Effects of Growth Stage and Cd Chemical Form on Cd and Zn Accumulation in Arabidopsis halleri ssp. gemmifera
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and Soil Preparation and Pot Experiment
2.2. Plants and Soil Analysis Subsection
3. Results
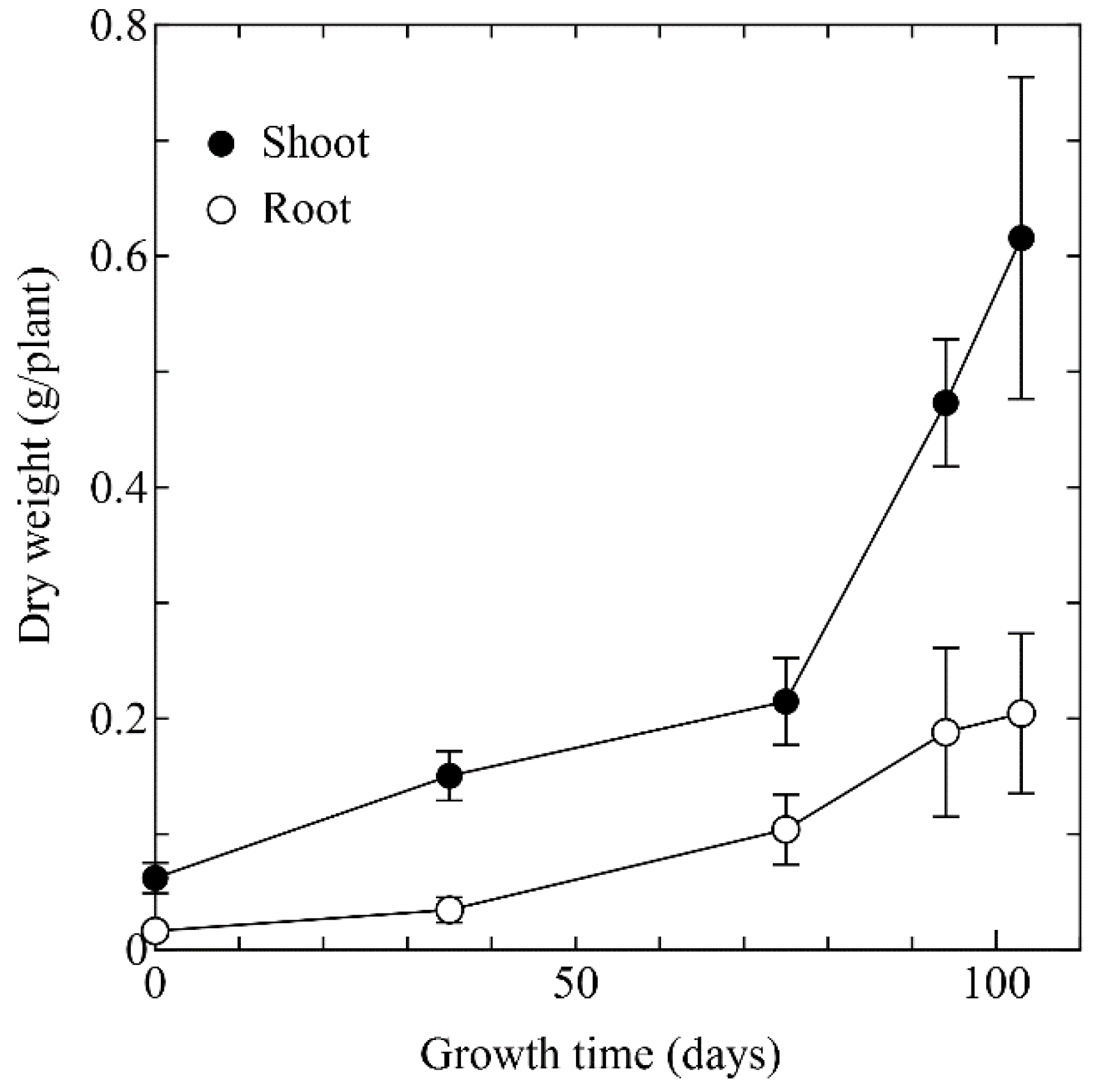
3.1. Biomass Yield and Heavy Metals Concentration of Arabidopsis halleri ssp. gemmifera
3.2. Cd and Zn Concentration of Contaminated Soil
3.3. Material Balance between Soil and Plant
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satarug, S.; Haswell-Elkins, M.R.; Moore, M.R. Safe levels of cadmium intake to prevent renal toxicity in human subjects. Br. J. Nutr. 2000, 84, 791–802. [Google Scholar] [CrossRef]
- Smolders, E. Cadmium uptake by plants. Int. J. Occup. Med. Environ. Health 2001, 14, 177–183. [Google Scholar]
- Klaassen, C.D.; Casarett, L.J.; Doull, J. Casarett and Doull’s Toxicology: The Basic Science of Poisons; McGraw-Hill: New York, NY, USA, 2013. [Google Scholar]
- Zhao, F.-J.; Ma, Y.; Zhu, Y.-G.; Tang, Z.; McGrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.; Li, H.; Wang, L.; Tudi, M.; Yang, L. Concentration, spatial distribution, contamination degree and human health risk assessment of heavy metals in urban soils across China between 2003 and 2019—A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 3099. [Google Scholar] [CrossRef]
- Abedi, T.; Mojiri, A. Cadmium uptake by wheat (Triticum aestivum L.): An overview. Plants 2020, 9, 500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishijo, M.; Nakagawa, H.; Suwazono, Y.; Nogawa, K.; Kido, T. Causes of death in patients with Itai-itai disease suffering from severe chronic cadmium poisoning: A nested case-control analysis of a follow-up study in Japan. BMJ Open 2017, 7, e015694. [Google Scholar] [CrossRef] [Green Version]
- The Ministry of Agriculture, Forestry and Fisheries in Japan, Hokuriku Regional Agricultural Administration Office. Restoration of Cd Contaminated Agricultural Soil around Jinzu River in Toyama Prefecture, Japan. 2013. Available online: http://www.maff.go.jp/hokuriku/news/print/50nen_ayumi/pdf/13_17-18_50ayumi.pdf (accessed on 10 February 2021).
- Lee, J.H. An overview of phytoremediation as a potentially promising technology for environmental pollution control. Biotechnol. Bioprocess Eng. 2013, 18, 431–439. [Google Scholar] [CrossRef]
- Kubota, H.; Takenaka, C. Arabis gemmifera is a hyperaccumulator of Cd and Zn. Int. J. Phytoremediat. 2003, 5, 197–201. [Google Scholar] [CrossRef]
- Kashem, A.; Singh, B.R.; Kubota, H.; Nagashima, R.S.; Kitajima, N.; Kondo, T.; Kawai, S. Assessing the potential of Arabidopsis halleri ssp. gemmifera as a new cadmium hyperaccumulator grown in hydroponics. Can. J. Plant Sci. 2007, 87, 499–502. [Google Scholar] [CrossRef]
