Advancement in Benthic Microbial Fuel Cells toward Sustainable Bioremediation and Renewable Energy Production
Abstract
1. Introduction
2. Configuration of Benthic Microbial Fuel Cell
2.1. Anode Chamber
2.2. Cathode Chamber
3. Bioremediation Mechanisms of BTX through BMFCs
4. Operating Factors
4.1. Influence of pH on BMFCs’ Performance
4.2. Effect of Temperature on BMFCs Performance
4.3. Internal Resistance
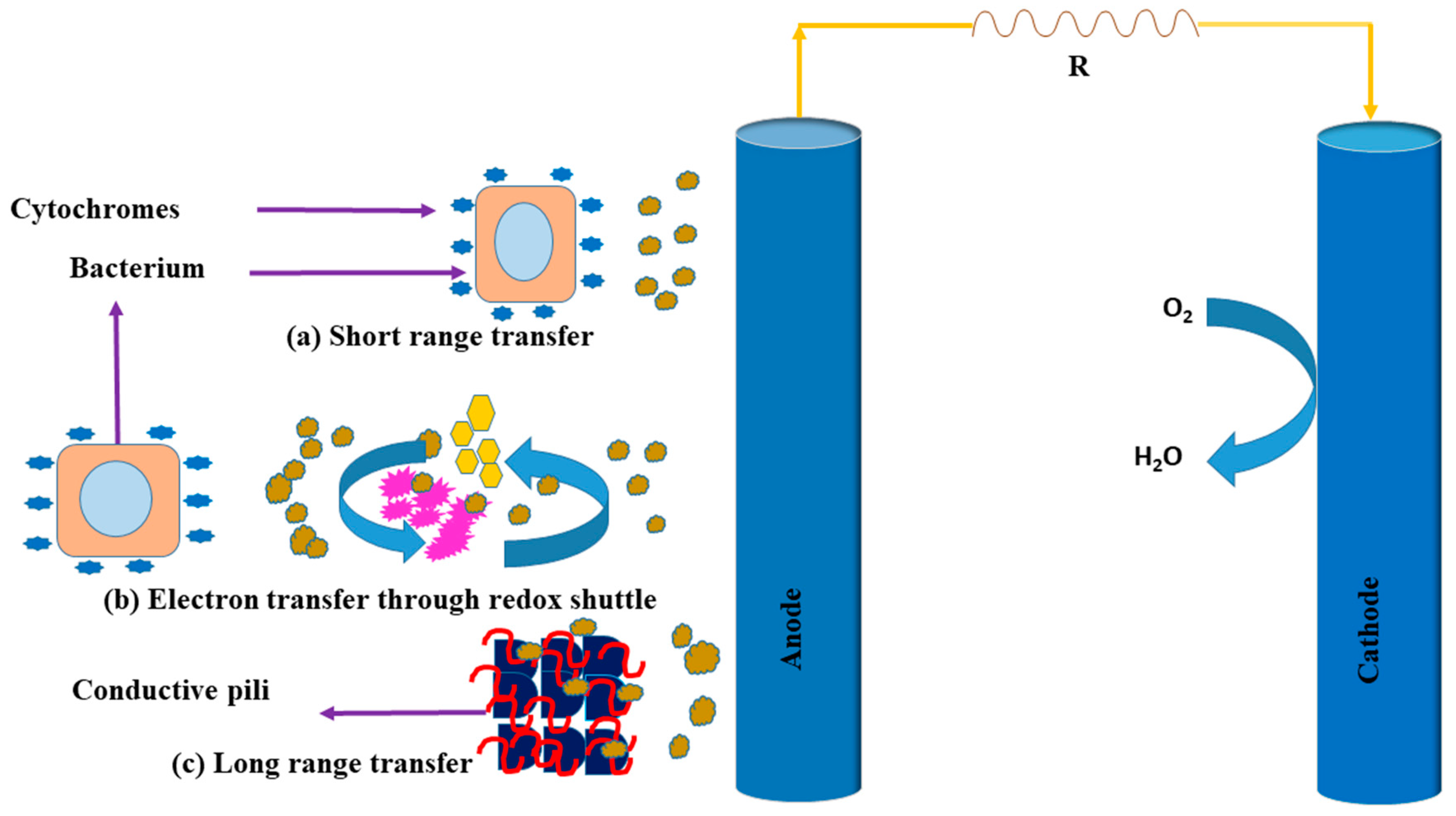
5. Bio-Electron Mechanism Pathways
5.1. Electron Shuttling to Electrodes
5.2. Short-Range Electron Conduction via Cytochromes
5.3. Electron Conduction through Conductive Pili
6. BMFCs Employment
6.1. Treatment of Wastewater
6.2. Bioenergy
6.3. Biosensors
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asono, T.; Cotruvo, J.A. Groundwater recharge with reclaimed municipal waste water: Health and regulatory considerations. Water Res. 2004, 38, 1941–1951. [Google Scholar] [CrossRef]
- Tengrui, L.; Al-Harbawi, A.F.; Bo, L.M.; Jun, Z.; Long, X.Y. Characteristics of nitrogen removal from old landfill leachate by sequencing batch biofilm reactor. Am. J. Appl. Sci. 2007, 4, 211–214. [Google Scholar] [CrossRef]
- Norton-Brandão, D.; Scherrenberg, S.M.; van Lier, J.B. Reclamation of used urban waters for irrigation purposes—A review of treatment technologies. J. Environ. Manag. 2013, 122, 85–98. [Google Scholar] [CrossRef]
- Adelaja, O.; Keshavarz, T.; Kyazze, G. Treatment of phenanthrene and benzene using microbial fuel cells operated continuously for possible in situ and ex situ applications. Int. Biodeterior. Biodegrad. 2017, 116, 91–103. [Google Scholar] [CrossRef]
- Lamichhane, S.; Krishna, K.C.B.; Sarukkalige, R. Polycyclic aromatic hydrocarbons (PAHs) removal by sorption: A review. Chemosphere 2016, 148, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Amini, H.; Schindler, C.; Hosseini, V.; Yunesian, M.; Künzli, N. Land use regression models for Alkylbenzenes in a middle eastern megacity: Tehran study of exposure prediction for environmental Health Research (Tehran SEPEHR). Environ. Sci. Technol. 2017, 51, 8481–8490. [Google Scholar] [CrossRef]
- Kuranchie, F.A.; Angnunavuri, P.N.; Attiogbe, F.; Nerquaye-Tetteh, E.N. Occupational exposure of benzene, toluene, ethylbenzene and xylene (BTEX) to pump attendants in Ghana: Implications for policy guidance. Cogent Environ. Sci. 2019, 5, 1603418. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Rafatullah, M.; Ismail, N.; Syakir, M.I. The behaviour of membrane less sediment microbial fuel cell in the terms of bioremediation and power generation. Malays. J. Microbiol. 2018, 14, 108–112. [Google Scholar] [CrossRef]
- Kugarajah, V.; Dharmalingam, S. Effect of silver incorporated sulphonated poly ether ether ketone membranes on microbial fuel cell performance and microbial community analysis. Chem. Eng. J. 2021, 415, 12896. [Google Scholar] [CrossRef]
- Girguis, P.R.; Nielsen, M.; Reimers, C. Fundamentals of benthic microbial fuel cells: Theory, development and application. In Bioelectrochemical Systems, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Yadav, R.K.; Chiranjeevi, P.; Sukarampal; Patil, S.A. Integrated drip hydroponics-microbial fuel cell system for wastewater treatment and resource recovery. Bioresour. Technol. Rep. 2020, 9, 100392. [Google Scholar] [CrossRef]
- Liu, B.; Weinstein, A.; Kolln, M.; Garrett, C.; Wang, L.; Bagtzoglou, A.; Karra, U.; Li, Y.; Li, B. Distributed multiple-anodes benthic microbial fuel cell as reliable power source for subsea sensors. J. Power Sources 2015, 286, 210–216. [Google Scholar] [CrossRef]
