Accuracy of Computer-Assisted Dynamic Navigation as a Function of Different Intraoral Reference Systems: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Implantation Planning and Models
2.2. Process Chains
2.3. Registering the Implant Position
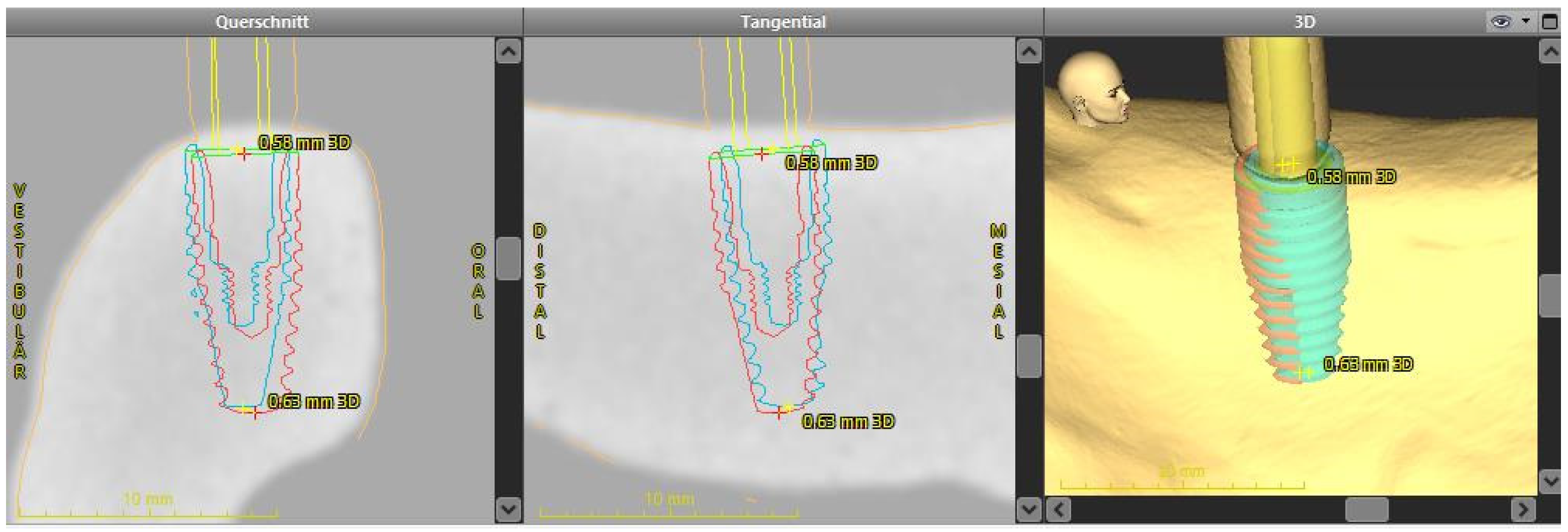
2.4. Analysis of the Implant Position
- 3D deviation: the three-dimensional deviation of the midpoints between implant planning and the clinically-achieved implant position, measured at the implant shoulder and apex (corresponding to the Euclidean distance).
- Apico-coronal deviation (height difference): vertical spatial offset measured at the center of the implant shoulder.
- Axis deviation: Angular deviation of the implant axes between the planned and clinically-achieved implant positions.
- The two-dimensional deviations in the mesio-distal and bucco-lingual directions were measured at the implant shoulder and at the implant axis.
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Haese, J.; Ackhurst, J.; Wismeijer, D.; De Bruyn, H.; Tahmaseb, A. Current state of the art of computer-guided implant surgery. Periodontology 2000 2016, 73, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000 2017, 73, 22–40. [Google Scholar] [CrossRef]
- Albrektsson, T.; Chrcanovic, B.; Östman, P.-O.; Sennerby, L. Initial and long-term crestal bone responses to modern dental implants. Periodontology 2000 2016, 73, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.E.; Delgado-Ruiz, R.; Sculean, A. Concepts for prevention of complications in implant therapy. Periodontology 2000 2019, 81, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Al Amri, M.D. Influence of interimplant distance on the crestal bone height around dental implants: A systematic review and meta-analysis. J. Prosthet. Dent. 2016, 115, 278–282. [Google Scholar] [CrossRef]
- Staubli, N.; Walter, C.; Schmidt, J.C.; Weiger, R.; Zitzmann, N.U. Excess cement and the risk of peri-implant disease—A systematic review. Clin. Oral Implant. Res. 2017, 28, 1278–1290. [Google Scholar] [CrossRef]
- Garber, D.A.; Belser, U.C. Restoration-driven implant placement with restoration-generated site development. Compend. Contin. Educ. Dent. (Jamesburg N.J. 1995) 1995, 16, 796–798. [Google Scholar]
- Vercruyssen, M.; Van De Wiele, G.; Teughels, W.; Naert, I.; Jacobs, R.; Quirynen, M. Implant- and patient-centred outcomes of guided surgery, a 1-year follow-up: An RCT comparing guided surgery with conventional implant placement. J. Clin. Periodontol. 2014, 41, 1154–1160. [Google Scholar] [CrossRef]
