Metastatic Neuroblastoma Presenting as a Submandibular Mass with Mandibular Bone Involvement in a Three-Year-Old Child
Abstract
1. Introduction
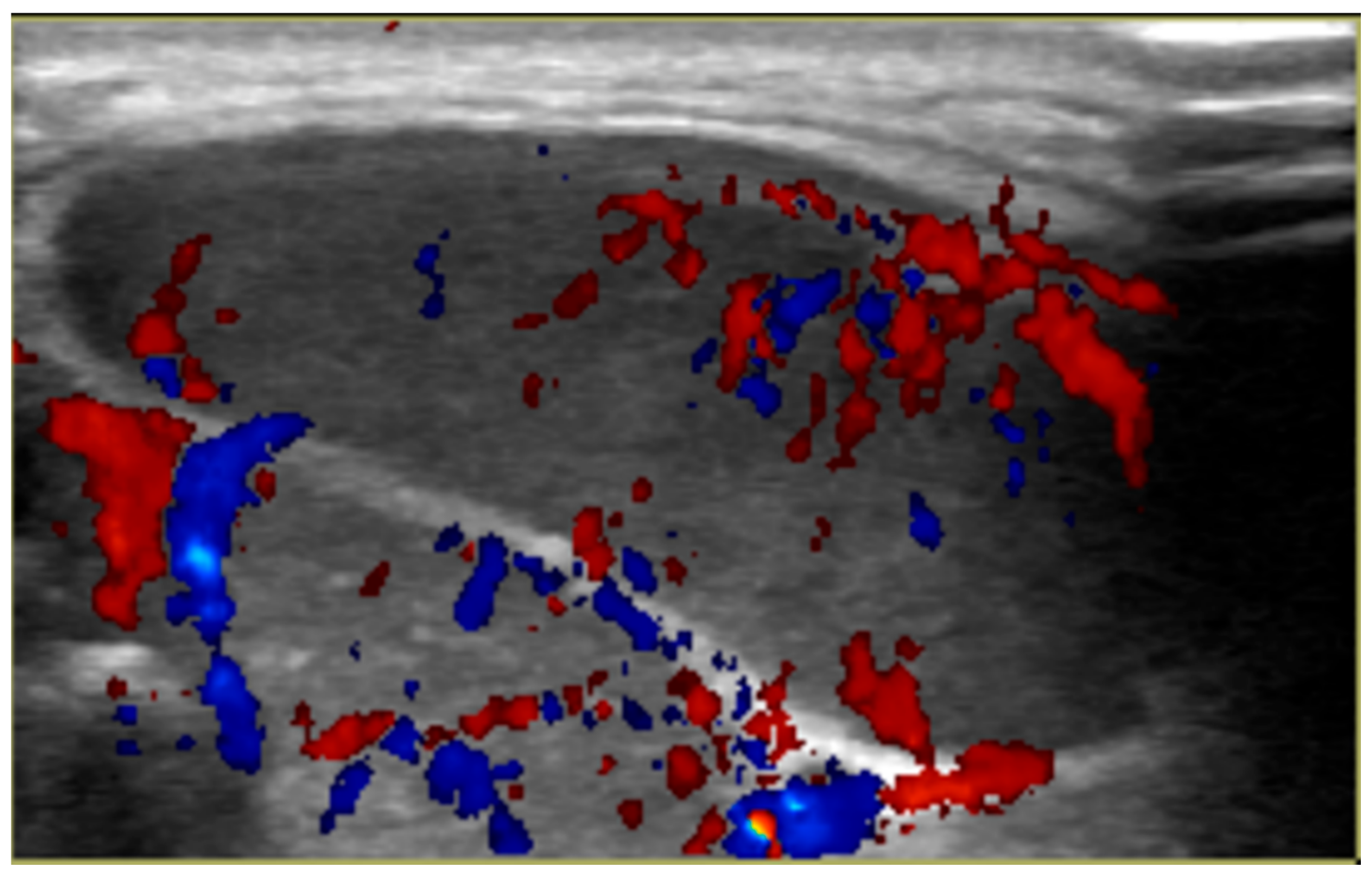
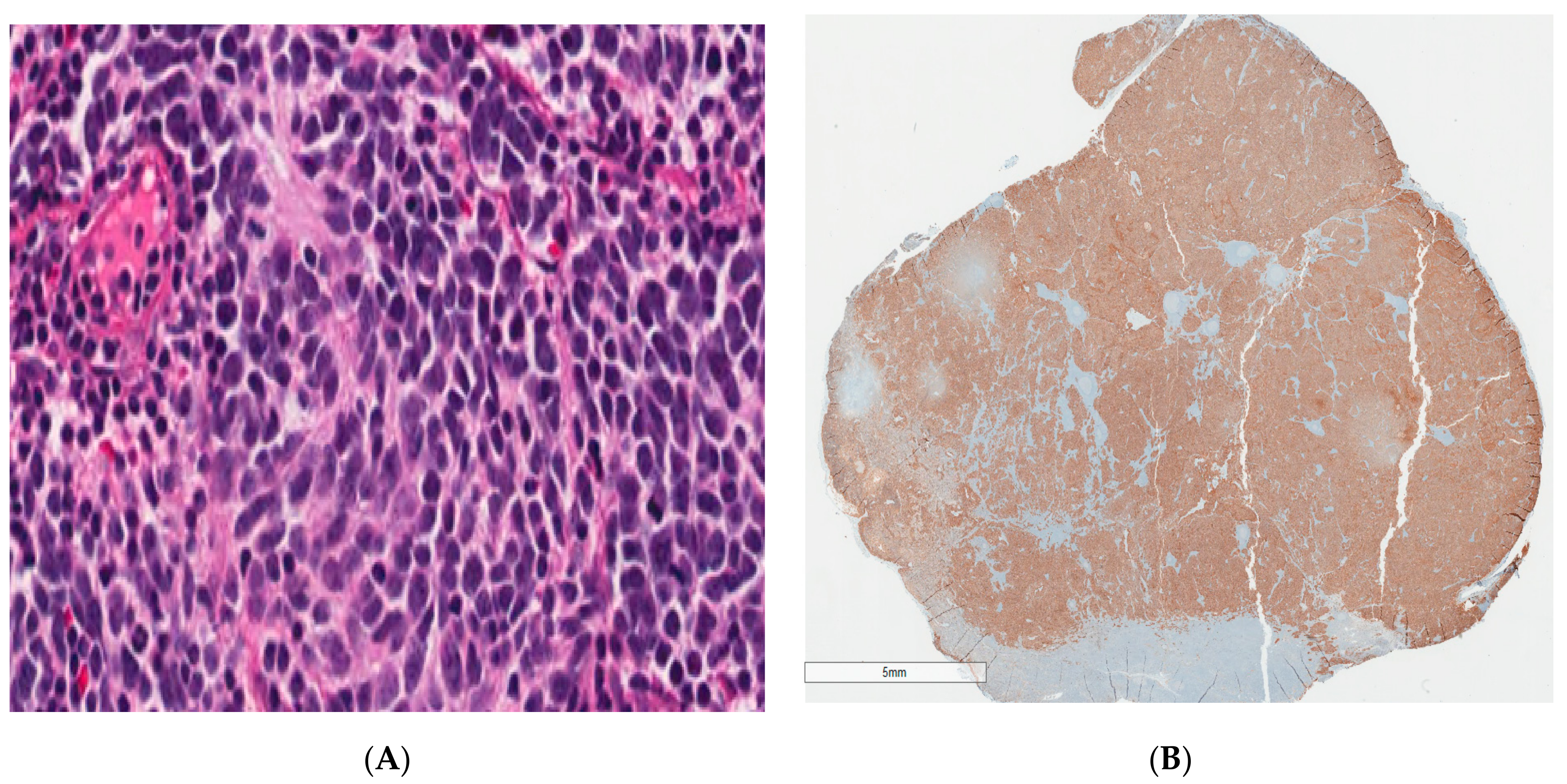
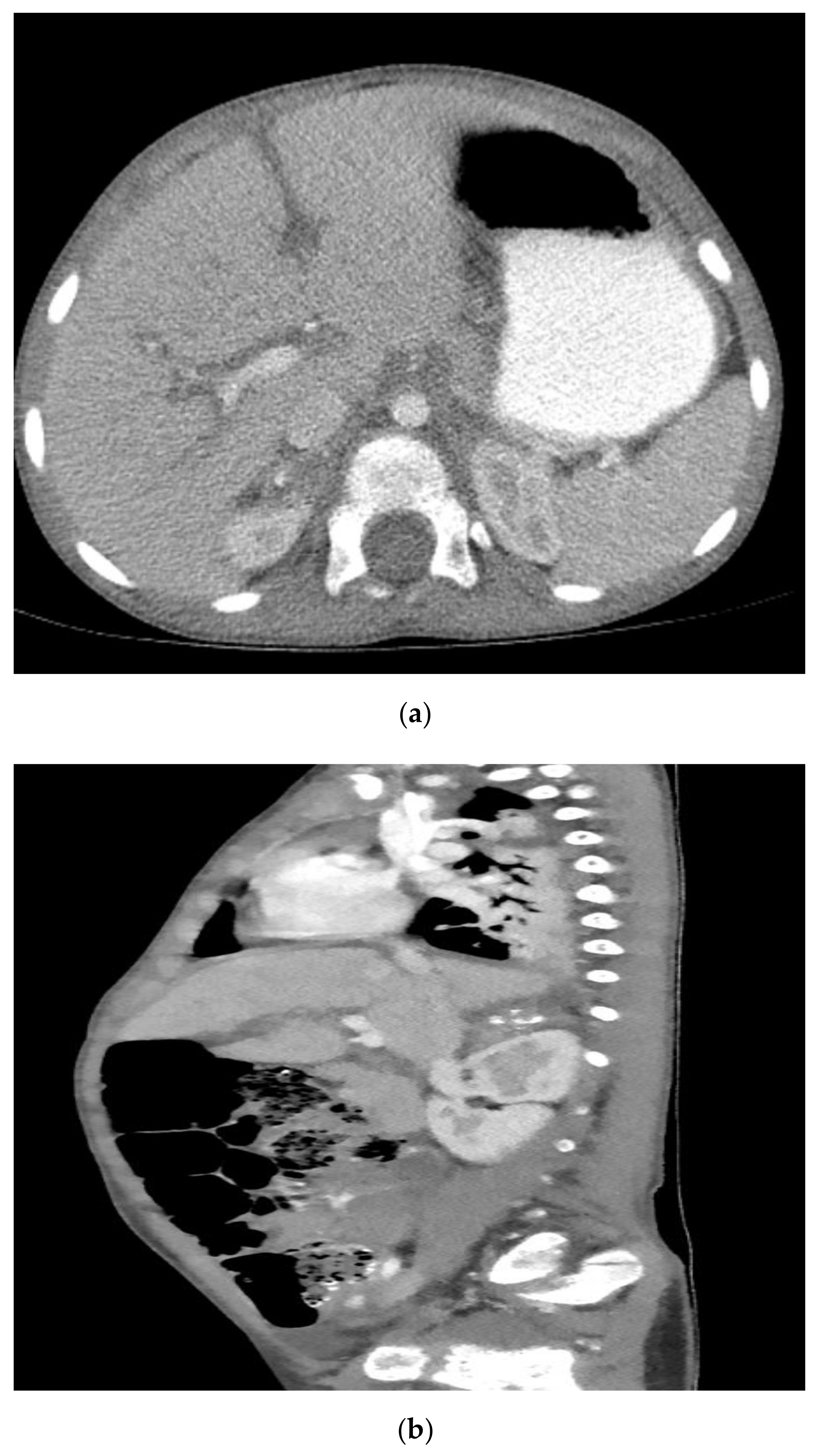
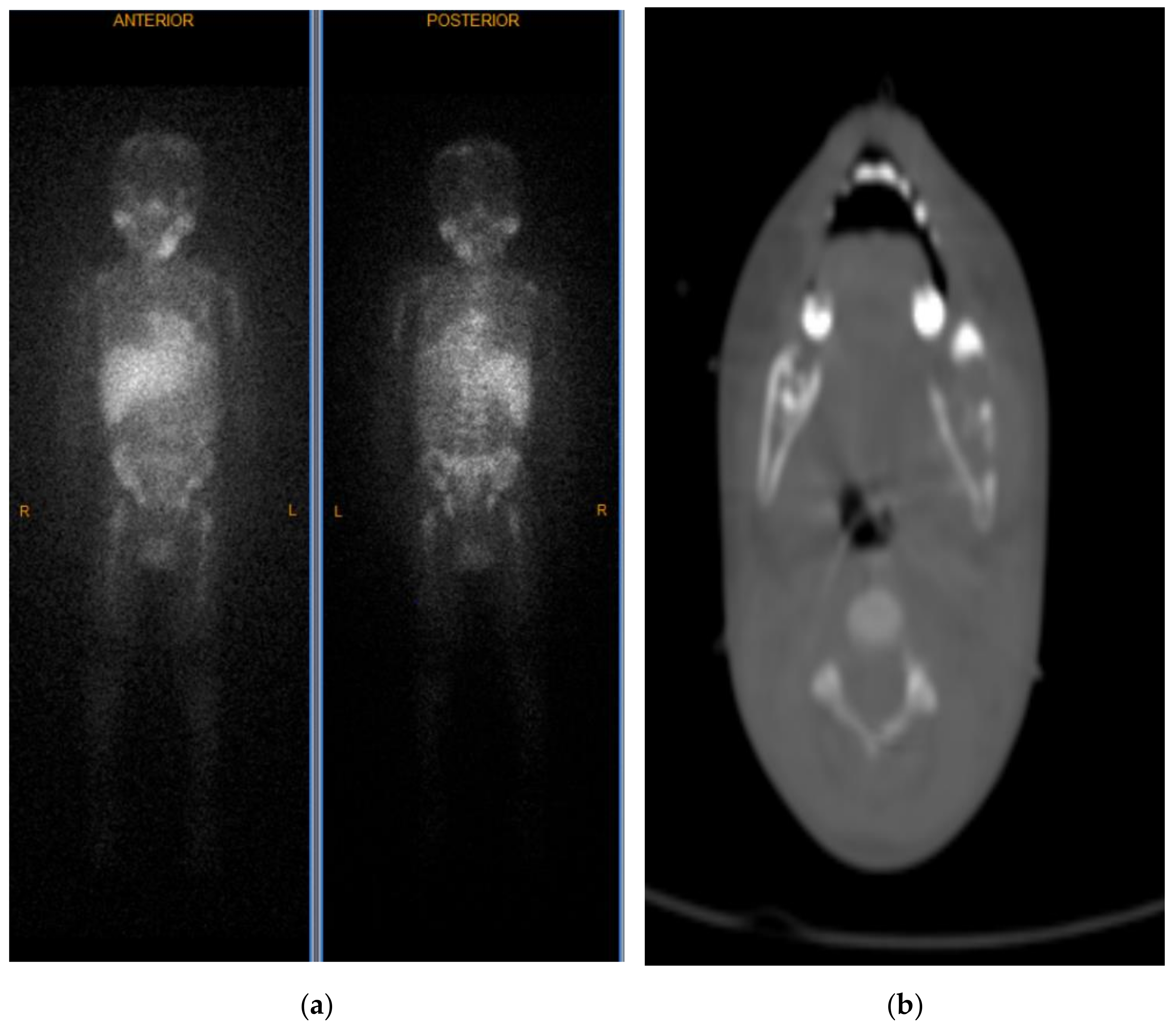
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Park, J.R.; Eggert, A.; Caron, H. Neuroblastoma: Biology, Prognosis, and Treatment. Hematol. Clin. N. Am. 2010, 24, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Brossard, J.; Bernstein, M.L.; Lemieux, B. Neuroblastoma: An enigmatic disease. Br. Med Bull. 1996, 52, 787–801. [Google Scholar] [CrossRef][Green Version]
- Barr, E.K.; Applebaum, M.A. Genetic Predisposition to Neuroblastoma. Children 2018, 5, 119. [Google Scholar] [CrossRef]
- Mosse, Y.P.; Laudenslager, M.; Khazi, D.; Carlisle, A.J.; Winter, C.L.; Rappaport, E.; Maris, J.M. Germline PHOX2B Mutation in Hereditary Neuroblastoma. Am. J. Hum. Genet. 2004, 75, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Azarova, A.M.; Gautam, G.; George, R.E. Emerging importance of ALK in neuroblastoma. Semin. Cancer Biol. 2011, 21, 267–275. [Google Scholar] [CrossRef]
