuzen, F.; Louis, J.; Fillard, J.R.; Barbiche, E.; Hausswirth, C. Time-course of changes in inflammatory response after whole-body cryotherapy multi exposures following severe exercise. PLoS ONE 2011, 6, e22748. [Google Scholar] [CrossRef]
- Ziemann, E.; Olek, R.A.; Grzywacz, T.; Kaczor, J.J.; Antosiewicz, J.; Skrobot, W.; Kujach, S.; Laskowski, R. Whole-body cryostimulation as an effective way of reducing exercise-induced inflammation and blood cholesterol in young men. Eur. Cytokine Netw. 2013, 25, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Junior, J.B.; Bottaro, M.; Vieira, A.; Siqueira, A.F.; Vieira, C.A.; Durigan, J.L.; Cadore, E.L.; Coelho, L.G.; Simoes, H.G.; Bemben, M.G. One session of partial-body cryotherapy (−110 °C) improves muscle damage recovery. Scand. J. Med. Sci. Sports 2014, 25, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Hausswirth, C.; Louis, J.; Bieuzen, F.; Pournot, H.; Fournier, J.; Filliard, J.R.; Brisswalter, J. Effects of whole-body cryotherapy vs. far-infrared vs. passive modalities on recovery from exercise-induced muscle damage in highly-trained runners. PLoS ONE 2011, 6, e27749. [Google Scholar] [CrossRef] [Green Version]
- Vieira, A.; Bottaro, M.; Ferreira-Junior, J.B.; Vieira, C.; Cleto, V.A.; Cadore, E.L.; Simões, H.G.; Carmo, J.D.; Brown, L.E. Does whole-body cryotherapy improve vertical jump recovery following a high-intensity exercise bout? Open Access J. Sports Med. 2015, 6, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krueger, M.; Costello, J.T.; Achtzehn, S.; Dittmar, K.H.; Mester, J. Whole-body cryotherapy (−110 °C) following high-intensity intermittent exercise does not alter hormonal, inflammatory or muscle damage biomarkers in trained males. Cytokine 2018, 113, 277–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, L.J.; Cockburn, E.; Paice, K.; Sinclair, S.; Faki, T.; Hills, F.A.; Gondek, M.B.; Wood, A.; Dimitriou, L. Recovery following a marathon: A comparison of cold water immersion, whole body cryotherapy and a placebo control. Eur. J. Appl. Physiol. 2018, 118, 153–163. [Google Scholar] [CrossRef]
- McCormick, R.; Vasilaki, A. Age-related changes in skeletal muscle: Changes to life-style as a therapy. Biogerontology 2018, 19, 519–536. [Google Scholar] [CrossRef] [Green Version]
- Young, A.J.; Sawka, M.N.; Pandolf, K.B. Physiology of cold exposure. In Nutritional Needs in Cold and High-Altitude Environments: Applications for Military Personnel in Field Operations; National Academies Press: Washington, DC, USA, 1996; pp. 127–148. [Google Scholar]
- Knight, J.; Nigam, Y. The Anatomy and Physiology of Ageing. Part 1—The Cardiovascular System. Nurs. Times 2008, 104, 26–27. [Google Scholar]
- Stephens, J.M.; Halson, S.L.; Miller, J.; Slater, G.J.; Chapman, D.W.; Askew, C.D. Effect of body composition on physiological responses to cold-water immersion and the recovery of exercise performance. Int. J. Sports Physiol. Perform. 2018, 13, 382–389. [Google Scholar] [CrossRef]
- Stocks, J.M.; Taylor, N.A.; Tipton, M.J.; Greenleaf, J.E. Human physiological responses to cold exposure. Aviat. Space Environ. Med. 2004, 75, 444–457. [Google Scholar] [PubMed]
- Otte, J.W.; Merrick, M.A.; Ingersoll, C.D.; Cordova, M.L. Subcutaneous adipose tissue thickness alters cooling time during cryotherapy. Arch. Phys. Med. Rehabil. 2002, 83, 1501–1505. [Google Scholar] [CrossRef]
- Cuttell, S.; Hammond, L.; Langdon, D.; Costello, J. Individualising the exposure of −110 °C whole body cryotherapy: The effects of sex and body composition. J. Therm. Biol. 2017, 65, 41–47. [Google Scholar] [CrossRef]