- Hultin, M.; Svensson, K.G.; Trulsson, M. Clinical advantages of computer-guided implant placement: A systematic review. Clin. Oral Implant. Res. 2012, 23, 124–135. [Google Scholar] [CrossRef]
- Wismeijer, D.; Joda, T.; Flügge, T.; Fokas, G.; Tahmaseb, A.; Bechelli, D.; Bohner, L.; Bornstein, M.; Burgoyne, A.; Caram, S.; et al. Group 5 ITI Consensus Report: Digital technologies. Clin. Oral Implant. Res. 2018, 29, 436–442. [Google Scholar] [CrossRef]
- Tahmaseb, A.; Wu, V.; Wismeijer, D.; Coucke, W.; Evans, C. The accuracy of static computer-aided implant surgery: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29, 416–435. [Google Scholar] [CrossRef]
- Al Yafi, F.; Camenisch, B.; Al-Sabbagh, M. Is Digital Guided Implant Surgery Accurate and Reliable? Dent. Clin. N. Am. 2019, 63, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Gargallo-Albiol, J.; Barootchi, S.; Salomó-Coll, O.; Wang, H.-L. Advantages and disadvantages of implant navigation surgery. A systematic review. Ann. Anat. Anat. Anz. 2019, 225, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Tahmaseb, A.; Wismeijer, D.; Coucke, W.; Derksen, W. Computer Technology Applications in Surgical Implant Dentistry: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2014, 29, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Gjelvold, B.; Mahmood, D.J.H.; Wennerberg, A. Accuracy of surgical guides from 2 different desktop 3D printers for computed tomography-guided surgery. J. Prosthet. Dent. 2019, 121, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Henprasert, P.; Dawson, D.V.; El-Kerdani, T.; Song, X.; Couso-Queiruga, E.; Holloway, J.A. Comparison of the Accuracy of Implant Position Using Surgical Guides Fabricated by Additive and Subtractive Techniques. J. Prosthodont. 2020, 29, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Pieralli, S.; Spies, B.C.; Hromadnik, V.; Nicic, R.; Beuer, F.; Wesemann, C. How Accurate Is Oral Implant Installation Using Surgical Guides Printed from a Degradable and Steam-Sterilized Biopolymer? J. Clin. Med. 2020, 9, 2322. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yuan, S.; Huan, J.; Zhang, Y.; Fang, C.; Li, J. In Vitro Experimental Study of the Effect of Adjusting the Guide Sleeve Height and Using a Visual Direction-Indicating Guide on Implantation Accuracy. J. Oral Maxillofac. Surg. 2019, 77, 2259–2268. [Google Scholar] [CrossRef]