- Umar, M.F.; Abbas, S.Z.; Mohamad Ibrahim, M.N.; Ismail, N.; Rafatullah, M. Insights into advancements and electrons transfer mechanisms of electrogens in benthic microbial fuel cells. Membranes 2020, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.Z.; Rafatullah, M. Recent advances in soil microbial fuel cells for soil contaminants remediation. Chemosphere 2021, 272. [Google Scholar] [CrossRef]
- Potter, M.C. Electrical effects accompanying the decomposition of organic compounds. Proc. R. Soc. Lond. Ser. Contain. Pap. Biol. Character 1911, 84, 260–276. [Google Scholar] [CrossRef]
- Logan, B.E. Microbial Fuel Cells; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Abbas, S.Z.; Rafatullah, M.; Ismail, N.; Shakoori, F.R. Electrochemistry and microbiology of microbial fuel cells treating marine sediments polluted with heavy metals. RSC Adv. 2018, 8, 8800–8813. [Google Scholar] [CrossRef]
- Kim, B.H.; Ikeda, T.; Park, H.S.; Kim, H.J.; Hyun, M.S.; Kano, K.; Takagi, K.; Tatsumi, H. Electrochemical activity of an Fe (III)-reducing bacterium, Shewanella putrefaciens IR-1, in the presence of alternative electron acceptors. Biotechnol. Tech. 1999, 13, 475–478. [Google Scholar] [CrossRef]
- Reimers, C.E.; Tender, L.M.; Fertig, S.; Wang, W. Harvesting energy from the marine sediment−water interface. Environ. Sci. Technol. 2001, 35, 192–195. [Google Scholar] [CrossRef]
- Martins, G.; Peixoto, L.; Ribeirov, D.C.; Parpot, P.; Brito, A.G.; Nogueira, R. Towards implementation of a benthic microbial fuel cell in lake Furnas (Azores): Phylogenetic affiliation and electrochemical activity of sediment bacteria. Bioelectrochemistry 2010, 78, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Tavakolian, M.; Taleghani, H.G.; Khorshidian, M. New design of benthic microbial fuel cell for bioelectricity generation: Comparative study. Int. J. Hydrog. Energy 2020, 45, 23533–23542. [Google Scholar] [CrossRef]
- Pushkar, P.; Mungray, A.K. Exploring the use of 3 dimensional low-cost sugar-urea carbon foam electrode in the benthic microbial fuel cell. Renew. Energy 2020, 147, 2032–2042. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Rafatullah, M.; Khan, M.A.; Siddiqui, M.R. Bioremediation and Electricity generation by using open and closed sediment microbial fuel cells. Front. Microbiol. 2019, 9, 3348. [Google Scholar] [CrossRef] [PubMed]
- Joiner, K.L.; Tukeman, G.L.; Obraztsova, A.Y.; Arias-Thode, Y.M. Impact of sediment parameters in the prediction of benthic microbial fuel cell performance. RSC Adv. 2020, 10, 26220–26228. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, F.; Li, X.; Lu, W. Contribution of influent rivers affected by different types of pollution to the changes of benthic microbial community structure in a large lake. Ecotoxicol. Environ. Saf. 2020, 198, 110657. [Google Scholar] [CrossRef] [PubMed]
- Algar, C.K.; Howard, A.; Ward, C.; Wange, G. Sediment microbial fuel cells as a barrier to sulfide accumulation and their potential for sediment remediation beneath aquaculture pens. Sci. Rep. 2020, 10, 13087. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wangv, W.; Caov, X.; Wang, Y.; Zou, L.; Gev, X.; Zhao, Y.; Si, Z.; Wang, Y. Chlorella vulgaris on the cathode promoted the performance of sediment microbial fuel cells for electrogenesis and pollutant removal. Sci. Total Environ. 2020, 728, 138011. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.; Chadwick, B.; Kagan, J.; Thacher, R.; Wotawa-Bergen, A.; Richter, K. Scale up considerations for sediment microbial fuel cells. RSC Adv. 2013, 3, 15947–15954. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Yang, Y.; Xu, M. Diffusion and filamentous bacteria jointly govern the spatiotemporal process of sulfide removal in sediment microbial fuel cells. Chem. Eng. J. 2021, 405, 126680. [Google Scholar] [CrossRef]