- Kashem, A.; Singh, B.R.; Kubota, H.; Sugawara, R.; Kitajima, N.; Kondo, T.; Kawai, S. Zinc tolerance and uptake by Arabidopsis halleri ssp. gemmifera grown in nutrient solution. Environ. Sci. Pollut. Res. 2010, 17, 1174–1176. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Hu, L.; McBride, M.B.; Cheng, H.; Wu, J.; Shi, J.; Xu, J.; Wu, L. Root-induced changes to cadmium speciation in the rhizosphere of two rice (Oryza sativa L.) genotypes. Environ. Res. 2011, 111, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Xian, X. Effect of chemical forms of cadmium, zinc, and lead in polluted soils on their uptake by cabbage plants. Plant Soil 1989, 113, 257–264. [Google Scholar] [CrossRef]
- Kubota, H.; Sugawara, R.; Kitajima, N.; Yajima, S.; Tani, S. Cadmium phytoremediation by Arabidopsis halleri ssp. gemmifera. J. Soil Sci. Plant Nutr. 2010, 81, 118–124. (In Japanese) [Google Scholar]
- Tani, S.; Kameyama, K. Phytoextraction with Arabidopsis halleri ssp. gemmifera to remediate Cd-contaminated soils. Global Environ. Res. 2009, 15, 55–62. (In Japanese) [Google Scholar]
- Lasat, M.M. Phytoextraction of toxic metals. J. Environ. Qual. 2002, 31, 109–120. [Google Scholar]
- Maeda, Y.; Konishi, M.; Kiba, T.; Sakuraba, Y.; Sawaki, N.; Kurai, T.; Ueda, Y.; Sakakibara, H.; Yanagisawa, S. A NIGT1-centered transcriptional cascade regulates nitrate signalling and incorporates phosphorus starvation signals in Arabidopsis. Nat. Commun. 2018, 9, 1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazoa, P.; Vidmar, J.J.; Tranbarger, T.J.; Mouline, K.; Damiani, I.; Tillard, P.; Zhuo, D.; Glass, A.D.; Touraine, B. Regulation of the nitrate transporter gene AtNRT2.1 in Arabidopsis thaliana: Responses to nitrate, amino acids and developmental stage. Plant Mol. Biol. 2003, 52, 689–703. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Hokura, A.; Kitajima, N.; Terada, Y.; Saito, H.; Abe, T.; Nakai, I. Mico X-xray fluorescence imaging and micro X-ray adsorption spectroscopy of cadmium hyper-accumulating plant, Arabidopsis halleri ssp. gemmifera, using high-energy synchrotron radiation. J. Anal. At. Spectrom. 2008, 23, 1068–1075. [Google Scholar] [CrossRef]
- Poorter, H.; Hler, J.B.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugawara, K.; Kobayashi, A.; Endo, G.; Hatayama, M.; Inoue, C. Evaluation of the effectiveness and salt stress of Pteris vittata in the remediation of arsenic contamination caused by tsunami sediments. J. Environ. Sci. Health Part A 2014, 49, 1631–1638. [Google Scholar] [CrossRef]
- Sugawara, K.; Wen, X.; Haung, Y.; Miyauchi, K.; Endo, G.; Kitajima, N.; Inoue, C. Evaluation ability to accumulate Cd and Zn of Arabidpsis halleri ssp. gemmifera in field and hydroponic study. In Proceedings of the 11th International Phytotechnologies Conference, Heraklion, Crete, Greece, 30 September–3 October 2014. [Google Scholar]
- Zhang, Z.; Wen, X.; Huang, Y.; Inoue, C.; Liang, Y. Higher accumulation capacity of cadmium than zinc by Arabidopsis halleri ssp. germmifera in the field using different sowing strategies. Plant Soil 2017, 418, 165–176. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Bert, V.; Meerts, P.; Saumitou-Laprade, P.; Salis, P.; Gruber, W.; Verbruggen, N. Genetic basis of Cd tolerance and hyperaccumulation in Arabidopsis halleri. Plant Soil 2003, 249, 9–18. [Google Scholar] [CrossRef]
