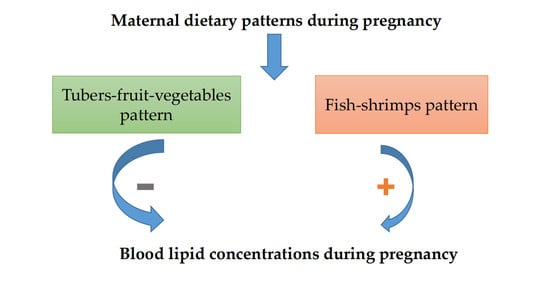
Relationships between Maternal Dietary Patterns and Blood Lipid Levels during Pregnancy: A Prospective Cohort Study in Shanghai, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Lipid Analysis
2.3. Dietary Assessment
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
3.2. Dietary Patterns
3.3. Dietary Patterns in Relation to Maternal Characteristics
3.4. Dietary Patterns in Relation to Blood Lipid Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butte, N.F. Carbohydrate and lipid metabolism in pregnancy: Normal compared with gestational diabetes mellitus. Am. J. Clin. Nutr. 2000, 71, 1256S–1261S. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Ortega-Senovilla, H. Lipid metabolism during pregnancy and its implications for fetal growth. Curr. Pharm. Biotechnol. 2014, 15, 24–31. [Google Scholar] [CrossRef]
- Adank, M.C.; Benschop, L.; Peterbroers, K.R.; Gregoor, A.M.S.; Kors, A.W.; Mulder, M.T.; Schalekamp-Timmermans, S.; Van Lennep, J.E.R.; Steegers, E.A. Is maternal lipid profile in early pregnancy associated with pregnancy complications and blood pressure in pregnancy and long term postpartum? Am. J. Obstet. Gynecol. 2019, 221, 150–151. [Google Scholar] [CrossRef] [Green Version]
- Ryckman, K.K.; Spracklen, C.N.; Smith, C.J.; Robinson, J.G.; Saftlas, A.F. Maternal lipid levels during pregnancy and gestational diabetes: A systematic review and meta-analysis. BJOG 2015, 122, 643–651. [Google Scholar] [CrossRef]
- Jin, W.-Y.; Lin, S.-L.; Hou, R.-L.; Chen, X.-Y.; Han, T.; Jin, Y.; Tang, L.; Zhu, Z.-W.; Zhao, Z.-Y. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: A population-based study from China. BMC Pregnancy Childbirth 2016, 16, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Liu, X.; Chen, Y.; He, B.; Cheng, W. Associations of lipid levels during gestation with hypertensive disorders of pregnancy and gestational diabetes mellitus: A prospective longitudinal cohort study. BMJ Open 2016, 6, e013509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mina, T.H.; Lahti, M.; Drake, A.J.; Forbes, S.; Denison, F.C.; Räikkönen, K.; Norman, J.E.; Reynolds, R.M. Maternal lipids in pregnancy are associated with increased offspring cortisol reactivity in childhood. Psychoneuroendocrinology 2017, 83, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.J.; Retterstøl, K.; Godang, K.; Roland, M.C.P.; Qvigstad, E.; Bollerslev, J.; Ueland, T.; Henriksen, T.; Holven, K.B. LDL cholesterol in early pregnancy and offspring cardiovascular disease risk factors. J. Clin. Lipidol. 2016, 10, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, A.A.; Alberdi, G.; O’Sullivan, E.J.; O’Brien, E.C.; Crosbie, B.; Twomey, P.J.; McAuliffe, F.M. Maternal and fetal blood lipid concentrations during pregnancy differ by maternal body mass index: Findings from the ROLO study. BMC Pregnancy Childbirth 2017, 17, 360. [Google Scholar] [CrossRef] [PubMed]