- Srivastava, P.; Abbassi, R.; Yadav, A.K.; Garaniya, V.; Khan, F. Microbial fuel cell–integrated wastewater treatment systems. In Integrated Microbial Fuel Cells for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 29–46. [Google Scholar] [CrossRef]
- Liu, X.; Yin, W.; Liu, X.; Zhao, X. Enhanced Cr reduction and bioelectricity production in microbial fuel cells using polypyrrole-coated MnO2 on carbon cloth. Environ. Chem. Lett. 2020, 18, 517–525. [Google Scholar] [CrossRef]
- Imran, M.; Prakash, O.; Pushkar, P.; Mungray, A.; Kailasa, S.K.; Chongdar, S.; Mungray, A.K. Performance enhancement of benthic microbial fuel cell by cerium coated electrodes. Electrochim. Acta 2019, 295, 58–66. [Google Scholar] [CrossRef]
- Prakash, O.; Pushkar, P.; Mungray, A.K.; Mungray, A.; Kailasa, S.K. Effect of geometrical position of a multi-anode system in power output and nutritional variation in benthic microbial fuel cells. J. Environ. Chem. Eng. 2018, 6, 1558–1568. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Ying, M.; Fu, Y.B.; Chen, W. Improving electrochemical performance of carbon felt anode by modifying with akaganeite in marine benthic microbial fuel cells. Fuel Cells 2019, 19, 190–199. [Google Scholar] [CrossRef]
- Capitaine, A.; Pillonnet, G.; Allard, B. Strategies of maximum power point tracking for sub-mw benthic microbial fuel cells. J. Low Psower Electron. 2019, 15, 351–360. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, D.; You, H.; Li, W.; Jiang, K. Benthic microbial fuel cell equipped with a photocatalytic Cu2O-coated cathode. J. Nanopart. Res. 2018, 21, 3. [Google Scholar] [CrossRef]
- Kaur, A.; Boghani, H.C.; Milner, E.M.; Kimber, R.L.; Michie, I.S.; Daalmans, R.; Dinsdale, R.M.; Guwy, A.J.; Head, I.M.; Lloyd, J.R.; et al. Bioelectrochemical treatment and recovery of copper from distillery waste effluents using power and voltage control strategies. J. Hazard. Mater. 2019, 371, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Ji, M.; Liang, B.; Zhao, Y.; Zhai, S.; Ma, Z.; Yang, Z. Bioelectrochemical degradation of monoaromatic compounds: Current advances and challenges. J. Hazard. Mater. 2020, 398, 122892. [Google Scholar] [CrossRef]
- Zhang, T.; Gannon, S.M.; Nevin, K.P.; Franks, A.E.; Lovley, D.R. Stimulating the anaerobic degradation of aromatic hydrocarbons in contaminated sediments by providing an electrode as the electron acceptor. Environ. Microbiol. 2010, 12, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.Z.; Rafatullah, M.; Ismail, N.; Syakir, M.I. A review on sediment microbial fuel cells as a new source of sustainable energy and heavy metal remediation: Mechanisms and future prospective. Int. J. Energy Res. 2017, 41, 1242–1264. [Google Scholar] [CrossRef]
- Wei, M.; Harnisch, F.; Vogt, C.; Ahlheim, J.; Neu, T.R.; Richnow, H.H. Harvesting electricity from benzene and ammonium-contaminated groundwater using a microbial fuel cell with an aerated cathode. RSC Adv. 2015, 5, 5321–5330. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Rafatullah, M.; Ismail, N.; Nastro, R.A. Enhanced bioremediation of toxic metals and harvesting electricity through sediment microbial fuel cell. Int. J. Energy Res. 2017, 41, 2345–2355. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Khatoon, A.; Mohd Setapar, S.H.; Umar, K.; Parveen, T.; Mohamad Ibrahim, M.N.; Ahmad, A.; Rafatullah, M. Outlook on the role of microbial fuel cells in remediation of environmental pollutants with electricity generation. Catalysts 2020, 10, 819. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Mohamad Ibrahim, M.N.; Rafatullah, M.; Chua, Y.S.; Ahmad, A.; Umar, K. Recent advances in anodes for microbial fuel cells: An overview. Materials 2020, 13, 2078. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, L.; Parpot, P.; Martins, G. Assessment of electron transfer mechanisms during a long-term sediment microbial fuel cell operation. Energies 2019, 12, 481. [Google Scholar] [CrossRef]