- Tallarico, M.; Martinolli, M.; Kim, Y.-J.; Cocchi, F.; Meloni, S.M.; Alushi, A.; Xhanari, E. Accuracy of Computer-Assisted Template-Based Implant Placement Using Two Different Surgical Templates Designed with or without Metallic Sleeves: A Randomized Controlled Trial. Dent. J. 2019, 7, 41. [Google Scholar] [CrossRef]
- Kuhl, S.; Payer, M.; Zitzmann, N.U.; Lambrecht, J.T.; Filippi, A. Technical accuracy of printed surgical templates for guided implant surgery with the coDiagnostiX software. Clin. Implant. Dent. Relat. Res. 2015, 17 (Suppl. 1), e177–e182. [Google Scholar] [CrossRef]
- Schnutenhaus, S.; Edelmann, C.; Rudolph, H.; Luthardt, R.G. Retrospective study to determine the accuracy of template-guided implant placement using a novel nonradiologic evaluation method. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 121, e72–e79. [Google Scholar] [CrossRef] [PubMed]
- Vercruyssen, M.; Fortin, T.; Widmann, G.; Jacobs, R.; Quirynen, M. Different techniques of static/dynamic guided implant surgery: Modalities and indications. Periodontology 2000 2014, 66, 214–227. [Google Scholar] [CrossRef]
- Du, Y.; Wangrao, K.; Liu, L.; Liu, L.; Yao, Y. Quantification of Image Artifacts from Navigation Markers in Dynamic Guided Implant Surgery and the Effect on Registration Performance in Different Clinical Scenarios. Int. J. Oral Maxillofac. Implant. 2019, 34, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Brief, J.; Edinger, D.; Hassfeld, S.; Eggers, G. Accuracy of image-guided implantology. Clin. Oral Implant. Res. 2005, 16, 495–501. [Google Scholar] [CrossRef]
- Hoffmann, J.; Westendorff, C.; Reinert, S.; Gomez-Roman, G. Accuracy of navigation-guided socket drilling before implant installation compared to the conventional free-hand method in a synthetic edentulous lower jaw model. Clin. Oral Implant. Res. 2005, 16, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Kramer, F.-J.; Baethge, C.; Swennen, G.; Rosahl, S. Navigated vs. conventional implant insertion for maxillary single tooth replacement. Clin. Oral Implant. Res. 2004, 16, 60–68. [Google Scholar] [CrossRef]
- Chen, J.T.-Y. A Novel Application of Dynamic Navigation System in Socket Shield Technique. J. Oral Implant. 2019, 45, 409–415. [Google Scholar] [CrossRef]
- Block, M.S.; Emery, R.W. Static or Dynamic Navigation for Implant Placement—Choosing the Method of Guidance. J. Oral Maxillofac. Surg. 2016, 74, 269–277. [Google Scholar] [CrossRef]