- Farias, D.R.; Franco-Sena, A.B.; Vilela, A.; Lepsch, J.; Mendes, R.H.; Kac, G. Lipid changes throughout pregnancy according to pre-pregnancy BMI: Results from a prospective cohort. BJOG 2016, 123, 570–578. [Google Scholar] [CrossRef]
- Eshriqui, I.; Franco-Sena, A.B.; Farias, D.R.; Freitas-Vilela, A.A.; Cunha, D.B.; Barros, E.G.; Emmett, P.M.; Kac, G. Prepregnancy Dietary Patterns Are Associated with Blood Lipid Level Changes During Pregnancy: A Prospective Cohort Study in Rio de Janeiro, Brazil. J. Acad. Nutr. Diet. 2017, 117, 1066–1079. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Dreibelbis, C.; Kingshipp, B.L.; Wong, Y.P.; Abrams, B.; Gernand, A.D.; Rasmussen, K.M.; Siega-Riz, A.M.; Stang, J.; Casavale, K.O.; et al. Dietary patterns before and during pregnancy and birth outcomes: A systematic review. Am. J. Clin. Nutr. 2019, 109, 729S–756S. [Google Scholar] [CrossRef] [PubMed]
- Kibret, K.T.; Chojenta, C.; Gresham, E.; Tegegne, T.K.; Loxton, D. Maternal dietary patterns and risk of adverse pregnancy (hypertensive disorders of pregnancy and gestational diabetes mellitus) and birth (preterm birth and low birth weight) outcomes: A systematic review and meta-analysis. Public Health Nutr. 2018, 1–15. [Google Scholar] [CrossRef]
- Nykjaer, C.; Higgs, C.; Greenwood, D.C.; Simpson, N.A.; Cade, J.E.; Alwan, N.A. Maternal Fatty Fish Intake Prior to and during Pregnancy and Risks of Adverse Birth Outcomes: Findings from a British Cohort. Nutrients 2019, 11, e643. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, Z.; Wang, H.; Du, W.; Su, C.; Zhang, J.; Jiang, H.; Jia, X.; Huang, F.; Zhai, F.; et al. Association between dietary patterns and blood lipid profiles among Chinese women. Public Health Nutr. 2016, 19, 3361–3368. [Google Scholar] [CrossRef] [Green Version]
- Htun, N.C.; Suga, H.; Imai, S.; Shimizu, W.; Ishikawa-Takata, K.; Takimoto, H. Dietary pattern and its association with blood pressure and blood lipid profiles among Japanese adults in the 2012 Japan National Health and Nutrition Survey. Asia Pac. J. Clin. Nutr. 2018, 27, 1048–1061. [Google Scholar] [PubMed]
- O’Connor, E.L.; Paddon-Jones, U.; Wright, A.J.; Campbell, W.W. A Mediterranean-style eating pattern with lean, unprocessed red meat has cardiometabolic benefits for adults who are overweight or obese in a randomized, crossover, controlled feeding trial. Am. J. Clin. Nutr. 2018, 108, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Willett, W. Nutritional Epidemiology, 2nd ed.; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- National Institute of Nutrition and Food Safety, China Centers for Disease Control. China Food Composition; Peking University Medical Press: Beijing, China, 2004.
- National Institute of Nutrition and Food Safety, China Centers for Disease Control. China Food Composition, 2nd ed.; Peking University Medical Press: Beijing, China, 2009.