- Ishola, T.A.; Chung, D.H. Neuroblastoma. Surg. Oncol. 2007, 16, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Colon, N.C.; Chung, D.H. Neuroblastoma. Adv. Pediatr. 2011, 58, 297–311. [Google Scholar]
- DuBois, S.G.; Kalika, Y.; Lukens, J.N.; Brodeur, G.M.; Seeger, R.C.; Atkinson, J.B.; Haase, G.M.; Black, C.T.; Perez, C.; Shimada, H.; et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J. Pediatr. Hematol. Oncol. 1999, 21, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Ambros, I.M.; Dehner, L.P.; Hata, J.-I.; Joshi, V.V.; Roald, B.; Stram, D.O.; Gerbing, R.B.; Lukens, J.N.; Matthay, K.K.; et al. The International Neuroblastoma Pathology Classification (the Shimada system). Cancer 1999, 86, 364–372. [Google Scholar] [CrossRef]
- Shimada, H.; Ambros, I.M.; Dehner, L.P.; Hata, J.-I.; Joshi, V.V.; Roald, B. Terminology and morphologic criteria of neuroblastic tumors. Cancer 1999, 86, 349–363. [Google Scholar] [CrossRef]
- Alexander, N.; Sullivan, K.; Shaikh, F.; Irwin, M.S. Characteristics and management of ganglioneuroma and ganglioneuroblastoma-intermixed in children and adolescents. Pediatr. Blood Cancer 2018, 65, e26964. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Modak, S.; Solano, A.K.; Kushner, B.H.; Wolden, S.; Huryn, J.; Estilo, C.L. Mandibular metastases in neuroblastoma: Outcomes and dental sequelae. Pediatr. Blood Cancer 2021, 68, e28918. [Google Scholar] [CrossRef]
- He, W.G.; Yan, Y.; Tang, W.; Cai, R.; Ren, G. Clinical and biological features of neuroblastic tumors: A comparison of neuroblastoma and ganglioneuroblastoma. Oncotarget 2017, 8, 37730–37739. [Google Scholar] [CrossRef]
- Otmani, N.; Khattab, M. Metastatic neuroblastoma to the mandible in a 3-year-old boy: A case report. Med. Oral Patol. Oral Cir. Bucal 2007, 12, 201–204. [Google Scholar]
- Kürklü, E.; Emiroğlu, H.H.; Kebudi, R.; Özdaş, D.Ö.; Ayan, I.; Görgün, Ö.; Zülfikar, B.; Yekeler, E.; Ak, G. Metastatic Mandibular Neuroblastoma: A Rare Cause of Tooth Mobility. J. Clin. Pediatr. Dent. 2011, 36, 203–206. [Google Scholar] [CrossRef]
- Mittal, D.; Mandelia, A.; Bajpai, M.; Agarwala, S. Adrenal neuroblastoma with metastatic mandibular mass: An unusual presentation. J. Cancer Res. Ther. 2015, 11, 645. [Google Scholar] [CrossRef]