- Schnutenhaus, S.; Edelmann, C.; Knipper, A.; Luthardt, R. Accuracy of Dynamic Computer-Assisted Implant Placement: A Systematic Review and Meta-Analysis of Clinical and In Vitro Studies. J. Clin. Med. 2021, 10, 704. [Google Scholar] [CrossRef]
- Ayoub, A.; Pulijala, Y. The application of virtual reality and augmented reality in Oral & Maxillofacial Surgery. BMC Oral Health 2019, 19, 1–8. [Google Scholar] [CrossRef]
- Hoffmann, J.; Westendorff, C.; Schneider, M.; Reinert, S. Accuracy assessment of image-guided implant surgery: An experimental study. Int. J. Oral Maxillofac. Implant. 2005, 20, 382–386. [Google Scholar]
- Kang, S.-H.; Lee, J.-W.; Lim, S.-H.; Kim, Y.-H.; Kim, M.-K. Verification of the usability of a navigation method in dental implant surgery: In vitro comparison with the stereolithographic surgical guide template method. J. Cranio Maxillofac. Surg. 2014, 42, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-G.; Lee, W.-J.; Lee, S.-S.; Heo, M.-S.; Huh, K.-H.; Choi, S.-C.; Kim, T.-I.; Yi, W.-J. An advanced navigational surgery system for dental implants completed in a single visit: An in vitro study. J. Cranio Maxillofac. Surg. 2015, 43, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Somogyi-Ganss, E.; Holmes, H.I.; Jokstad, A. Accuracy of a novel prototype dynamic computer-assisted surgery system. Clin. Oral Implant. Res. 2014, 26, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla Guzman, A.; Riad Deglow, E.; Zubizarreta-Macho, A.; Agustin-Panadero, R.; Hernandez Montero, S. Accuracy of Computer-Aided Dynamic Navigation Compared to Computer-Aided Static Navigation for Dental Implant Placement: An In Vitro Study. J. Clin. Med. 2019, 8, 2123. [Google Scholar] [CrossRef]
- Pellegrino, G.; Bellini, P.; Cavallini, P.F.; Ferri, A.; Zacchino, A.; Taraschi, V.; Marchetti, C.; Consolo, U. Dynamic Navigation in Dental Implantology: The Influence of Surgical Experience on Implant Placement Accuracy and Operating Time. An in Vitro Study. Int. J. Environ. Res. Public Health 2020, 17, 2153. [Google Scholar] [CrossRef]
- Emery, R.W.; A Merritt, S.; Lank, K.; Gibbs, J.D. Accuracy of Dynamic Navigation for Dental Implant Placement–Model-Based Evaluation. J. Oral Implant. 2016, 42, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Forna, N.; Agop-Forna, D. Esthetic aspects in implant-prosthetic rehabilitation. Med. Pharm. Rep. 2019, 92, S6–S13. [Google Scholar] [CrossRef]