- Joseph, F.; Hair, J.; Black, W.C.; Babin, B.J.; Anderson, R.E. Multivariate Data Analysis, 7th ed.; Pearson Education, Inc.: New York, NY, USA, 2010; pp. 91–146. [Google Scholar]
- Zhou, B.; Cooperative Meta-Analysis Group of China Obesity Task Force. Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population. Zhonghua Liu Xing Bing Xue Za Zhi 2002, 23, 5–10. [Google Scholar]
- Bassett, D.J. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1396. [Google Scholar] [CrossRef]
- Fan, M.; Lyu, J.; He, P. Chinese guidelines for data processing and analysis concerning the International Physical Activity Questionnaire. Zhonghua Liu Xing Bing Xue Za Zhi 2014, 35, 961–964. [Google Scholar]
- Peter, D.; Patrick, H.; Kung-Yee, L.; Scott, Z. Analysis of Longitudinal Data, 2nd ed.; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- Eberg, M.; Platt, R.W.; Filion, K.B. The Estimation of Gestational Age at Birth in Database Studies. Epidemiology 2017, 28, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Gulati, S.; Misra, A.; Pandey, R.M. Effects of 3 g of soluble fiber from oats on lipid levels of Asian Indians—A randomized controlled, parallel arm study. Lipids Health Dis. 2017, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Zhang, W.; Zhang, R.; Jiao, J.; Fu, C.; Tong, X.; Zhang, W.; Qin, L. Cereal fiber improves blood cholesterol profiles and modulates intestinal cholesterol metabolism in C57BL/6 mice fed a high-fat, high-cholesterol diet. Food Nutr. Res. 2019, 63, 59. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.E.M.; De Oliveira, E.P.; Moreto, F.; Gabriel, G.F.; Corrente, J.E.; Burini, R.C. Dietary intake and blood lipid profile in overweight and obese schoolchildren. BMC Res. Notes 2012, 5, 598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mente, A.; Dehghan, M.; Rangarajan, S.; McQueen, M.; Dagenais, G.; Wielgosz, A.; Lear, S.; Li, W.; Chen, H.; Yi, S.; et al. Association of dietary nutrients with blood lipids and blood pressure in 18 countries: A cross-sectional analysis from the PURE study. Lancet Diabetes Endocrinol. 2017, 5, 774–787. [Google Scholar] [CrossRef] [Green Version]
- Kwon, Y.-J.; Lee, H.S.; Chang, H.-J.; Koh, S.-B.; Lee, J.-W. Association of dietary lipid intake with low-density lipoprotein cholesterol levels: Analysis of two independent population-based studies. Eur. J. Nutr. 2019, 59, 2557–25679. [Google Scholar] [CrossRef] [PubMed]
