Cocaine-Induced Midline Destructive Lesions: A Real Challenge in Oral Rehabilitation
Abstract
:1. Introduction
2. Materials and Methods
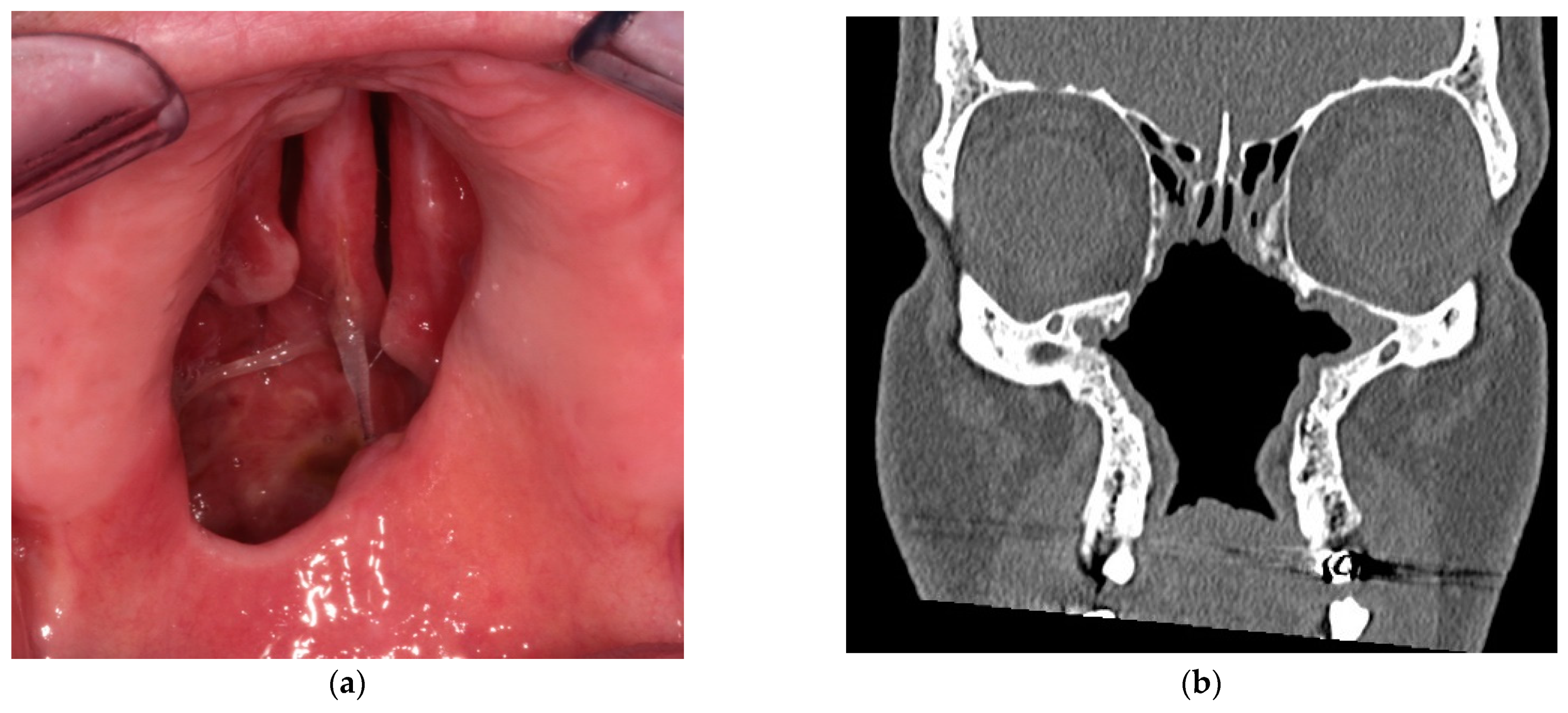
2.1. Diagnosis
2.2. Preliminary Evaluation and Production of the Prosthesis
2.3. Post-Delivery Care
3. Results
3.1. Main Findings
3.2. Symptoms and Post-Delivery Care
3.3. Patient Presentation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report 2019: Trends and Developments; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
- Nord, G.A.; Rock, A.; Murphy, F.J.; Miloslavskiy, I.; Miller, D.J.; Wasserman, B.S. Prosthetic and surgical management of oronasal communications secondary to cocaine abuse. N. Y. State Dent. J. 2012, 78, 22–25. [Google Scholar] [PubMed]
- Trimarchi, M.; Bussi, M.; Sinico, R.A.; Meroni, P.; Specks, U. Cocaine-induced midline destructive lesions—An autoimmune disease? Autoimmun. Rev. 2013, 12, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, M.; Sykopetrites, V.; Bussi, M. Management of a cocaine-induced palatal perforation with a nasal septal button. Ear. Nose. Throat J. 2016, 95, E36–E38. [Google Scholar] [CrossRef] [PubMed]
- Trimarchi, M.; Bertazzoni, G.; Bussi, M. Cocaine induced midline destructive lesions. Rhinology 2014, 52, 104–111. [Google Scholar] [CrossRef] [Green Version]
- Molteni, M.; Saibene, A.M.; Luciano, K.; Maccari, A. Snorting the clivus away: An extreme case of cocaine-induced midline destructive lesion. BMJ Case Rep. 2016, bcr2016216393. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, D.; Trimarchi, M.; Galli, A.; Bussi, M. Columella reconstruction with an inferiorly-based philtral advancement flap in a cocaine abuser. Indian J. Plast. Surg. 2017, 50, 96–99. [Google Scholar] [CrossRef]