- Hammond, L.E.; Cuttell, S.; Nunley, P.; Meyler, J. Anthropometric characteristics and sex influence magnitude of skin cooling following exposure to whole body cryotherapy. Biomed Res. Int. 2014, 2014, 628724. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Nosaka, K.; Lin, M.J.; Chen, H.L.; Wu, C.J. Changes in running economy at different intensities following downhill running. J. Sports Sci. 2009, 27, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Malm, C.; Sjödin, B.; Sjöberg, B.; Lenkei, R.; Renström, P.; Lundberg, I.; Ekblom, B. Leukocytes, cytokines, growth factors and hormones in human skeletal muscle and blood after uphill or downhill running. J. Physiol. 2004, 556, 983–1000. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Suzuki, K.; Hordern, M.; Wilson, G.; Nosaka, K.; Coombes, J.S. Plasma cytokine changes in relation to exercise intensity and muscle damage. Eur. J. Appl. Physiol. 2005, 95, 514–521. [Google Scholar] [CrossRef]
- Smith, L.L.; McKune, A.J.; Semple, S.J.; Silbanda, E.; Steel, H.; Anderson, R. Changes in serum cytokines after repeated bouts of downhill running. Appl. Physiol. Nutr. Metab. 2007, 32, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Assumpção, C.O.; Lima, L.C.R.; Oliveira, F.R.D.; Greco, C.C.; Denadai, B.S. Exercise-induced muscle damage and running economy in humans. Sci. World J. 2013, 2013, 189149. [Google Scholar] [CrossRef]
- Cheung, K.; Hume, P.; Maxwell, L. Delayed onset muscle soreness: Treatment strategies and performance factors. Sports Med. 2003, 33, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Costello, J.T.; Algar, L.A.; Donnelly, A.E. Effects of whole-body cryotherapy (−110 °C) on proprioception and indices of muscle damage. Scand. J. Med. Sci. Sports 2012, 22, 190–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayers, S.P.; Clarkson, P.M. Force recovery after eccentric exercise in males and females. Eur. J. Appl. Physiol. 2001, 84, 122–126. [Google Scholar] [CrossRef]
- Yoon, E.J.; Kim, J. Effect of body fat percentage on muscle damage induced by high-intensity eccentric exercise. Int. J. Environ. Res. Public Health 2020, 17, 3476. [Google Scholar] [CrossRef]
- Baross, A.W.; Wiles, J.D.; Swaine, I.L. Double-leg isometric exercise training in older men. Open Access J. Sports Med. 2013, 4, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Durnin, J.V.; Womersley, J. Body fat assessed from total body density and its estimation from skinfold thickness: Measurements on 481 men and women aged from 16 to 72 years. Br. J. Nutr. 1974, 32, 77–97. [Google Scholar] [CrossRef] [Green Version]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Ramanthan, N.L. A new weighting system for mean surface temperature of the human body. J. Appl. Physiol. 1964, 19, 531–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortes, M.B.; Di Felice, U.; Dolci, A.; Junglee, N.A.; Crockford, M.J.; West, L.; Hillier-Smith, R.; Macdonald, J.H.; Walsh, N.P. Muscle-damaging exercise increases heat strain during subsequent exercise heat stress. Med. Sci. Sports Exerc. 2013, 45, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Dolci, A.; Fortes, M.B.; Walker, F.S.; Haq, A.; Riddle, T.; Walsh, N.P. Repeated muscle damage blunts the increase in heat strain during subsequent exercise heat stress. Eur. J. Appl. Physiol. 2015, 115, 1577–1588. [Google Scholar] [CrossRef] [Green Version]
- Bouzigon, R.; Grappe, F.; Ravier, G.; Dugue, B. Whole- and partial-body cryostimulation/cryotherapy: Current technologies and practical applications. J. Therm. Biol. 2016, 61, 67–81. [Google Scholar] [CrossRef]