- Hollænder, P.L.B.; Ross, A.B.; Kristensen, M. Whole-grain and blood lipid changes in apparently healthy adults: A systematic review and meta-analysis of randomized controlled studies. Am. J. Clin. Nutr. 2015, 102, 556–572. [Google Scholar] [CrossRef] [Green Version]
- Layton, J.; Powe, C.; Allard, C.; Battista, M.-C.; Doyon, M.; Bouchard, L.; Perron, P.; Wessel, J.; Hivert, M.-F. Maternal lipid profile differs by gestational diabetes physiologic subtype. Metabolism 2019, 91, 39–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huffman, K.M.; Hawk, V.H.; Henes, S.T.; Ocampo, C.I.; Orenduff, M.C.; Slentz, C.A.; Johnson, J.L.; Houmard, J.A.; Samsa, G.P.; Kraus, W.E.; et al. Exercise effects on lipids in persons with varying dietary patterns-does diet matter if they exercise? Responses in Studies of a Targeted Risk Reduction Intervention through Defined Exercise I. Am. Hear. J. 2012, 164, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Shaw, K.; Gennat, H.; O’Rourke, P.; Del, M.C. Exercise for overweight or obesity. Cochrane Database Syst. Rev. 2006, D3817. [Google Scholar] [CrossRef] [Green Version]
- Whitton, C.; Rebello, S.A.; Lee, J.; Tai, E.S.; Van Dam, R.M. A healthy Asian a posteriori dietary pattern correlates with a priori dietary patterns and is associated with cardiovascular disease risk factors in a multiethnic Asian population. J. Nutr. 2018, 148, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Zock, P.L.; Kester, A.D.M.; Katan, M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: A meta-analysis of 60 controlled trials. Am. J. Clin. Nutr. 2003, 77, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Lecerf, J.M.; de Lorgeril, M. Dietary cholesterol: From physiology to cardiovascular risk. Br. J. Nutr. 2011, 106, 6–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristics | Values |
|---|---|
| Age (years) | |
| ≤29 | 384 (38.1) |
| 30–34 | 486 (48.2) |
| ≥35 | 138 (13.7) |
| Ethnicity | |
| Han Chinese | 980 (97.2) |
| Others | 28 (2.8) |
| Education | |
| ≤middle school | 21 (2.1) |
| Senior high school | 64 (6.3) |
| College | 205 (20.3) |
| Graduate, | 507 (50.3) |
| Postgraduate or above | 211 (20.9) |
| Household income | |
| <¥10,000 | 81 (8.0) |
| ¥10,000–30,000 | 649 (64.4) |
| >¥30,000 | 278 (27.6) |
| Smoking 3 months preconception * | |
| No | 984 (97.6) |
| Yes | 24 (2.4) |
| Passive smoking 3 months preconception ‡ | |
| No | 892 (88.5) |
| Yes | 116 (11.5) |
| Alcohol drinking 3 months preconception † | |
| No | 915 (90.8) |
| Yes | 93 (9.2) |
| Pre-BMI(kg/m2) | |
| <18.5 | 150 (14.9) |
| 18.5–23.9 | 722 (71.6) |
| 24.0–27.9 | 102 (10.1) |
| ≥28.0 | 34 (3.4) |