- Zain, S.M.; Ching, N.L.; Jusoh, S.; Yunus, S.Y. Different types of microbial fuel cell (MFC) systems for simultaneous electricity generation and pollutant removal. J. Teknol. 2015, 74, 13–19. [Google Scholar] [CrossRef][Green Version]
- Marashi, S.K.F.; Kariminia, H.-R. Performance of a single chamber microbial fuel cell at different organic loads and pH values using purified terephthalic acid wastewater. J. Environ. Health Sci. Eng. 2015, 13, 27. [Google Scholar] [CrossRef]
- Tender, L.M.; Gray, S.A.; Groveman, E.; Lowy, D.A.; Kauffman, P.; Melhado, J.; Tyce, R.C.; Flynn, D.; Petrecca, R.; Dobarro, J. The first demonstration of a microbial fuel cell as a viable power supply: Powering a meteorological buoy. J. Power Sources 2008, 179, 571–575. [Google Scholar] [CrossRef]
- Guo, F.; Shi, Z.; Yang, K.; Wu, Y.; Liu, H. Enhancing the power performance of sediment microbial fuel cells by novel strategies: Overlying water flow and hydraulic-driven cathode rotating. Sci. Total Environ. 2019, 678, 533–542. [Google Scholar] [CrossRef]
- Mahmoud, M.; El-Khatib, K.M. Three-dimensional graphitic mesoporous carbon-doped carbon felt bioanodes enables high electric current production in microbial fuel cells. Int. J. Hydrog. Energy 2020, 56, 32413–32422. [Google Scholar] [CrossRef]
- Espinoza-Tofalos, A.; Daghio, M.; Palma, E.; Aulenta, F.; Franzetti, A. Structure and functions of hydrocarbon-degrading microbial communities in bioelectrochemical systems. Water 2020, 12, 343. [Google Scholar] [CrossRef]
- Khan, N.; Anwer, A.H.; Ahmad, A.; Sabir, S.; Khan, M.Z. Investigating microbial fuel cell aided bio-remediation of mixed phenolic contaminants under oxic and anoxic environments. Biochem. Eng. J. 2020, 155, 107485. [Google Scholar] [CrossRef]
- Singh, H.M.; Pathak, A.K.; Chopra, K.; Tyagi, V.V.; Anand, S.; Kothari, R. Microbial fuel cells: A sustainable solution for bioelectricity generation and wastewater treatment. Biofuels 2019, 10, 11–31. [Google Scholar] [CrossRef]
- Babauta, J.T.; Kerber, M.; Hsu, L.; Phipps, A.; Chadwick, D.B.; Arias-Thode, Y.M. Scaling up benthic microbial fuel cells using flyback converters. J. Power Sources 2018, 395, 98–105. [Google Scholar] [CrossRef]
- Nawaz, A.; Hafeez, A.; Abbas, S.Z.; Haq, I.; Mukhtar, H.; Rafatullah, M. A state of the art review on electron transfer mechanisms, characteristics, applications and recent advancements in microbial fuel cells technology. Green Chem. Lett. Rev. 2020, 365–381. [Google Scholar] [CrossRef]
- Titov, D.V.; Cracan, V.; Goodman, R.P.; Peng, J.; Grabarek, Z.; Mootha, V.K. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science 2016, 352, 231–235. [Google Scholar] [CrossRef]
- Wrighton, K.C.; Thrash, J.C.; Melnyk, R.A.; Bigi, J.P.; Byrne-Bailey, K.G.; Remis, J.P.; Schichnes, D.; Auer, M.; Chang, C.J.; Coates, J.D. Evidence for direct electron transfer by a gram-positive bacterium isolated from a microbial fuel cell. Appl. Environ. Microbiol. 2011, 77, 7633. [Google Scholar] [CrossRef]
- Mathis, B.J.; Marshall, C.W.; Milliken, C.E.; Makkar, R.S.; Creager, S.E.; May, H.D. Electricity generation by thermophilic microorganisms from marine sediment. Appl. Microbiol. Biotechnol. 2008, 78, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, H.; Yu, R.; Cao, X.; Fang, Z.; Li, X. New process for copper migration by bioelectricity generation in soil microbial fuel cells. Environ. Sci. Pollut. Res. 2016, 23, 13147–13154. [Google Scholar] [CrossRef]