- Cosola, S.; Marconcini, S.; Boccuzzi, M.; Fabris, G.B.M.; Covani, U.; Peñarrocha-Diago, M.; Peñarrocha-Oltra, D. Radiological Outcomes of Bone-Level and Tissue-Level Dental Implants: Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 6920. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Liu, Z.; Song, L.; Kuo, C.-L.; Shafer, D.M. Clinical Factors Affecting the Accuracy of Guided Implant Surgery—A Systematic Review and Meta-analysis. J. Evid. Based Dent. Pr. 2018, 18, 28–40. [Google Scholar] [CrossRef]
- Yeung, M.; Abdulmajeed, A.; Carrico, C.K.; Deeb, G.R.; Bencharit, S. Accuracy and precision of 3D-printed implant surgical guides with different implant systems: An in vitro study. J. Prosthet. Dent. 2020, 123, 821–828. [Google Scholar] [CrossRef]
- Cassetta, M.; Altieri, F.; Giansanti, M.; Bellardini, M.; Brandetti, G.; Piccoli, L. Is there a learning curve in static computer-assisted implant surgery? A prospective clinical study. Int. J. Oral Maxillofac. Surg. 2020, 49, 1335–1342. [Google Scholar] [CrossRef]
- Bover-Ramos, F.; Viña-Almunia, J.; Cervera-Ballester, J.; Peñarrocha-Diago, M.; García-Mira, B. Accuracy of Implant Placement with Computer-Guided Surgery: A Systematic Review and Meta-Analysis Comparing Cadaver, Clinical, and In Vitro Studies. Int. J. Oral Maxillofac. Implant. 2018, 33, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Schneider, D.; Marquardt, P.; Zwahlen, M.; Jung, R.E. A systematic review on the accuracy and the clinical outcome of computer-guided template-based implant dentistry. Clin. Oral Implant. Res. 2009, 20, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Jung, R.E.; Schneider, D.; Ganeles, J.; Wismeijer, D.; Zwahlen, M.; Hämmerle, C.H.F.; Tahmaseb, A. Computer technology applications in surgical implant dentistry: A systematic review. Int. J. Oral Maxillofac. Implant. 2009, 24, 25–42. [Google Scholar]
- Aydemir, C.A.; Arısan, V. Accuracy of dental implant placement via dynamic navigation or the freehand method: A split-mouth randomized controlled clinical trial. Clin. Oral Implant. Res. 2020, 31, 255–263. [Google Scholar] [CrossRef]
- Block, M.S.; Emery, R.W.; Cullum, D.R.; Sheikh, A. Implant Placement Is More Accurate Using Dynamic Navigation. J. Oral Maxillofac. Surg. 2017, 75, 1377–1386. [Google Scholar] [CrossRef]
- Block, M.S.; Emery, R.W.; Lank, K.; Ryan, J. Implant Placement Accuracy Using Dynamic Navigation. Int. J. Oral Maxillofac. Implant. 2017, 32, 92–99. [Google Scholar] [CrossRef]