| First-degree family history of diabetes | |
| No | 883 (87.6) |
| Yes | 122 (12.1) |
| Not clear | 3 (0.3) |
| Parity | |
| 0 | 806 (80.0) |
| ≥1 | 202 (20.0) |
| Food Groups | Dietary Patterns | ||||
|---|---|---|---|---|---|
| Tubers-Fruit-Vegetables | Beans-Fungi-Algae | Fish-Shrimps | Refined Grains-Red Meat-Organs | Confectionery-Sugared Beverages | |
| Tubers | 0.690 | - | - | - | - |
| Fruits | 0.647 | - | - | - | - |
| Vegetables | 0.516 | - | - | - | - |
| Dairy products ‡ | 0.489 | - | - | - | - |
| Fungi and Mushrooms | - | 0.653 | - | - | - |
| Beans and bean products † | - | 0.645 | - | - | - |
| algae | - | 0.539 | |||
| Shrimps and shellfishes | - | - | 0.648 | - | - |
| Marine fish | - | - | 0.552 | - | - |
| Freshwater fishes | - | - | 0.541 | ||
| Refined grains | - | - | - | 0.728 | - |
| Red meat, animal organs and blood | - | - | - | 0.568 | - |
| Whole grains and pulses Δ | 0.423 | - | - | −0.560 | |
| Confectionery | - | - | - | - | 0.754 |
| Sugared beverages | - | - | - | - | 0.677 |
| Variance explained | 15.580 | 9.098 | 8.581 | 7.877 | 6.822 |
| Cumulative variance explained (%) § | 15.580 | 24.678 | 33.259 | 41.136 | 47.958 |
| Characteristics | Dietary Patterns Scores | ||||
|---|---|---|---|---|---|
| Tubers-Fruit-Vegetables | Beans-Fungi-Algae | Fish-Shrimps | Refined Grains-Red Meat-Organs | Confectionery-Sugared Beverages | |
| Age (years) | |||||
| <25 | 0.15 ± 1.24 | 0.12 ± 1.23 | 0.03 ± 1.05 | 0.04 ± 1.14 | |
| 25–29 | −0.01 ± 0.95 | −0.03 ± 0.95 | −0.01 ± 0.97 | 0.00 ± 0.98 | −0.02 ± 1.00 |
| 30–34 | −0.13 ± 0.94 | −0.07 ± 0.90 | −0.01 ± 0.82 | 0.06 ± 1.03 | 0.02 ± 0.85 |
| ≥35 | −0.17 ± 0.58 | 0.22 ± 1.14 | −0.24 ± 0.76 | −0.42 ± 1.11 | 0.08 ± 0.75 |
| p value | 0.102 | 0.187 | 0.363 | 0.238 | 0.906 |
| Ethnicity | |||||
| Han Chinese | −0.02 ± 0.99 | −0.01 ± 0.98 | 0.00 ± 0.98 | 0.00 ± 0.99 | 0.00 ± 0.97 |
| Others | 0.56 ± 1.16 | 0.48 ± 1.59 | −0.14 ± 1.64 | −0.09 ± 1.20 | 0.10 ± 1.81 |
| p value | 0.007 | 0.021 | 0.514 | 0.661 | 0.633 |
| Education | |||||
| ≤Middle school | −0.13 ± 0.57 | −0.14 ± 0.59 | −0.03 ± 0.90 | 0.17 ± 0.83 | −0.25 ± 0.90 |
| Senior high school | 0.00 ± 0.93 | 0.02 ± 1.60 | 0.09 ± 1.28 | 0.43 ± 1.24 | −0.25 ± 1.39 |
| College | −0.09 ± 0.93 | −0.19 ± 0.88 | −0.12 ± 0.93 | 0.07 ± 0.96 | 0.13 ± 1.05 |
| Graduate, | 0.00 ± 1.11 | 0.03 ± 0.98 | 0.08 ± 1.03 | −0.04 ± 1.00 | 0.03 ± 0.96 |
| ≥Postgraduate | 0.10 ± 0.82 | 0.12 ± 0.94 | −0.11 ± 0.90 | −0.10 ± 0.93 | −0.10 ± 0.90 |
| p value | 0.508 | 0.048 | 0.087 | 0.009 | 0.060 |
| Household income | |||||