- Bond, D.R.; Holmes, D.E.; Tender, L.M.; Lovley, D.R. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 2002, 295, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Jangir, Y.; El-Naggar, M.Y. Disentangling the roles of free and cytochrome-bound flavins in extracellular electron transport from Shewanella oneidensis MR-1. Electrochim. Acta 2016, 198, 49–55. [Google Scholar] [CrossRef]
- Xu, Y.-S.; Zheng, T.; Yong, X.-Y.; Zhai, D.-D.; Si, R.-W.; Li, B.; Yu, Y.-Y.; Yong, Y.-C. Trace heavy metal ions promoted extracellular electron transfer and power generation by Shewanella in microbial fuel cells. Bioresour. Technol. 2016, 211, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, D.V.P.; Jacobs, D.; Gregoryv, K.; Huang, J.; Hu, Y.; Vidic, R.; Yun, M. Changes in carbon electrode morphology affect microbial fuel cell performance with Shewanella oneidensis MR-1. Energies 2015, 8, 1817–1829. [Google Scholar] [CrossRef]
- Pirbadian, S.; Barchinger, S.E.; Leung, K.M.; Byun, H.S.; Jangir, Y.; Bouhenni, R.A.; Reed, S.B.; Romine, M.F.; Saffarini, D.A.; Shi, L.; et al. Shewanella oneidensis MR-1 nanowires are outer membrane and periplasmic extensions of the extracellular electron transport components. Proc. Natl. Acad. Sci. USA 2014, 111, 12883. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Jiang, H.; Cai, H.; Zhou, Y.; Krumholz, L.R. Complex interactions between the macrophyte Acorus calamus and microbial fuel cells during pyrene and benzo a pyrene degradation in sediments. Sci. Rep. 2015, 5, 10709. [Google Scholar] [CrossRef] [PubMed]
- Busalmen, J.P.; Esteve-Núñez, A.; Berná, A.; Feliu, J.M. C-type cytochromes wire electricity-producing bacteria to electrodes. Angew. Chem. Int. Ed. 2008, 47, 4874–4877. [Google Scholar] [CrossRef] [PubMed]
- Estevez-Canales, M.; Kuzume, A.; Borjas, Z.; Füeg, M.; Lovley, D.; Wandlowski, T.; Esteve-Núñez, A. A severe reduction in the cytochrome C content of Geobacter sulfurreducens eliminates its capacity for extracellular electron transfer. Environ. Microbiol. Rep. 2015, 7, 219–226. [Google Scholar] [CrossRef]
- Kokhan, O.; Ponomarenko, N.S.; Pokkuluri, P.R.; Schiffer, M.; Mulfort, K.L.; Tiede, D.M. Bidirectional photoinduced electron transfer in Ruthenium(II)-Tris-bipyridyl-Modified PpcA.; a multi-heme c-type cytochrome from Geobacter sulfurreducens. J. Phys. Chem. B 2015, 119, 7612–7624. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.C.; Silva, M.A.; Morgado, L.; Dantas, J.M.; Salgueiro, C.A. Diving into the redox properties of Geobacter sulfurreducens cytochromes: A model for extracellular electron transfer. Dalton Trans. 2015, 44, 9335–9344. [Google Scholar] [CrossRef]
- Nair, R.R.; Silveira, C.M.; Diniz, M.S.; Almeida, M.G.; Moura, J.J.G.; Rivas, M.G. Changes in metabolic pathways of Desulfovibrio alaskensis G20 cells induced by molybdate excess. J. Biol. Inorg. Chem. 2015, 20, 311–322. [Google Scholar] [CrossRef]
- Verma, J.; Kumar, D.; Singh, N.; Katti, S.S.; Shah, Y.T. Electricigens and microbial fuel cells for bioremediation and bioenergy production: A review. Environ. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Hernández-Eligio, A.; Andrade, Á.; Soto, L.; Morett, E.; Juárez, K. The unphosphorylated form of the PilR two-component system regulates pilA gene expression in Geobacter sulfurreducens. Environ. Sci. Pollut. Res. 2017, 24, 25693–25701. [Google Scholar] [CrossRef]
- White, G.F.; Edwards, M.J.; Gomez-Perez, L.; Richardson, D.J.; Butt, J.N.; Clarke, T.A. Chapter three—Mechanisms of bacterial extracellular electron exchange. In Advances in Microbial Physiology; Poole, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 87–138. [Google Scholar]