- Kaewsiri, D.; Panmekiate, S.; Subbalekha, K.; Mattheos, N.; Pimkhaokham, A. The accuracy of static vs. dynamic computer-assisted implant surgery in single tooth space: A randomized controlled trial. Clin. Oral Implant. Res. 2019, 30, 505–514. [Google Scholar] [CrossRef]
- Pellegrino, G.; Taraschi, V.; Andrea, Z.; Ferri, A.; Marchetti, C. Dynamic navigation: A prospective clinical trial to evaluate the accuracy of implant placement. Int. J. Comput. Dent. 2019, 22, 139–147. [Google Scholar]
- Stefanelli, L.V.; DeGroot, B.S.; I Lipton, D.; A Mandelaris, G. Accuracy of a Dynamic Dental Implant Navigation System in a Private Practice. Int. J. Oral Maxillofac. Implant. 2019, 34, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Stefanelli, L.V.; Mandelaris, G.A.; Franchina, A.; Pranno, N.; Pagliarulo, M.; Cera, F.; Maltese, F.; De Angelis, F.; Di Carlo, S. Accuracy of Dynamic Navigation System Workflow for Implant Supported Full Arch Prosthesis: A Case Series. Int. J. Environ. Res. Public Health 2020, 17, 5038. [Google Scholar] [CrossRef] [PubMed]
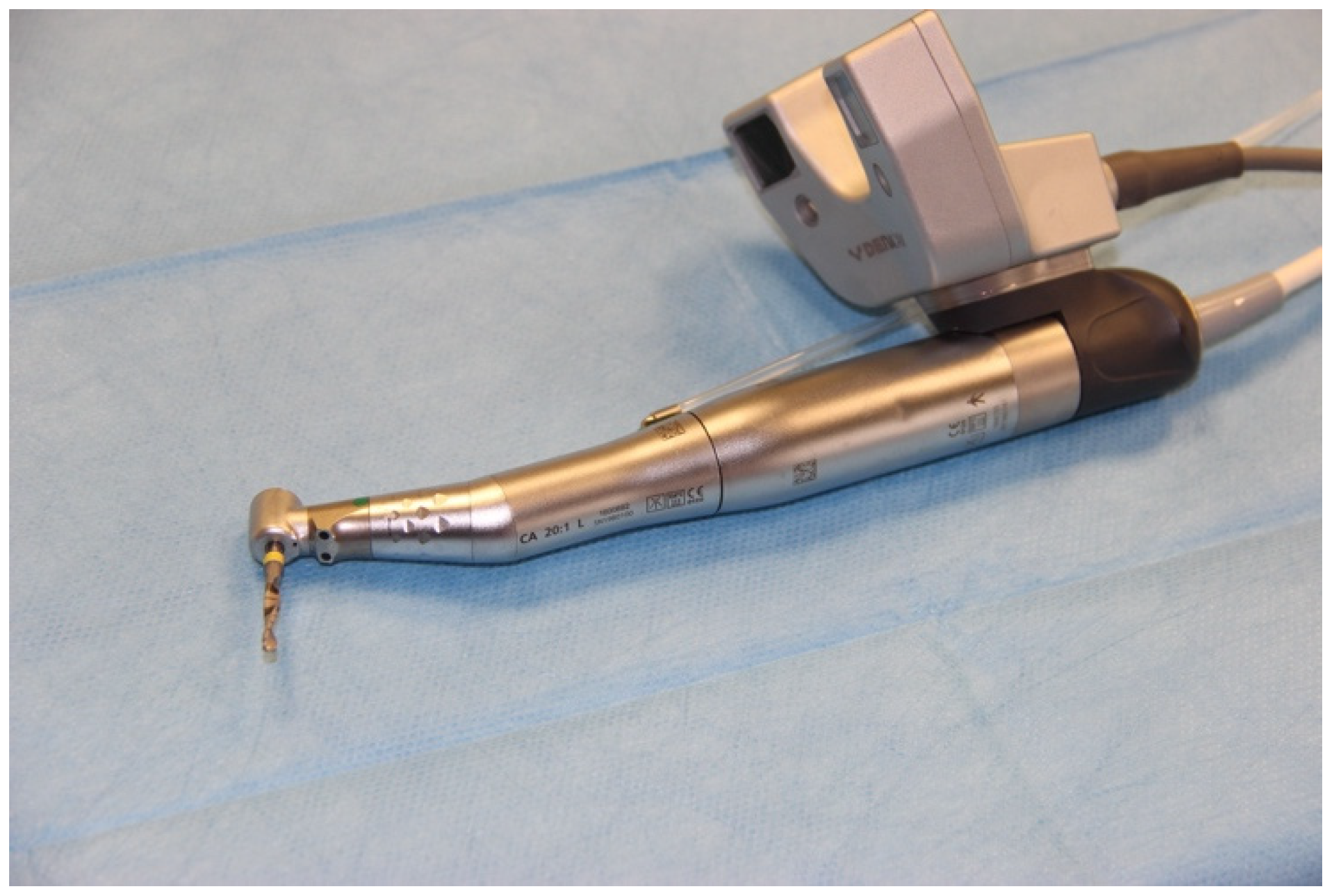
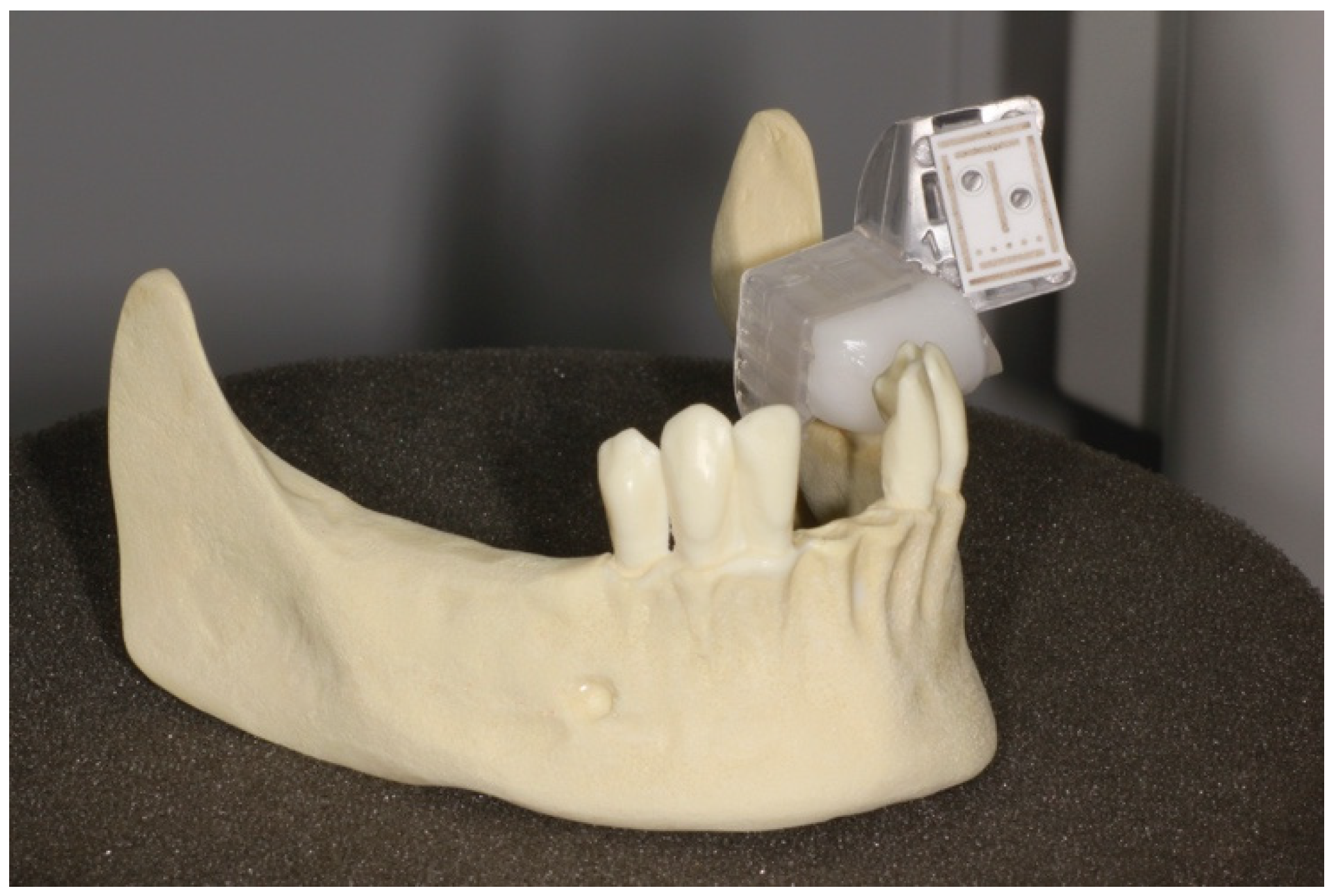

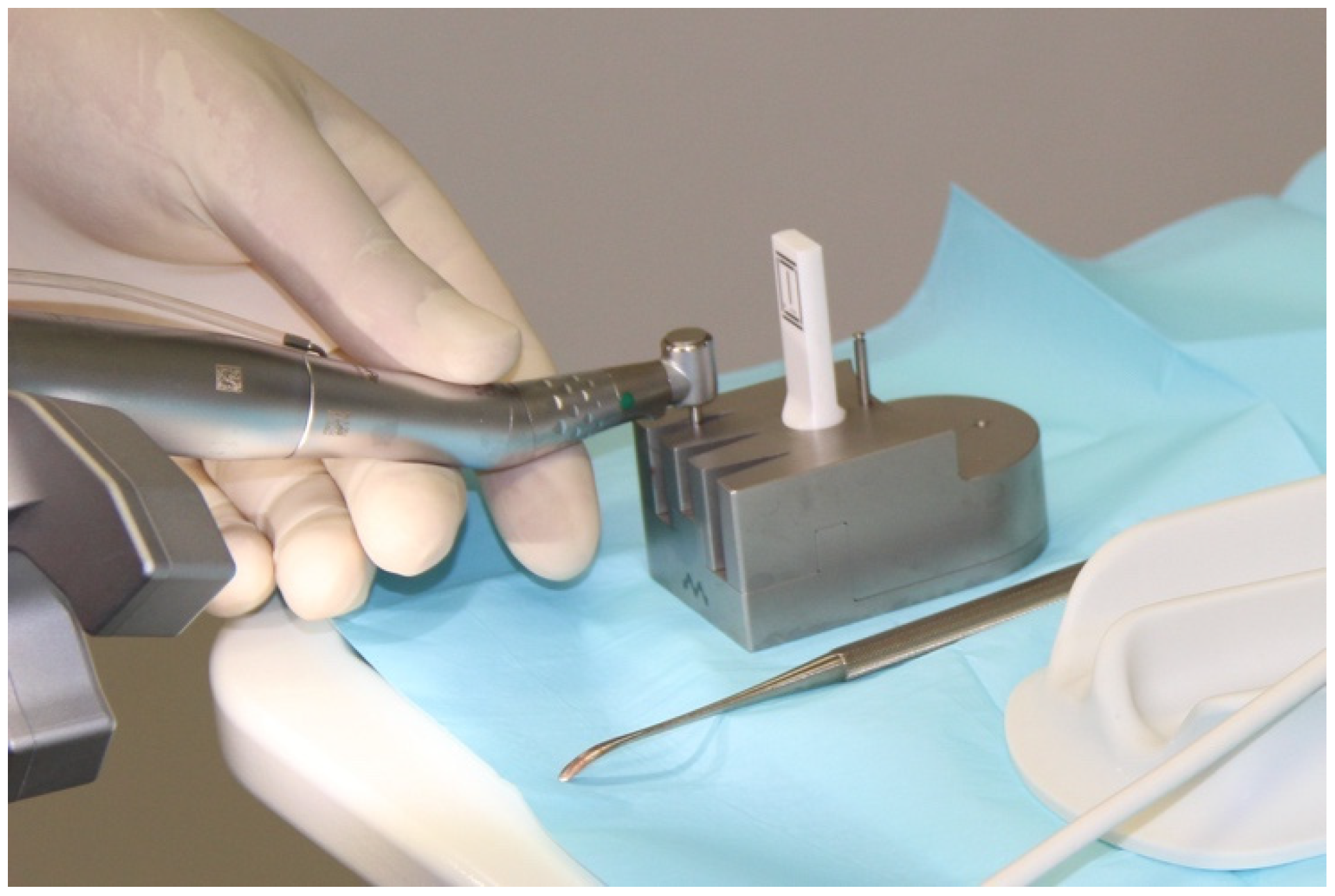
| Workflow | |||||
|---|---|---|---|---|---|
| A | B_1 | B_2 | C | ||
| Data Generation | CBCT | CBCT image with marker | CBCT image | CBCT image | CBCT image |
| On the patient | Intraoral scan | Two intraoral scans (with and without marker) | Alginate impression | Intraoral scan | |
| Virtually | Creation of a digital marker template | ||||
| In the laboratory | Two model scans (with and without marker) | 3D printing of a marker template | |||
| Reference marker in surgery | Surgery with Denatray | Surgery with Denatray | Surgery with Denatray | Surgery with marker template | |
| Total n = 60 Models/120 Implants | Region 45 n = 60 Models/60 Implants | Region 47 n = 60 Models/60 Implants | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Min–Max | Mean (SD) | 95% CI | Min–Max | Mean (SD) | 95% CI | Min–Max | ||
| Deviation at implant shoulder (mm) | ||||||||||
| 3D | 1.53 (0.70) | 1.40–1.66 | 0.20–4.02 | 1.52 (0.64) | 1.36–1.69 | 0.26–4.02 | 1.54 (0.77) | 1.34–1.74 | 0.20–3.75 | 0.923 |
| Mesio-distal | 0.70 (0.59) | 0.59–0.80 | 0.02–3.06 | 0.63 (0.57) | 0.48–0.78 | 0.02–2.37 | 0.77 (0.62) | 0.61–0.93 | 0.04–3.06 | 0.208 |
| Bucco-lingual | 0.98 (0.68) | 0.86–1.11 | 0.00–2.81 | 1.09 (0.66) | 0.91–1.25 | 0.00–2.52 | 0.87 (0.70) | 0.71–1.07 | 0.00–2.81 | 0.116 |
| Apico-coronal | 0.57 (0.50) | 0.48–0.67 | 0.00–2.34 | 0.48 (0.43) | 0.37–0.60) | 0.00–2.17 | 0.66 (0.54) | 0.51–0.80 | 0.00–2.34 | 0.059 |