| <¥10,000 | 0.01 ± 1.27 | −0.16 ± 0.91 | 0.07 ± 1.05 | 0.11 ± 0.85 | −0.03 ± 1.00 |
| ¥10,000–30,000 | −0.02 ± 0.92 | −0.05 ± 0.96 | −0.03 ± 0.94 | 0.01 ± 1.05 | 0.00 ± 0.94 |
| >¥30,000 | 0.04 ± 1.11 | 0.17 ± 1.11 | 0.05 ± 1.12 | −0.05 ± 0.91 | 0.02 ± 1.15 |
| p value | 0.761 | 0.012 | 0.556 | 0.489 | 0.94 |
| Smoking 3 months preconception * | |||||
| No | 0.00 ± 1.00 | 0.00 ± 0.98 | −0.01 ± 0.97 | −0.01 ± 1.00 | −0.01 ± 0.96 |
| Yes | 0.16 ± 1.15 | 0.09 ± 1.70 | 0.27 ± 1.86 | 0.41 ± 1.11 | 0.30 ± 2.03 |
| p value | 0.472 | 0.695 | 0.223 | 0.065 | 0.172 |
| Passive smoking 3 months preconception ‡ | |||||
| No | 0.02 ± 1.01 | 0.00 ± 1.02 | −0.01 ± 0.99 | −0.01 ± 1.01 | −0.04 ± 0.99 |
| Yes | −0.18 ± 0.94 | −0.01 ± 0.87 | 0.07 ± 1.09 | 0.10 ± 0.92 | 0.28 ± 1.03 |
| p value | 0.060 | 0.952 | 0.471 | 0.295 | 0.004 |
| Alcohol drinking 3 months preconception † | |||||
| No | −0.01 ± 1.00 | 0.02 ± 0.99 | 0.00 ± 0.99 | 0.01 ± 0.98 | −0.03 ± 0.96 |
| Yes | 0.07 ± 1.00 | −0.20 ± 1.13 | −0.02 ± 1.14 | −0.08 ± 1.19 | 0.31 ± 1.33 |
| p value | 0.507 | 0.071 | 0.873 | 0.459 | 0.006 |
| Pre-BMI (kg/m2) | |||||
| <18.5 | 0.18 ± 1.27 | 0.15 ± 1.24 | 0.12 ± 1.23 | 0.03 ± 1.05 | 0.04 ± 1.14 |
| 18.5–23.9 | −0.01 ± 0.95 | −0.03 ± 0.95 | −0.01 ± 0.97 | 0.00 ± 0.98 | −0.02 ± 1.00 |
| 24.0–27.9 | −0.13 ± 0.94 | −0.07 ± 0.90 | −0.01 ± 0.82 | 0.06 ± 1.03 | 0.02 ± 0.85 |
| ≥28.0 | −0.17 ± 0.58 | 0.22 ± 1.14 | −0.24 ± 0.76 | −0.42 ± 1.11 | 0.08 ± 0.75 |
| p value | 0.102 | 0.187 | 0.363 | 0.238 | 0.906 |
| First-degree family history of diabetes | |||||
| No | 0.01 ± 1.00 | 0.00 ± 0.99 | 0.00 ± 1.00 | −0.01 ± 0.98 | −0.01 ± 1.02 |
| Yes | −0.04 ± 1.00 | 0.03 ± 1.10 | 0.02 ± 1.00 | 0.05 ± 1.14 | 0.05 ± 0.88 |
| Not clear | −0.91 ± 0.15 | −0.60 ± 0.32 | 0.34 ± 0.45 | 0.20 ± 0.34 | −0.06 ± 0.25 |
| p value | 0.260 | 0.556 | 0.825 | 0.812 | 0.885 |
| Parity | |||||
| 0 | 0.04 ± 1.04 | 0.02 ± 1.03 | 0.02 ± 1.00 | −0.05 ± 1.01 | 0.02 ± 1.04 |
| 1 | −0.18 ± 0.75 | −0.07 ± 0.86 | −0.10 ± 1.02 | 0.22 ± 0.95 | −0.11 ± 0.82 |
| 2 | −0.68 ± 0.59 | −0.57 ± 0.93 | −0.31 ± 0.37 | 0.18 ± 0.83 | 0.16 ± 0.03 |
| p value | 0.031 | 0.427 | 0.362 | 0.013 | 0.351 |
| Outcomes | Tubers-Fruit-Vegetables | p Value | Beans-Fungi-Algae | p Value | Fish-Shrimps | p Value | Refined Grains-Red Meat-Organs | p Value | Confectionery-Sugared Beverages | p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| β (95%CI) | β (95%CI) | β (95%CI) | β (95%CI) | β (95%CI) | ||||||
| First trimester | ||||||||||