- Lovley, D.R. Powering microbes with electricity: Direct electron transfer from electrodes to microbes. Environ. Microbiol. Rep. 2011, 3, 27–35. [Google Scholar] [CrossRef]
- El-Naggar, M.Y.; Wanger, G.; Leung, K.M.; Yuzvinsky, T.D.; Southam, G.; Yang, J.; Lau, W.M.; Nealson, K.H.; Gorby, Y.A. Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. Proc. Natl. Acad. Sci. USA 2010, 107, 18127. [Google Scholar] [CrossRef] [PubMed]
- Summers, Z.M.; Fogarty, H.E.; Leang, C.; Franks, A.E.; Malvankar, N.S.; Lovley, D.R. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 2010, 330, 1413. [Google Scholar] [CrossRef]
- He, Z.; Minteer, S.D.; Angenent, L.T. Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environ. Sci. Technol. 2005, 39, 5262–5267. [Google Scholar] [CrossRef]
- Habermann, W.; Pommer, E.H. Biological fuel cells with sulphide storage capacity. Appl. Microbiol. Biotechnol. 1991, 35, 128–133. [Google Scholar] [CrossRef]
- Bier, R.L.; Wernegreen, J.J.; Vilgalys, R.J.; Ellis, J.C.; Bernhardt, E.S. Subsidized or stressed? Shifts in freshwater benthic microbial metagenomics along a gradient of alkaline coal mine drainage. Limnol. Oceanogr. 2020, 65, 277–292. [Google Scholar] [CrossRef]
- González-Gamboa, N.; Domínguez-Benetton, X.; Pacheco-Catalán, D.; Kumar-Kamaraj, S.; Valdés-Lozano, D.; Domínguez-Maldonadov, J.; Alzate-Gaviria, L. Effect of operating parameters on the performance evaluation of benthic microbial fuel cells using sediments from the Bay of Campeche, Mexico. Sustainability 2018, 10, 2446. [Google Scholar] [CrossRef]
- Corbella, C.; Puigagut, J. Improving domestic wastewater treatment efficiency with constructed wetland microbial fuel cells: Influence of anode material and external resistance. Sci. Total Environ. 2018, 631–632, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Lai, C.-Y.; Ye, J.-W.; Lin, C.-W. Increasing removal of benzene from groundwater using stacked tubular air-cathode microbial fuel cells. J. Clean. Prod. 2018, 194, 78–84. [Google Scholar] [CrossRef]
- Wu, C.H.; Lai, C.Y.; Lin, C.W.; Kao, M.H. Generation of power by microbial fuel cell with ferricyanide in biodegradation of benzene. Clean Soil Air Water 2013, 41, 390–395. [Google Scholar] [CrossRef]
- Chang, S.-H.; Wu, C.-H.; Wang, R.-C.; Lin, C.-W. Electricity production and benzene removal from groundwater using low-cost mini tubular microbial fuel cells in a monitoring well. J. Environ. Manag. 2017, 193, 551–557. [Google Scholar] [CrossRef]
- Rakoczy, J.; Feisthauer, S.; Wasmund, K.; Bombachv, P.; Neu, T.R.; Vogt, C.; Richnow, H.H. Benzene and sulfide removal from groundwater treated in a microbial fuel cell. Biotechnol. Bioeng. 2013, 110, 3104–3113. [Google Scholar] [CrossRef] [PubMed]
- Safwat, S.M.; Rozaik, E.; Abdel-Halim, H. A comparative study on treatment of wastewaters with various biodegradability and various pH values using single-chamber microbial fuel cells. Water Environ. J. 2019, 33, 409–417. [Google Scholar] [CrossRef]
- Wu, C.-H.; Yet-Pole, I.; Chiu, Y.-H.; Lin, C.-W. Enhancement of power generation by toluene biodegradation in a microbial fuel cell in the presence of pyocyanin. J. Taiwan Inst. Chem. Eng. 2014, 45, 2319–2324. [Google Scholar] [CrossRef]
- Zhang, S.; You, J.; An, N.; Zhaov, J.; Wangv, L.; Cheng, Z.; Ye, J.; Chenv, D.; Chen, J. Gaseous toluene powered microbial fuel cell: Performance.; microbial community.; and electron transfer pathway. Chem. Eng. J. 2018, 351, 515–522. [Google Scholar] [CrossRef]