- Vert, G.; Grotz, N.; Dédaldéchamp, F.; Gaymard, F.; Guerinot, M.L.; Briat, J.-F.; Curie, C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 2002, 14, 1223–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kameyama, K.; Tani, S.; Sugawara, R.; Ishikawa, Y. Applicability of phytoextraction with Arabidopsis halleri ssp. gemmifera to remediate Cd-contaminated Andisols. Trans. Jpn. Soc. Irrig. 2009, 259, 99–106. (In Jpananese) [Google Scholar]
- Tlustoš, P.; Száková, J.; Hrubý, J.; Hartman, I.; Najmanová, J.; Nedělník, J.; Pavlíková, D.; Batysta, M. Removal of As, Cd, Pb, and Zn from contaminated soil by high biomass producing plants. Plant Soil Environ. 2018, 52, 413–423. [Google Scholar] [CrossRef] [Green Version]
- Degryse, F.; Buekers, J.; Smolders, E. Radio-labile cadmium and zinc in soils as affected by pH and source of contamination. Eur. J. Soil Sci. 2004, 55, 113–122. [Google Scholar] [CrossRef]
- Gradd, G.M. Microbial influence on metal mobility and application for bioremediation. Geoderma 2004, 122, 109–119. [Google Scholar]
- Makino, T.; Hasegawa, S.; Sakurai, Y.; Ohno, S.; Utagawa, H.; Maejima, Y.; Momohara, K. Influence of soil-drying under field conditions on exchangeable manganese, cobalt, and copper contents. Soil Sci. Plant Nutr. 2000, 46, 581–590. [Google Scholar] [CrossRef]
- McGrath, S.P.; Zhao, F.; Lombi, E. Plant and rhizosphere processes involved in phytoremediation of metal-contaminated soils. Plant Soil 2001, 232, 207–214. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Wang, Y.; Yeh, K.-C. Role of root exudates in metal acquisition and tolerance. Curr. Opin. Plant Biol. 2017, 39, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: Beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. A review. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Wassmann, R.; Bueno, C.; Kreuzwieser, J.; Rennenberg, H. Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant Biol. 2001, 3, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Gransee, A.; Wittenmayer, L. Qualitative and quantitative analysis of water-soluble root exudates in relation to plant species and development. J. Plant Nutr. Soil Sci. 2000, 163, 381–385. [Google Scholar] [CrossRef]
- Hoffland, E. Quantitative evaluation of the role of organic acid exudation in the mobilization of rock phosphate by rape. Plant Soil 1992, 140, 279–289. [Google Scholar] [CrossRef]
- Dinkelaker, B.; Romheld, V.; Marschner, H. Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus L.). Plant Cell Environ. 1989, 12, 285–292. [Google Scholar] [CrossRef]
- Gaume, A.; Machler, F.; De Leon, C.; Narro, L.; Frossard, E. Low-P tolerance by maize (Zea mays L.) genotypes: Significance of root growth, and organic acids and acid phosphatase root exudation. Plant Soil 2001, 228, 253–264. [Google Scholar] [CrossRef]