| Deviation at implant apex (mm) | ||||||||||
| 3D | 1.79 (0.80) | 1.64–1.94 | 0.29–4.05 | 1.81 (0.74) | 1.78–2.00 | 0.29–4.05 | 1.77 (0.86) | 1.54–1.99 | 0.29–3.74 | 0.766 |
| Mesio-distal | 0.81 (0.70) | 0.68–0.93 | 0.01–3.73 | 0.80 (0.71) | 0.62–0.98 | 0.04–3.73 | 0.82 (0.69) | 0.64–1.00 | 0.01–3.26 | 0.890 |
| Bucco-lingual | 1.25 (0.75) | 1.12–1.39 | 0.01–3.10 | 1.36 (0.66) | 1.19–1.53 | 0.20–2.79 | 1.15 (0.83) | 0.93–1.36 | 0.01–3.10 | 0.126 |
| Apico-coronal | 0.58 (0.50) | 0.49–0.67 | 0.00–2.35 | 0.50 (0.43) | 0.39–0.61 | 0.00–2.19 | 0.66 (0.55) | 0.52–0.81 | 0.00–2.35 | 0.068 |
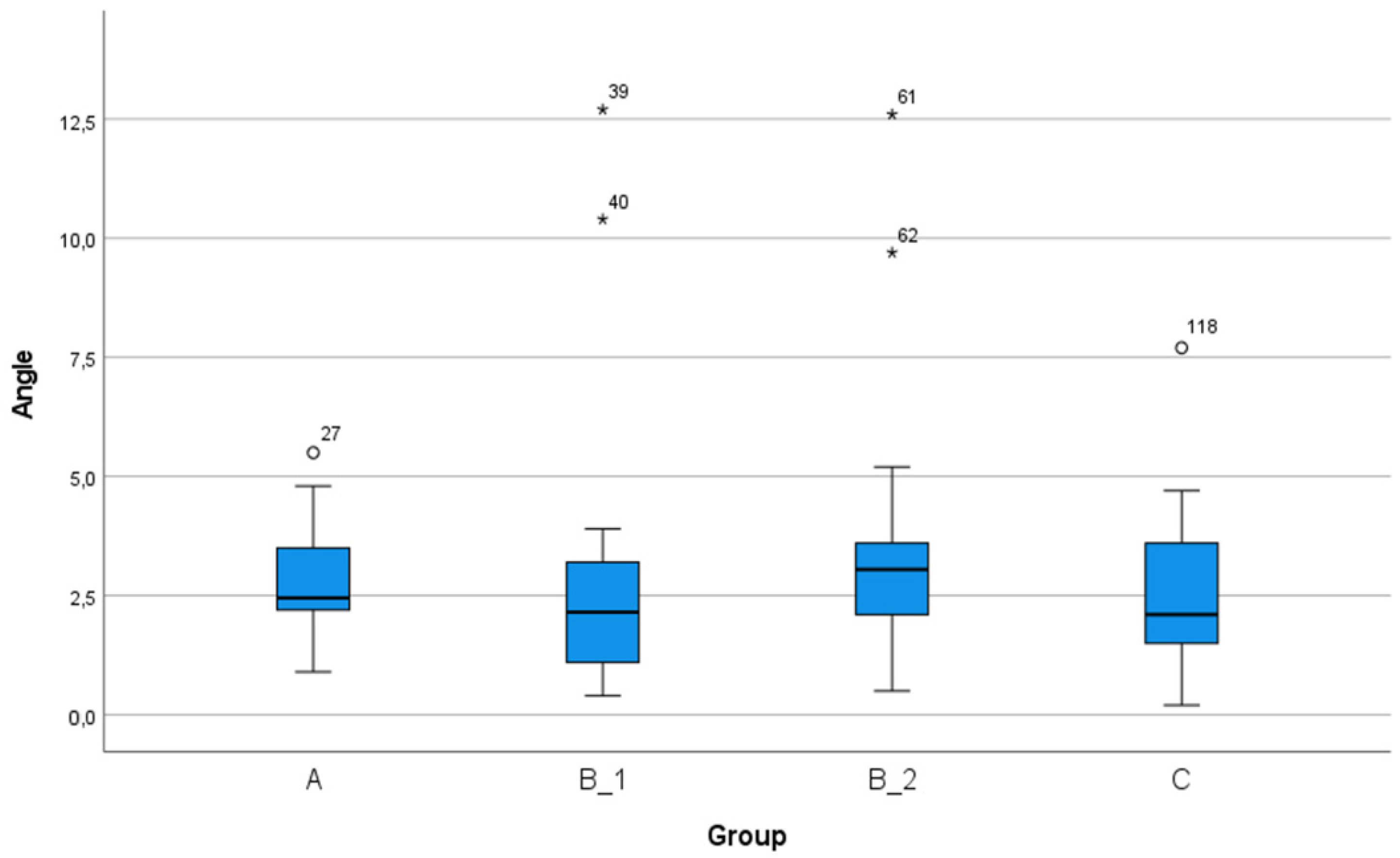
| Angular deviation (°) | 2.88 (2.03) | 2.51–3.25 | 0.20–12.70 | 2.87 (2.22) | 2.30–3.44 | 0.20–12.70 | 2.89 (1.83) | 2.41–3.36 | 0.40–10.40 | 0.964 |
| Process Chain A n = 15 Models/30 Implants | Process Chain B_1 n = 15 Models/30 Implants | Process Chain B_2 n = 15 Models/30 Implants | Process Chain C n = 15 Models/30 Implants | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Min–Max | Mean (SD) | 95% CI | Min–Max | Mean (SD) | 95% CI | Min–Max | Mean (SD) | 95% CI | Min–Max | ||
| Deviation at implant shoulder (mm) | |||||||||||||
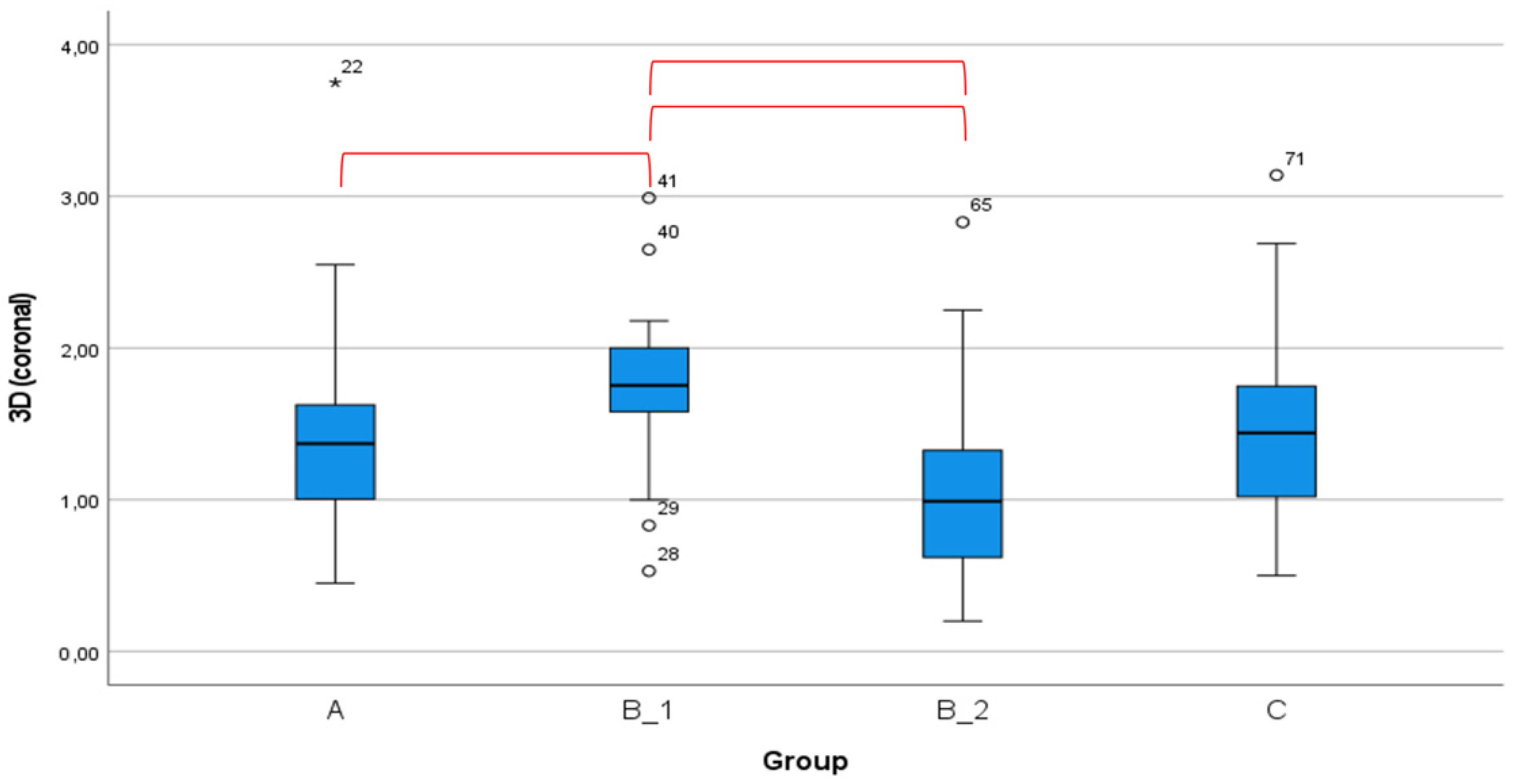
| 3D | 1.40 (0.65) | 1.16–1.64 | 0.41–3.75 | 1.85 (0.52) | 1.66–2.04 | 0.53–2.99 | 1.48 (0.92) | 1.14–1.83 | 0.20–4.02 | 1.39 (0.59) | 1.17–1.61 | 0.50–3.14 | 0.034 |
| Mesio-distal | 0.80 (0.63) | 0.55–1.04 | 0.06–3.06 | 0.64 (0.50) | 0.45–0.83 | 0.04–1.70 | 0.68 (0.66) | 0.44–0.93 | 0.02–2.37 | 0.67 (0.56) | 0.46–0.88 | 0.04–2.63 | 0.763 |
| Bucco-lingual | 0.60 (0.48) | 0.42–0.78 | 0.06–1.91 | 1.47 (0.65) | 1.47–1.72 | 0.14–2.6 | 0.91 (0.75) | 0.63–1.19 | 0.00–2.81 | 0.95 (0.53) | 0.76–1.15 | 0.00–1.81 | <0.005 |
| Apico-coronal | 0.67 (0.53) | 0.47–0.87 | 0.00–2.10 | 0.56 (0.38) | 0.41–0.70) | 0.01–1.29 | 0.60 (0.65) | 0.36–0.84 | 0.01–2.34 | 0.45 (0.37) | 0.31–0.59 | 0.02–1.65 | 0.303 |
| Deviation at implant apex (mm) | |||||||||||||
| 3D | 1.54 (0.72) | 1.27–1.81 | 0.75–3.56 | 2.13 (0.62) | 1.90–2.09 | 0.69–3.49 | 1.80 (1.06) | 1.41–2.20 | 0.29–4.05 | 1.68 (0.65) | 1.44–1.92 | 0.63–3.68 | 0.029 |
| Mesio-distal | 0.80 (0.66) | 0.55–1.05 | 0.04–2.87 | 0.67 (0.51) | 0.48–0.86 | 0.04–1.80 | 0.83 (0.88) | 0.50–1.16 | 0.04–3.73 | 0.93 (0.70) | 0.66–1.19 | 0.01–3.26 | 0.553 |
| Bucco-lingual | 0.86 (0.59) | 0.64–1.08 | 0.01–2.41 | 1.82 (0.68) | 1.57–2.07 | 0.08–3.10 | 1.23 (0.79) | 0.93–1.20 | 0.12–2.93 | 1.10 (0.60) | 0.88–1.32 | 0.18–2.31 | <0.005 |