- Trimarchi, M.; Gregorini, G.; Facchetti, F.; Morassi, M.L.; Manfredini, C.; Maroldi, R.; Nicolai, P.; Russel, K.A.; Mcdonald, T.J.; Specks, U. Cocaine-Induced Midline Destructive Lesions: Clinical, radiographic, histopathologic, and serologic features and their differentiation from Wegener granulomatosis. Medicine 2001, 80, 391–404. [Google Scholar] [CrossRef]
- Silvestre, F.J.; Perez-Herbera, A.; Puente-Sandoval, A.; Bagán, J.V. Hard palate perforation in cocaine abusers: A systematic review. Clin. Oral Investig. 2010, 14, 621–628. [Google Scholar] [CrossRef]
- Hofstede, T.M.; Jacob, R.F. Diagnostic considerations and prosthetic rehabilitation of a cocaine-induced midline destructive lesion: A clinical report. J. Prosthet. Dent. 2010, 103, 1–5. [Google Scholar] [CrossRef]
- Di Cosola, M.; Turco, M.; Acero, J.; Navarro-Vila, C.; Cortelazzi, R. Cocaine-related syndrome and palatal reconstruction: Report of a series of cases. Int. J. Oral Maxillofac. Surg. 2007, 36, 721–727. [Google Scholar] [CrossRef]
- Morassi, M.L.; Trimarchi, M.; Nicolai, P.; Gregorini, G.; Maroldi, R.; Specks, U.; Facchetti, F. Cocaina, ANCA e granulomatosi di Wegener. Pathologica 2001, 93, 581–583. [Google Scholar] [PubMed]
- Alamino, R.P.; Espinoza, L.R. Vasculitis Mimics: Cocaine-induced Midline Destructive Lesions. Am. J. Med. Sci. 2013, 346, 430–431. [Google Scholar] [CrossRef]
- Lanzillotta, M.; Campochiaro, C.; Trimarchi, M.; Arrigoni, G.; Gerevini, S.; Milani, R.; Bozzolo, E.; Biafora, M.; Venturini, E.; Cicalese, M.P.; et al. Deconstructing IgG4-related disease involvement of midline structures: Comparison to common mimickers. Mod. Rheumatol. 2017, 27, 638–645. [Google Scholar] [CrossRef]
- United States Department of Health and Human Services-Substance Abuse and Mental Health Service Administration. National Household Survey on Drug Abuse 1998; Inter-University Consortium for Political and Social Research: Ann Arbor, MI, USA, 1998.