- Lin, C.-W.; Chen, J.; Zhao, J.; Liu, S.-H.; Lin, L.-C. Enhancement of power generation with concomitant removal of toluene from artificial groundwater using a mini microbial fuel cell with a packed-composite anode. J. Hazard. Mater. 2020, 387, 121717. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Wu, C.-H.; Chiu, Y.-H.; Tsa, S.-L. Effects of different mediators on electricity generation and microbial structure of a toluene powered microbial fuel cell. Fuel 2014, 125, 30–35. [Google Scholar] [CrossRef]
- Palma, E.; Daghio, M.; Tofalos, A.E.; Franzetti, A.; Viggi, C.C.; Fazi, S.; Papini, M.P.; Aulenta, F. Anaerobic electrogenic oxidation of toluene in a continuous-flow bioelectrochemical reactor: Process performance, microbial community analysis and biodegradation pathways. Environ. Sci. Water Res. Technol. 2018, 4, 2136–2145. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Zhang, J.; Ye, D.; Zhu, X.; Liao, Q. A microbial fuel cell capable of converting gaseous toluene to electricity. Biochem. Eng. J. 2013, 75, 39–46. [Google Scholar] [CrossRef]
- Liu, S.-H.; Chang, C.-M.; Lin, C.-W. Modifying proton exchange membrane in a microbial fuel cell by adding clay mineral to improve electricity generation without reducing removal of toluene. Biochem. Eng. J. 2018, 134, 101–107. [Google Scholar] [CrossRef]
- You, J.; Deng, Y.; Chen, H.; Ye, J.; Zhang, S.; Zhao, J. Enhancement of gaseous o-xylene degradation in a microbial fuel cell by adding Shewanella oneidensis MR-1. Chemosphere 2020, 252, 126571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; You, J.; Chen, H.; Ye, J.; Cheng, Z.; Chen, J. Gaseous toluene, ethylbenzene, and xylene mixture removal in a microbial fuel cell: Performance, biofilm characteristics, and mechanisms. Chem. Eng. J. 2020, 386, 123916. [Google Scholar] [CrossRef]
- Sun, Z.; Ding, C.; Xi, J.; Lu, L.; Yang, B. Enhancing biofilm formation in biofilters for benzene.; toluene.; ethylbenzene.; and xylene removal by modifying the packing material surface. Bioresour. Technol. 2020, 296, 122335. [Google Scholar] [CrossRef]
- Li, L.; Goel, R. Biodegradation of naphthalene, benzene, toluene, ethyl benzene, and xylene in batch and membrane bioreactors. Environ. Eng. Sci. 2012, 29, 42–51. [Google Scholar] [CrossRef]
- Daghio, M.; Tofalos, A.E.; Leoni, B.; Cristiani, P.; Papacchini, M.; Jalilnejad, E.; Bestetti, G.; Franzetti, A. Bioelectrochemical BTEX removal at different voltages: Assessment of the degradation and characterization of the microbial communities. J. Hazard. Mater. 2018, 341, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Prakash, O.; Mungray, A.; Chongdar, S.; Kailasa, S.K.; Mungray, A.K. Performance of polypyrrole coated metal oxide composite electrodes for benthic microbial fuel cell (BMFC). J. Environ. Chem. Eng. 2020, 8, 102757. [Google Scholar] [CrossRef]
- Prakash, O.; Mungray, A.; Kailasa, S.K.; Chongdar, S.; Mungray, A.K. Comparison of different electrode materials and modification for power enhancement in benthic microbial fuel cells (BMFCs). Process. Saf. Environ. Prot. 2018, 117, 11–21. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Wang, Y.-Z.; Fang, Z.; Shi, Y.-T.; Cheng, Q.-W.; Chen, Y.-X.; Shi, W.; Yong, Y.-C. Single cell electron collectors for highly efficient wiring-up electronic abiotic/biotic interfaces. Nat. Commun. 2020, 11, 4087. [Google Scholar] [CrossRef]
- Shantaram, A.; Beyenal, H.; Veluchamy, R.R.A.; Lewandowski, Z. Wireless sensors powered by microbial fuel cells. Environ. Sci. Technol. 2005, 39, 5037–5042. [Google Scholar] [CrossRef] [PubMed]
- Karra, U.; Muto, E.; Umaz, R.; Kölln, M.; Santoro, C.; Wang, L.; Li, B. Performance evaluation of activated carbon-based electrodes with novel power management system for long-term benthic microbial fuel cells. Int. J. Hydrog. Energy 2014, 39, 21847–21856. [Google Scholar] [CrossRef]