| Apico-coronal | 0.69 (0.53) | 0.49–0.89 | 0.00–2.12 | 0.57 (0.39) | 0.42–0.71 | 0.01–1.35 | 0.62 (0.65) | 0.38–0.86 | 0.01–2.35 | 0.45 (0.37) | 0.31–0.59 | 0.00–1.62 | 0.303 |
| Angular deviation (°) | 2.77 (1.06) | 2.37–3.16 | 0.90–5.50 | 2.70 (2.61) | 1.73–3.68 | 0.40–12.70 | 3.43 (2.44) | 2.52–3.13 | 0.50–12.6 | 2.62 (1.60) | 2.02–3.22 | 0.20–7.70 | 0.394 |
| A-B_1 | A-B_2 | A-C | B_1-B_2 | B_1-C | B_2-C | |
|---|---|---|---|---|---|---|
| Deviation at implant shoulder (mm) | ||||||
| 3D | 0.001 * | 0.722 | 0.873 | 0.003 * | 0.002 * | 0.844 |
| Mesio-distal | 0.747 | 0.887 | 0.849 | 0.992 | 0.997 | 1.000 |
| Bucco-lingual | <0.001 * | 0.220 | 0.125 | 0.003 * | 0.007 * | 0.992 |
| Apico-coronal | 0.800 | 0.937 | 0.303 | 0.988 | 0.835 | 0.648 |
| Deviation at implant apex (mm) | ||||||
| 3D | 0.022 * | 0.573 | 0.905 | 0.366 | 0.119 | 0.929 |
| Mesio-distal | 0.884 | 0.998 | 0.897 | 0.805 | 0.481 | 0.951 |
| Bucco-lingual | <0.001 * | 0.147 | 0.502 | 0.005 * | <0.001 * | 0.881 |
| Apico-coronal | 0.796 | 0.958 | 0.255 | 0.976 | 0.787 | 0.536 |
| Angular deviation (degree) | 0.999 | 0.590 | 0.992 | 0.513 | 0.998 | 0.413 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnutenhaus, S.; Knipper, A.; Wetzel, M.; Edelmann, C.; Luthardt, R. Accuracy of Computer-Assisted Dynamic Navigation as a Function of Different Intraoral Reference Systems: An In Vitro Study. Int. J. Environ. Res. Public Health 2021, 18, 3244. https://doi.org/10.3390/ijerph18063244
Schnutenhaus S, Knipper A, Wetzel M, Edelmann C, Luthardt R. Accuracy of Computer-Assisted Dynamic Navigation as a Function of Different Intraoral Reference Systems: An In Vitro Study. International Journal of Environmental Research and Public Health. 2021; 18(6):3244. https://doi.org/10.3390/ijerph18063244
Chicago/Turabian StyleSchnutenhaus, Sigmar, Anne Knipper, Martin Wetzel, Cornelia Edelmann, and Ralph Luthardt. 2021. "Accuracy of Computer-Assisted Dynamic Navigation as a Function of Different Intraoral Reference Systems: An In Vitro Study" International Journal of Environmental Research and Public Health 18, no. 6: 3244. https://doi.org/10.3390/ijerph18063244
APA StyleSchnutenhaus, S., Knipper, A., Wetzel, M., Edelmann, C., & Luthardt, R. (2021). Accuracy of Computer-Assisted Dynamic Navigation as a Function of Different Intraoral Reference Systems: An In Vitro Study. International Journal of Environmental Research and Public Health, 18(6), 3244. https://doi.org/10.3390/ijerph18063244