- Trimarchi, M.; Bondi, S.; Della Torre, E.; Terreni, M.R.; Bussi, M. Palate perforation differentiates cocaine-induced midline destructive lesions from granulomatosis with polyangiitis. Acta Otorhinolaryngol. Ital. 2017, 37, 281–285. [Google Scholar] [CrossRef]
- Bacciu, A.; Ghirelli, M.; Ingegnoli, A.; Bozzetti, F. Cocaine-Induced Midline Destructive Lesions Associated With Erosion of the Eustachian Tube. JAMA Otolaryngol. Neck Surg. 2018, 144, 846. [Google Scholar] [CrossRef]
- Trimarchi, M.; Nicolai, P.; Lombardi, D.; Facchetti, F.; Morassi, M.L.; Maroldi, R.; Gregorini, G.; Specks, U. Sinonasal Osteocartilaginous Necrosis in Cocaine Abusers: Experience in 25 Patients. Am. J. Rhinol. 2003, 17, 33–43. [Google Scholar] [CrossRef]
- Cottrell, D.A.; Mehra, P.; Malloy, J.C.; Ghali, G. Midline palatal perforation. J. Oral Maxillofac. Surg. 1999, 57, 990–995. [Google Scholar] [CrossRef]
- Della-Torre, E.; Campochiaro, C.; Cassione, E.B.; Albano, L.; Gerevini, S.; Bianchi-Marzoli, S.; Bozzolo, E.; Passerini, G.; Lanzillotta, M.; Terreni, M.; et al. Intrathecal rituximab for IgG 4 -related hypertrophic pachymeningitis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 441–444. [Google Scholar] [CrossRef]
- Trimarchi, M.; Bellini, C.; Fabiano, B.; Gerevini, S.; Bussi, M. Un caso di pemfigoide mucosinechiante con coinvolgimento nasale e laringeo. Acta Otorhinolaryngol. Ital. 2009, 29, 222–225. [Google Scholar]
- Trimarchi, M.; Miluzio, A.; Nicolai, P.; Morassi, M.L.; Bussi, M.; Marchisio, P.C. Massive Apoptosis Erodes Nasal Mucosa of Cocaine Abusers. Am. J. Rhinol. 2006, 20, 160–164. [Google Scholar] [CrossRef]
- Alfano, M.; Grivel, J.-C.; Ghezzi, S.; Corti, D.; Trimarchi, M.; Poli, G.; Margolis, L. Pertussis toxin B-oligomer dissociates T cell activation and HIV replication in CD4 T cells released from infected lymphoid tissue. AIDS 2005, 19, 1007–1014. [Google Scholar] [CrossRef]
- Wiesner, O.; Russell, K.A.; Lee, A.S.; Jenne, D.E.; Trimarchi, M.; Gregorini, G.; Specks, U. Antineutrophil cytoplasmic antibodies reacting with human neutrophil elastase as a diagnostic marker for cocaine-induced midline destructive lesions but not autoimmune vasculitis. Arthritis Rheum. 2004, 50, 2954–2965. [Google Scholar] [CrossRef]
- Peikert, T.; Finkielman, J.D.; Hummel, A.M.; McKenney, M.E.; Gregorini, G.; Trimarchi, M.; Specks, U. Functional characterization of antineutrophil cytoplasmic antibodies in patients with cocaine-induced midline destructive lesions. Arthritis Rheum. 2008, 58, 1546–1551. [Google Scholar] [CrossRef]
- Trimarchi, M.; Mortini, P. Cocaine-Induced Midline Destructive Lesion and Wegener Granulomatosis. Neurosurgery 2012, 70, E1339. [Google Scholar] [CrossRef]
- Rachapalli, S.M.; Kiely, P.D.W. Cocaine-induced midline destructive lesions mimicking ENT-limited Wegener’s granulomatosis. Scand. J. Rheumatol. 2008, 37, 477–480. [Google Scholar] [CrossRef]
- Simsek, S.; de Vries, X.H.; Jol, J.A.D.; Spoelstra-de Man, A.M.E.; Nanayakkara, P.W.B.; Smulders, Y.M.; Mahieu, H.F.; ter Wee, P.M. Sino-nasal bony and cartilaginous destruction associated with cocaine abuse, S. aureus and antineutrophil cytoplasmic antibodies. Neth. J. Med. 2006, 64, 248–251. [Google Scholar]
- Zwang, N.A.; Van Wagner, L.B.; Rose, S. A Case of Levamisole-Induced Systemic Vasculitis and Cocaine-Induced Midline Destructive Lesion. J. Clin. Rheumatol. 2011, 17, 197–200. [Google Scholar] [CrossRef]
- Bains, M.K.; Hosseini-Ardehali, M. Palatal perforations: Past and present. Two case reports and a literature review. Br. Dent. J. 2005, 199, 267–269. [Google Scholar] [CrossRef]
- Cintra, H.L.; Basile, F.V.; Tournieux, T.T.; Pitanguy, I.; Basile, A.R. Midline palate perforation secondary to cocaine abuse. J. Plast. Reconstr. Aesthetic Surg. 2008, 61, 588–590. [Google Scholar] [CrossRef]
- Jackson, I.T.; Kelly, C.; Bello-Rojas, G. Palatal Fistulae Resulting From Cocaine Abuse. Ann. Plast. Surg. 2009, 62, 67–69. [Google Scholar] [CrossRef]
- Silvestre, F.J.; Salort-Llorca, C.; Mínguez-Serra, M.P.; Silvestre-Rangil, J. Cocaine-related oronasal communication and hard palate destruction. J. Investig. Clin. Dent. 2012, 3, 157–160. [Google Scholar] [CrossRef]