- Wade, G.; Revels, J.; Hartman, L.; Brown, W. Pediatric mandibular metastasis: A rare finding of neuroblastoma. Radiol. Case Rep. 2018, 13, 289–294. [Google Scholar] [CrossRef]
- Haddad, M.; Triglia, J.M.; Helardot, P.; Couanet, D.; Gauthier, F.; Neuenschwander, S.; Bourlière, B.; Bergeron, C.; Munzer, C.; Rubie, H.; et al. Localized cervical neuroblastoma: Prevention of surgical complications. Int. J. Pediatr. Otorhinolaryngol. 2003, 67, 1361–1367. [Google Scholar] [CrossRef]
- Castleberry, R.P.; Pritchard, J.; Ambros, P.; Berthold, F.; Brodeur, G.M.; Castel, V.; Cohn, S.L.; De Bernardi, B.; Dicks-Mireaux, C.; Frappaz, D.; et al. The International Neuroblastoma Risk Groups (INRG): A preliminary report. Eur. J. Cancer 1997, 33, 2113–2116. [Google Scholar] [CrossRef]
- Twist, C.J.; Schmidt, M.L.; Naranjo, A.; London, W.B.; Tenney, S.C.; Marachelian, A.; Shimada, H.; Collins, M.H.; Esiashvili, N.; Adkins, E.S.; et al. Maintaining Outstanding Outcomes Using Response- and Biology-Based Therapy for Intermediate-Risk Neuroblastoma: A Report From the Children’s Oncology Group Study ANBL0531. J. Clin. Oncol. 2019, 37, 3243–3255. [Google Scholar] [CrossRef]