| S. N. | Electrode Materials | Target Pollutants | Inoculum Medium | Removal Efficiency | Operation Time (days) | pH | Temperature (°C) | Power Density (mW/m2) | References | |
|---|---|---|---|---|---|---|---|---|---|---|
| Anode | Cathode | |||||||||
| 1. | Carbon felt | Carbon felt | Benzene | Wastewater | 81.6% | 4 | 7.0 | 30 | 12.7 | [82] |
| 2. | Carbon felt | Carbon felt | Benzene | Wastewater | 80% | - | - | 28–30 | 0.0205 | [83] |
| 3. | Carbon brush | Carbon brush | Benzene | Wastewater | 95% | 195 | - | - | 38 | [84] |
| 4. | Carbon cloth | Carbon cloth | Benzene | Wastewater | 80% | 770 | 6.9–7.0 | 12–16 | - | [85] |
| 5. | Carbon felt | Carbon felt | Benzene | Wastewater | 80% | 160 | 7.5 ± 0.3 | 10–12 | 316 | [45] |
| 6. | Carbon brush | Carbon felt | Benzene | Minimal medium | 97.10% | 60 | - | 40 | 1.06 | [45] |
| 7. | Carbon rod | Carbon rod | Benzene | Wastewater | 90% | 120 | - | - | 32 | [86] |
| 8. | Carbon cloth | Carbon cloth | Toluene | xenobiotics-contaminated wastewater | 96% | 5 | 7.0 | 28 | 4.69 | [87] |
| 9. | Carbon felt | Carbon felt | Toluene | Wastewater sludge | 88% | 10 | 7.0 | 30 | 18.3 | [88] |
| 10. | Carbon brush | Carbon brush | Toluene | Groundwater | 76% | 45 | - | 30 | 103 | [89] |
| 11. | Carbon plate | Carbon plate | Toluene | Wastewater | - | 3 | 6.0 | 30 | 2.6 | [90] |
| 12. | Carbon felt | Carbon felt | Toluene | - | 88 | - | 7.0 | 30 | 18.3 | [88] |
| 13. | Carbon paper | Carbon paper | Toluene | Pyocyanin Wastewater | 96 | 5 | 7.0 | 80 | 21.76 | [75] |
| 14. | Carbon sheet | Carbon sheet | Toluene | Groundwater | 67.2 ± 5.7% | 165 | 7.0 | 20 ± 20.5 | 0.001 | [91] |
| 15. | Carbon cloth | Carbon cloth | Toluene | Groundwater | 91.2 ± 2.4% | - | - | - | 6.19 ± 0.45 | [92] |
| 16. | Carbon rod | Carbon rod | Toluene | Coke slurry mixture | 99% | - | - | 25.14 | [93] | |
| 17. | Carbon felt | Carbon felt | Xylene | Volatile organic compounds | 35–76% | 36 | - | 30 ± 1 °C | 92.5 | [94] |
| 18. | Carbon paper | Carbon paper | Xylene | Wastewater | 60.3% | - | - | - | - | [95] |
| 19. | Reticulated carbon paper | Reticulated carbon paper | Xylene | Wastewater | 61% | - | - | - | - | [96] |
| 20. | Carbon felt | Carbon felt | Xylene | Wastewater | 90% | - | - | - | - | [97] |
| 21. | Graphite plates | Graphite plates | Xylene | Wastewater | 7 ± 4 mg/L | 0.34 ± 0.09 | - | - | 220 mA/m2 | [98] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umar, M.F.; Rafatullah, M.; Abbas, S.Z.; Mohamad Ibrahim, M.N.; Ismail, N. Advancement in Benthic Microbial Fuel Cells toward Sustainable Bioremediation and Renewable Energy Production. Int. J. Environ. Res. Public Health 2021, 18, 3811. https://doi.org/10.3390/ijerph18073811
Umar MF, Rafatullah M, Abbas SZ, Mohamad Ibrahim MN, Ismail N. Advancement in Benthic Microbial Fuel Cells toward Sustainable Bioremediation and Renewable Energy Production. International Journal of Environmental Research and Public Health. 2021; 18(7):3811. https://doi.org/10.3390/ijerph18073811
Chicago/Turabian StyleUmar, Mohammad Faisal, Mohd Rafatullah, Syed Zaghum Abbas, Mohamad Nasir Mohamad Ibrahim, and Norli Ismail. 2021. "Advancement in Benthic Microbial Fuel Cells toward Sustainable Bioremediation and Renewable Energy Production" International Journal of Environmental Research and Public Health 18, no. 7: 3811. https://doi.org/10.3390/ijerph18073811
APA StyleUmar, M. F., Rafatullah, M., Abbas, S. Z., Mohamad Ibrahim, M. N., & Ismail, N. (2021). Advancement in Benthic Microbial Fuel Cells toward Sustainable Bioremediation and Renewable Energy Production. International Journal of Environmental Research and Public Health, 18(7), 3811. https://doi.org/10.3390/ijerph18073811