| TC | −0.09 (−0.14, −0.04) | 0.001 | −0.02 (−0.07, 0.03) | 0.481 | 0.09 (0.04, 0.14) | 0.001 | −0.03 (−0.08, 0.02) | 0.238 | 0.03 (−0.03, 0.08) | 0.351 |
| HDL-C | −0.02 (−0.04, −0.01) | <0.001 | −0.01 (−0.02, 0.01) | 0.436 | 0.02 (0.01, 0.04) | <0.001 | −0.01 (−0.02, 0.01) | 0.276 | 0.01 (−0.01,0.02) | 0.209 |
| LDL-C | −0.05 (−0.08, −0.02) | 0.001 | −0.02 (−0.05, 0.01) | 0.170 | 0.04 (0.01, 0.07) | 0.012 | −0.01 (−0.04, 0.02) | 0.617 | 0.01 (−0.02, 0.04) | 0.501 |
| TG | −0.02 (−0.06, 0.03) | 0.551 | 0.03 (−0.02, 0.08) | 0.200 | −0.02 (−0.07, 0.03) | 0.354 | −0.02 (−0.06, 0.03) | 0.539 | −0.02 (−0.07, 0.03) | 0.393 |
| Second trimester | ||||||||||
| TC | −0.15 (−0.22, −0.07) | <0.001 | −0.04 (−0.11, 0.03) | 0.311 | 0.11 (0.04, 0.18) | 0.003 | −0.03 (−0.10, 0.05) | 0.501 | 0.08 (0.01, 0.16) | 0.028 |
| HDL-C | −0.03 (−0.05, −0.01) | 0.001 | −0.01 (−0.03, 0.01) | 0.164 | 0.03 (0.01, 0.05) | 0.001 | −0.01 (−0.03, 0.01) | 0.575 | 0.02 (0.01, 0.04) | 0.015 |
| LDL-C | −0.08 (−0.12, −0.03) | 0.001 | −0.02 (−0.07, 0.02) | 0.259 | 0.07 (0.02, 0.11) | 0.002 | −0.01 (−0.05, 0.04) | 0.740 | 0.05 (0.00, 0.09) | 0.031 |
| TG | −0.03 (−0.11, 0.04) | 0.368 | 0.03 (−0.04, 0.10) | 0.360 | −0.04 (−0.11, 0.03) | 0.292 | 0.03 (−0.05, 0.10) | 0.454 | 0.02 (−0.05, 0.10) | 0.510 |
| Third trimester | ||||||||||
| TC | −0.17 (−0.25, −0.08) | <0.001 | −0.03 (−0.11, 0.05) | 0.476 | 0.14 (0.06, 0.23) | 0.001 | −0.05 (−0.14, 0.04) | 0.242 | 0.12 (0.03, 0.20) | 0.011 |
| HDL-C | −0.05 (−0.07, −0.03) | <0.001 | 0.00 (−0.03, 0.02) | 0.697 | 0.04 (0.02, 0.06) | <0.001 | −0.01 (−0.04, 0.01) | 0.215 | 0.03 (0.01, 0.05) | 0.013 |
| LDL-C | −0.10 (−0.16, −0.05) | <0.001 | −0.01 (−0.07, 0.04) | 0.572 | 0.09 (0.03, 0.14) | 0.001 | −0.04 (−0.09, 0.02) | 0.173 | 0.07 (0.02, 0.12) | 0.012 |
| TG | −0.06 (−0.15, 0.04) | 0.250 | 0.04 (−0.05, 0.13) | 0.428 | −0.05 (−0.14, 0.05) | 0.340 | −0.04 (−0.14, 0.05) | 0.391 | 0.02 (−0.08, 0.11) | 0.703 |
| Outcome | Tubers-Fruit-Vegetables | p Value | Beans-Fungi-Algae | p Value | Fish-Shrimps | p Value | Refined Grains-Red Meat-Organs | p Value | Confectionery-Sugared Beverages | p Value |
|---|---|---|---|---|---|---|---|---|---|---|
| b (95%CI) | b (95%CI) | b (95%CI) | b (95%CI) | b (95%CI) | ||||||
| Model 1 | ||||||||||
| TC | −0.14 (−0.20, −0.08) | <0.001 | −0.03 (−0.08, 0.03) | 0.330 | 0.10 (0.04, 0.15) | 0.001 | −0.02 (−0.07, 0.04) | 0.497 | 0.07 (0.01, 0.13) | 0.025 |
| HDL-C | −0.04 (−0.05, −0.02) | <0.001 | −0.01 (−0.02, 0.01) | 0.353 | 0.03 (0.01, 0.04) | <0.001 | 0.00 (−0.02, 0.01) | 0.565 | 0.02 (0.00, 0.03) | 0.013 |