- Gastaldi, G.; Palumbo, L.; Moreschi, C.; Gherlone, E.F.; Capparé, P. Prosthetic management of patients with oro-maxillo-facial defects: A long-term follow-up retrospective study. Oral Implantol. 2017, 10, 276–282. [Google Scholar] [CrossRef]
- Crespi, R.; Vinci, R.; Capparé, P.; Romanos, G.E.; Gherlone, E. A clinical study of edentulous patients rehabilitated according to the “all on four” immediate function protocol. Int. J. Oral Maxillofac. Implants 2012, 27, 428–434. [Google Scholar]
- Capparé, P.; Teté, G.; Romanos, G.E.; Nagni, M.; Sannino, G.; Gherlone, E.F. The “All-on-four” protocol in HIV-positive patients: A prospective, longitudinal 7-year clinical study. Int. J. Oral Implantol. 2019, 12, 501–510. [Google Scholar]
- Gherlone, E.F.; Capparé, P.; Tecco, S.; Polizzi, E.; Pantaleo, G.; Gastaldi, G.; Grusovin, M.G. Implant Prosthetic Rehabilitation in Controlled HIV-Positive Patients: A Prospective Longitudinal Study with 1-Year Follow-Up. Clin. Implant Dent. Relat. Res. 2016, 18, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Tecco, S.; Parisi, M.R.; Gastaldi, G.; Polizzi, E.; D’Amicantonio, T.; Zilocchi, I.; Gardini, I.; Gherlone, E.F.; Lazzarin, A.; Capparè, P. Point-of-care testing for hepatitis C virus infection at an Italian dental clinic: Portrait of the pilot study population. New Microbiol. 2019, 42, 133–138. [Google Scholar] [PubMed]
- Crespi, R.; Capparé, P.; Romanos, G.E.; Mariani, E.; Benasciutti, E.; Gherlone, E. Corticocancellous porcine bone in the healing of human extraction sockets: Combining histomorphometry with osteoblast gene expression profiles in vivo. Int. J. Oral Maxillofac. Implants 2011, 26, 866–872. [Google Scholar] [PubMed]
- Crespi, R.; Capparè, P.; Gherlone, E. Sinus floor elevation by osteotome: Hand mallet versus electric mallet. A prospective clinical study. Int. J. Oral Maxillofac. Implants 2012, 27, 1144–1150. [Google Scholar]
- Smith, J.C.; Kacker, A.; Anand, V.K. Midline nasal and hard palate destruction in cocaine abusers and cocaine’s role in rhinologic practice. Ear. Nose Throat J. 2002, 81, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Artero, E.; Querol-Cisneros, E.; Rodríguez-Garijo, N.; Tomás-Velázquez, A.; Antoñanzas, J.; Secundino, F.; Pilar Gil-Sánchez, M.; España, A. Mucocutaneous manifestations of cocaine abuse: A review. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.D.T.; Aspden, T.J.; Adler, J.; Jones, N.S.; Illum, L.; Davis, S.S. Measurement of nasal mucociliary transport rates on the isolated human inferior turbinate. Clin. Otolaryngol. 1995, 20, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.; Belloso, A.; Wilson, C.A.; McCormick, M. Rare case of naso-oral fistula with extensive osteocartilaginous necrosis secondary to cocaine abuse: Review of otorhinolaryngological presentations in cocaine addicts. J. Laryngol. Otol. 2000, 114, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Marquez, J.; Aguirre, L.; Muñoz, C.; Echeverri, A.; Restrepo, M.; Pinto, L.F. Cocaine-Levamisole-Induced Vasculitis/Vasculopathy Syndrome. Curr. Rheumatol. Rep. 2017, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Harper, L.; Cockwell, P.; Adu, D.; Savage, C.O.S. Neutrophil priming and apoptosis in anti-neutrophil cytoplasmic autoantibody-associated vasculitis. Kidney Int. 2001, 59, 1729–1738. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rampi, A.; Vinciguerra, A.; Bondi, S.; Policaro, N.S.; Gastaldi, G. Cocaine-Induced Midline Destructive Lesions: A Real Challenge in Oral Rehabilitation. Int. J. Environ. Res. Public Health 2021, 18, 3219. https://doi.org/10.3390/ijerph18063219
Rampi A, Vinciguerra A, Bondi S, Policaro NS, Gastaldi G. Cocaine-Induced Midline Destructive Lesions: A Real Challenge in Oral Rehabilitation. International Journal of Environmental Research and Public Health. 2021; 18(6):3219. https://doi.org/10.3390/ijerph18063219
Chicago/Turabian StyleRampi, Andrea, Alessandro Vinciguerra, Stefano Bondi, Nicoletta Stella Policaro, and Giorgio Gastaldi. 2021. "Cocaine-Induced Midline Destructive Lesions: A Real Challenge in Oral Rehabilitation" International Journal of Environmental Research and Public Health 18, no. 6: 3219. https://doi.org/10.3390/ijerph18063219