- Bleakley, C.M.; Bieuzen, F.; Davison, G.W.; Costello, J.T. Whole-body cryotherapy: Empirical evidence and theoretical perspectives. Open Access J. Sports Med. 2014, 10, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Costello, J.T.; Baker, P.R.; Minett, G.M.; Bieuzen, F.; Stewart, I.B.; Bleakley, C. Cochrane review: Whole-body cryotherapy (extreme cold air exposure) for preventing and treating muscle soreness after exercise in adults. Cochrane Database Syst. Rev. 2016, 18, CD010789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81 (Suppl. 11), S52–S69. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Melegati, G.; Barassi, A.; Dogliotti, G.; Melzi d’Eril, G.; Dugué, B.; Corsi, M.M. Effects of whole-body cryotherapy on serum mediators of inflammation and serum muscle enzymes in athletes. J. Therm. Biol. 2009, 34, 55–59. [Google Scholar] [CrossRef]
- Peake, J.M.; Neubauer, O.; Gatta, P.A.D.; Nozaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef]
- Tee, J.C.; Bosch, A.N.; Lambert, M.I. Metabolic consequences of exercise-induced muscle damage. Sports Med. 2007, 37, 27–36. [Google Scholar] [CrossRef]
- Pawik, M.; Kowalska, J.; Rymaszewska, J. The effectiveness of whole-body cryotherapy and physical exercises on the psychological well-being of patients with multiple sclerosis: A comparative analysis. Adv. Clin. Exp. Med. 2019, 28, 1477–1483. [Google Scholar] [CrossRef] [Green Version]
- Szczepańska-Gieracha, J.; Borsuka, P.; Pawika, M.; Rymaszewskaa, J. Mental state and quality of life after 10 session whole-body cryotherapy. Psychol. Health Med. 2014, 19, 40–46. [Google Scholar] [CrossRef]
- Costello, J.T.; Culligan, K.; Selfe, S.; Donnelly, A.E. Muscle, skin and core temperature after −110 °C cold air and 8 °C water treatment. PLoS ONE 2012, 7, e48190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruger, M.; de Marees, M.; Dittmar, K.H.; Sperlich, B.; Mester, J. Whole-body cryotherapy’s enhancement of acute recovery of running performance in well-trained athletes. Int. J. Sports Physiol. Perform. 2015, 10, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.L.; Lowe, D.A.; Armstrong, R.B. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999, 27, 43–59. [Google Scholar] [CrossRef]
- Crystal, N.J.; Townson, D.H.; Cook, S.B.; LaRoche, D.P. Effect of cryotherapy on muscle recovery and inflammation following a bout of damaging exercise. Eur. J. Appl. Physiol. 2013, 113, 2577–2586. [Google Scholar] [CrossRef] [PubMed]
- Rossato, M.; de Souza Bezerraa, E.; de Ceselles Seixas da Silvaa, D.A.; Santanab, T.A.; Malezamc, W.R.; Carpes, F.P. Effects of cryotherapy on muscle damage markers and perception of delayed onset muscle soreness after downhill running: A Pilot study. Rev. Andal. Med. del Deporte 2015, 8, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Close, G.L.; Ashton, T.; Cable, T.; Doran, D.; Noyes, C.; McArdle, F.; MacLaren, D.P. Effects of dietary carbohydrate on delayed onset muscle soreness and reactive oxygen species after contraction induced muscle damage. Br. J. Sports Med. 2005, 39, 948–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, M.R.; Conlee, R.K.; Parcell, A.C. Inadequate carbohydrate intake following prolonged exercise does not increase muscle soreness after 15 minutes of downhill running. Int. J. Sports Nutr. Exerc. Metab. 2004, 14, 171–184. [Google Scholar] [CrossRef]
- Baumann, C.W.; Green, M.S.; Doyle, J.A.; Rupp, J.C.; Ingalls, C.P.; Corona, B.T. Muscle torque loss and muscle damage after downhill running. J. Strength Cond. Res. 2014, 28, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Nosaka, K.; Tu, J.H. Changes in running economy following downhill running. J. Sports Sci. 2007, 25, 55–63. [Google Scholar] [CrossRef]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sports Sci. 2009, 19, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Miyama, M.; Nosaka, K. Muscle damage and soreness following repeated bouts of consecutive drop jumps. Adv. Exerc. Sports Physiol. 2004, 10, 63–69. [Google Scholar]