| LDL-C | −0.08 (−0.11, −0.05) | <0.001 | −0.02 (−0.05, 0.02) | 0.362 | 0.06 (0.03, 0.09) | <0.001 | −0.01 (−0.04, 0.02) | 0.605 | 0.04 (0.01, 0.08) | 0.016 |
| TG | −0.04 (−0.10, 0.03) | 0.257 | 0.02 (−0.05, 0.09) | 0.520 | −0.03 (−0.09, 0.02) | 0.247 | 0.01 (−0.05, 0.06) | 0.803 | 0.01 (−0.05, 0.07) | 0.648 |
| Model 2 | ||||||||||
| TC | −0.14 (−0.19, −0.08) | <0.001 | −0.03 (−0.08, 0.03) | 0.344 | 0.10 (0.04,0.15) | 0.001 | −0.02 (−0.07, 0.04) | 0.504 | 0.06 (0.00, 0.12) | 0.055 |
| HDL-C | −0.04 (−0.05, −0.02) | <0.001 | −0.01 (−0.02, 0.01) | 0.371 | 0.03 (0.01, 0.04) | <0.001 | 0.00 (−0.02, 0.01) | 0.574 | 0.02 (0.00, 0.03) | 0.036 |
| LDL-C | −0.08 (−0.11, −0.04) | <0.001 | −0.02 (−0.05, 0.02) | 0.343 | 0.06 (0.03,0.09) | <0.001 | −0.01 (−0.04, 0.02) | 0.573 | 0.04 (0.00, 0.07) | 0.041 |
| TG | −0.02 (−0.08, 0.03) | 0.419 | 0.04 (−0.03, 0.11) | 0.309 | −0.03 (−0.09, 0.02) | 0.242 | 0.01 (−0.04, 0.07) | 0.676 | 0.02 (−0.04, 0.07) | 0.587 |
| Model 3 | ||||||||||
| TC | −0.12 (−0.18, −0.06) | <0.001 | −0.02 (−0.08, 0.03) | 0.447 | 0.11(0.05, 0.17) | <0.001 | −0.04 (−0.10, 0.03) | 0.251 | 0.06 (0.00, 0.12) | 0.065 |
| HDL-C | −0.03 (−0.05, −0.02) | <0.001 | 0.00 (−0.02, 0.01) | 0.568 | 0.03 (0.02, 0.04) | <0.001 | −0.01(−0.02, 0.01) | 0.241 | 0.01 (0.00, 0.03) | 0.060 |
| LDL-C | −0.07 (−0.11, −0.04) | <0.001 | −0.01(−0.04, 0.02) | 0.489 | 0.07 (0.03, 0.10) | <0.001 | −0.02 (−0.06, 0.01) | 0.211 | 0.03 (0.00, 0.07) | 0.073 |
| TG | −0.03 (−0.09, 0.03) | 0.285 | 0.03(−0.03, 0.10) | 0.331 | −0.04 (−0.09, 0.02) | 0.232 | −0.03 (−0.08, 0.03) | 0.379 | 0.00 (−0.06, 0.06) | 0.964 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Deng, Z.; Wen, L.; Ding, Y.; He, G. Relationships between Maternal Dietary Patterns and Blood Lipid Levels during Pregnancy: A Prospective Cohort Study in Shanghai, China. Int. J. Environ. Res. Public Health 2021, 18, 3701. https://doi.org/10.3390/ijerph18073701
Wang N, Deng Z, Wen L, Ding Y, He G. Relationships between Maternal Dietary Patterns and Blood Lipid Levels during Pregnancy: A Prospective Cohort Study in Shanghai, China. International Journal of Environmental Research and Public Health. 2021; 18(7):3701. https://doi.org/10.3390/ijerph18073701
Chicago/Turabian StyleWang, Na, Zequn Deng, Liming Wen, Yan Ding, and Gengsheng He. 2021. "Relationships between Maternal Dietary Patterns and Blood Lipid Levels during Pregnancy: A Prospective Cohort Study in Shanghai, China" International Journal of Environmental Research and Public Health 18, no. 7: 3701. https://doi.org/10.3390/ijerph18073701