- Dong, D.; Peng, X.; Yan, X. Organic acid exudation induced by phosphorus deficiency and/or aluminium toxicity in two contrasting soybean genotypes. Physiol. Plant. 2004, 122, 190–199. [Google Scholar] [CrossRef]
- Mench, M.; Martin, E. Mobilization of cadmium and other metals from two soils by root exudates of Zea mays L., Nicotiana tabacum L. and Nicotiana rustica L. Plant Soil 1991, 132, 187–196. [Google Scholar] [CrossRef]
- Zhao, F.J.; Hamon, R.E.; McLaughlin, M.J. Root exudates of the hyperaccumulator Thlaspi caerulescens do not enhance metal mobilization. New Phytol. 2001, 151, 613–620. [Google Scholar] [CrossRef] [Green Version]





| Method of Extraction | Solvent | Soil Weight | Solvent Volume | Solvent Concentration | Shaking Speed | Shaking Time |
|---|---|---|---|---|---|---|
| Water extraction * | Milli-Q water | 3 g | 30 mL | - | 200 rpm | 6 h |
| Exchangeable extraction | (NH4)2SO4 | 1 g | 20 mL | 0.05 mol/L | 200 rpm | 4 h |
| HCl-soluble extraction * | HCl | 5 g | 25 mL | 1.0 mol/L | 100 rpm | 30 min |
| Growth Time (Days) | 35 Days | 75 Days | 94 Days | 103 Days | ||||
|---|---|---|---|---|---|---|---|---|
| Cd decrement in soil (mg) | −0.01 | ±0.08 | 0.18 | ±0.11 | 1.17 | ±0.33 | 1.47 | ±0.39 |
| Cd increment in plant (mg) | 0.03 | ±0.08 | 0.11 | ±0.06 | 0.93 | ±0.08 | 1.20 | ±0.27 |
| Cd Rp/s (%) | 17.6 | ±58.5 | 63.3 | ±16.7 | 83.7 | ±24.3 | 83.2 | ±10.1 |
| Cd TF | 2.03 | ±0.75 | 3.04 | ±0.92 | 7.49 | ±1.56 | 6.32 | ±0.28 |
| Cd BCF | 31.2 | ±18.1 | 95.2 | ±44.1 | 410 | ±19.1 | 424 | ±80.3 |
| Cd AF | 28.0 | ±15.3 | 72.7 | ±29.9 | 311 | ±19.9 | 336 | ±59.6 |
| Zn TF | 3.54 | ±1.01 | 6.98 | ±4.17 | 10.9 | ±3.87 | 8.65 | ±2.14 |
| Zn BCF | 5.65 | ±2.17 | 14.6 | ±5.84 | 43.1 | ±10.2 | 43.8 | ±7.36 |
| Zn AF | 4.88 | ±1.78 | 10.5 | ±3.89 | 32.5 | ±9.77 | 34.3 | ±4.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudo, H.; Inoue, C.; Sugawara, K. Effects of Growth Stage and Cd Chemical Form on Cd and Zn Accumulation in Arabidopsis halleri ssp. gemmifera. Int. J. Environ. Res. Public Health 2021, 18, 4214. https://doi.org/10.3390/ijerph18084214
Kudo H, Inoue C, Sugawara K. Effects of Growth Stage and Cd Chemical Form on Cd and Zn Accumulation in Arabidopsis halleri ssp. gemmifera. International Journal of Environmental Research and Public Health. 2021; 18(8):4214. https://doi.org/10.3390/ijerph18084214
Chicago/Turabian StyleKudo, Hiroshi, Chihiro Inoue, and Kazuki Sugawara. 2021. "Effects of Growth Stage and Cd Chemical Form on Cd and Zn Accumulation in Arabidopsis halleri ssp. gemmifera" International Journal of Environmental Research and Public Health 18, no. 8: 4214. https://doi.org/10.3390/ijerph18084214
APA StyleKudo, H., Inoue, C., & Sugawara, K. (2021). Effects of Growth Stage and Cd Chemical Form on Cd and Zn Accumulation in Arabidopsis halleri ssp. gemmifera. International Journal of Environmental Research and Public Health, 18(8), 4214. https://doi.org/10.3390/ijerph18084214