- Park, K.S.; Lee, M.G. Effects of unaccustomed downhill running on muscle damage, oxidative stress, and leukocyte apoptosis. J. Exerc. Nutr. Biochem. 2015, 19, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Mikkelsen, U.R.; Raastad, T.; Peake, J.M. Leucocytes, cytokines and satellite cells: What role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 2012, 18, 42–97. [Google Scholar]




| WBC (n = 26) | CON (n = 15) | OVERALL (n = 41) | T Test between WBC and CON | |
|---|---|---|---|---|
| Age (yrs) | 41.8 ± 15.5 | 42.3 ± 10.4 | 42.0 ± 13.7 | p = 0.93 |
| Height (m) | 1.78 ± 0.09 | 1.75 ± 0.06 | 1.76 ± 0.08 | p = 0.21 |
| Body mass (kg) | 74.9 ± 10.8 | 75.6 ± 11.1 | 75.2 ± 10.8 | p = 0.85 |
| Body mass index (kg/m2) | 23.7 ± 2.2 | 24.7 ± 2.9 | 24.1 ± 2.5 | p = 0.22 |
| Body fat % | 18.8 ± 4.3 | 20.0 ± 4.9 | 19.2 ± 4.5 | p = 0.4 |
| Absolute VO2 max (L/min) | 3.61 ± 0.55 | 3.53 ± 0.63 | 3.58 ± 0.57 | p = 0.67 |
| Relative VO2 max (mL/min/kg) | 48.4 ± 5.1 | 46.8 ± 6.5 | 47.8 ± 5.6 | p = 0.32 |
| Pre | Post-Run | 1 h Post | 24 h Post | ANOVA Time Effect | |
|---|---|---|---|---|---|
| WBC | 83.8% ± 16.7 | 82.3% ± 16.0 | 85.5% ± 15.0 | 81.1% ± 20.2 | p = 0.06 |
| CON | 78.9% ± 21.6 | 80.8% ± 18.4 | 81.2% ± 19.2 | 78.1% ± 16.9 | p = 0.44 |
| Variable | WBC Age Group | Pre | Post-Run | 1 h Post (Post-WBC) | 24 h Post | p Value for Time × Group Interaction |
|---|---|---|---|---|---|---|
| Muscle Soreness | OLD | 8.7% ± 7.2 | 28.6% ± 16.6 | 21.6% ± 15.8 | 42.6% ± 21.4 | 0.68 |
| YNG | 10.1% ± 10.7 | 40.2% ± 22.7 | 31.3% ± 14.1 | 49.5% ± 21.8 | ||
| CK (UI/L) | OLD | 149.5 ± 106.2 | 142.6 ± 94.5 | 175.9 ± 119.7 | 351.0 ± 283.3 | 0.22 |
| YNG | 177.0 ± 111.9 | 196.1 ± 124.7 | 212.7 ± 194.2 | 502.1 ± 349.1 | ||
| Tympanic Temp | OLD | 36.4 °C ± 0.5 | 36.7 °C ± 0.6 | 36.1 °C ± 0.3 * | n/a | 0.17 |
| YNG | 36.8 °C ± 0.4 | 36.8 °C ± 0.5 | 36.5 °C ± 0.4 * | n/a | ||
| Skin Temp | OLD | 32.6 °C ± 0.6 | 32.2 °C ± 0.7 | 27.4 °C ± 1.7 | n/a | 0.21 |
| YNG | 32.8 °C ± 0.6 | 33.3 °C ± 0.7 | 27.4 °C ± 1.4 | n/a | ||
| VAS Wellbeing | OLD | 88.5% ± 16.1 | 84.8% ± 13.3 | 87.7% ± 13.4 | 82.6% ± 21.6 | 0.17 |
| YNG | 85.7% ± 10.5 | 84.8% ± 11.8 | 88.9% ± 8.2 | 85.1% ± 9.5 |
| Variable | WBC Body Fat Group | Pre | Post-Run | 1 h Post (Post-WBC) | 24 h Post | p Value for Time × Group Interaction |
|---|---|---|---|---|---|---|
| Muscle Soreness | HFAT | 9.1% ± 6.8 | 31.9% ± 16.7 | 24.8% ± 17.0 | 44.0% ± 22.0 | 0.78 |
| LFAT | 12.25% ± 12.4 | 41.9% ± 20.8 | 34.1% ± 14.3 | 55.8% ± 17.2 | ||
| CK (UI/L) | HFAT | 153.7 ± 103.3 | 147.1 ± 93.2 | 181.1 ± 118.3 | 391.2 ± 254.4 | 0.59 |
| LFAT | 205.6 ± 131.4 | 233.7 ± 141.9 | 249.1 ± 234.2 | 588.3 ± 413.4 | ||
| Tympanic Temp | HFAT | 36.5 °C ± 0.4 | 36.6 °C ± 0.6 | 36.2 °C ± 0.4 | n/a | 0.44 |
| LFAT | 36.8 °C ± 0.4 | 36.7 °C ± 0.5 | 36.5 °C ± 0.4 | n/a | ||
| Skin Temp | HFAT | 32.7 °C ± 0.7 | 32.4 °C ± 1.0 | 27.1 °C ± 2.0 | n/a | 0.6 |
| LFAT | 32.9 °C ± 0.6 | 33.1 °C ± 0.7 | 27.3 °C ± 0.9 | n/a | ||
| VAS Wellbeing | HFAT | 82.2% ± 22.8 | 80.7% ± 21.6 | 81.7% ± 21.9 | 79.9% ± 25.9 | 0.22 |
| LFAT | 89.5% ± 9.4 | 80.8% ± 14.3 | 89.3% ± 9.6 | 80.1% ± 20.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haq, A.; Ribbans, W.; Baross, A.W. The Effects of Age and Body Fat Content on Post-Downhill Run Recovery Following Whole Body Cryotherapy. Int. J. Environ. Res. Public Health 2021, 18, 2906. https://doi.org/10.3390/ijerph18062906
Haq A, Ribbans W, Baross AW. The Effects of Age and Body Fat Content on Post-Downhill Run Recovery Following Whole Body Cryotherapy. International Journal of Environmental Research and Public Health. 2021; 18(6):2906. https://doi.org/10.3390/ijerph18062906
Chicago/Turabian StyleHaq, Adnan, William Ribbans, and Anthony W. Baross. 2021. "The Effects of Age and Body Fat Content on Post-Downhill Run Recovery Following Whole Body Cryotherapy" International Journal of Environmental Research and Public Health 18, no. 6: 2906. https://doi.org/10.3390/ijerph18062906