- McGregor, L.M.; Rao, B.N.; Davidoff, A.M.; Billups, C.A.; Hongeng, S.; Santana, V.M.; Hill, D.A.; Fuller, C.; Furman, W.L. The impact of early resection of primary neuroblastoma on the survival of children older than 1 year of age with stage 4 disease. Cancer 2005, 104, 2837–2846. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, C.S.; London, W.B.; Look, A.T.; Brodeur, G.M.; Altmiller, D.H.; Thorner, P.S.; Joshi, V.V.; Rowe, S.T.; Nash, M.B.; Smith, E.I.; et al. Natural history and biology of stage A neuroblastoma: A Pediatric Oncology Group Study. J. Pediatr. Hematol. Oncol. 2000, 22, 197–205. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, A.; Doroodchi, A.; Velasquez Zarate, L.; Mattox, S.N.; Sliker, T.; Willhite, D.K.; Khan, J.; Owen, H.C.; Rajpurohit, S.K.; Patel, N.G.; et al. Metastatic Neuroblastoma Presenting as a Submandibular Mass with Mandibular Bone Involvement in a Three-Year-Old Child. Int. J. Environ. Res. Public Health 2021, 18, 4157. https://doi.org/10.3390/ijerph18084157
Ullah A, Doroodchi A, Velasquez Zarate L, Mattox SN, Sliker T, Willhite DK, Khan J, Owen HC, Rajpurohit SK, Patel NG, et al. Metastatic Neuroblastoma Presenting as a Submandibular Mass with Mandibular Bone Involvement in a Three-Year-Old Child. International Journal of Environmental Research and Public Health. 2021; 18(8):4157. https://doi.org/10.3390/ijerph18084157
Chicago/Turabian StyleUllah, Asad, Atbin Doroodchi, Luis Velasquez Zarate, Samantha N. Mattox, Taylor Sliker, Dorian K. Willhite, Jaffar Khan, Harry C. Owen, Surendra K. Rajpurohit, Nikhil G. Patel, and et al. 2021. "Metastatic Neuroblastoma Presenting as a Submandibular Mass with Mandibular Bone Involvement in a Three-Year-Old Child" International Journal of Environmental Research and Public Health 18, no. 8: 4157. https://doi.org/10.3390/ijerph18084157
APA StyleUllah, A., Doroodchi, A., Velasquez Zarate, L., Mattox, S. N., Sliker, T., Willhite, D. K., Khan, J., Owen, H. C., Rajpurohit, S. K., Patel, N. G., & Hatley, R. M. (2021). Metastatic Neuroblastoma Presenting as a Submandibular Mass with Mandibular Bone Involvement in a Three-Year-Old Child. International Journal of Environmental Research and Public Health, 18(8), 4157. https://doi.org/10.3390/ijerph18084157









