Can Male Patient’s Age Affect the Cortical Bone Thickness of Jawbone for Dental Implant Placement? A Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection and Cone-Beam Computed Tomography (CBCT) Scanning
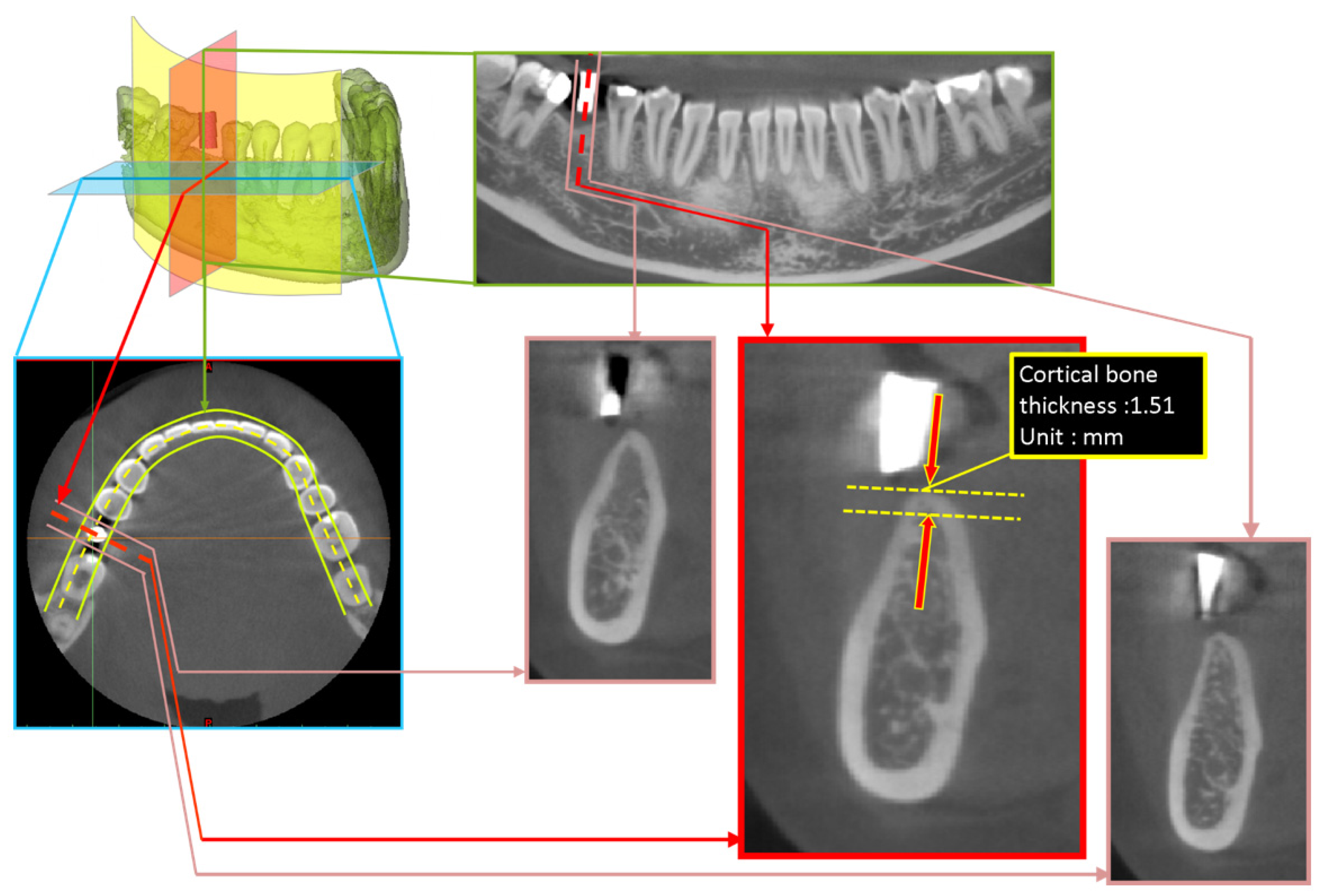
2.2. Measurement Approach of Cortical Bone Thickness at Dental Implant Sites
2.3. Statistical Analysis
3. Results
3.1. Cortical Bone Thickness at Dental Implant Sites
3.2. Relationship between Age and Cortical Bone Thickness at Dental Implant Sites
4. Discussion
5. Conclusions
- (1)
- Male patients were grouped by the jawbone regions where their dental implants were placed. The cortical bone thickness of the jawbone regions in descending order was as follows: posterior mandible: 1.07 ± 0.44 mm, anterior mandible: 0.99 ± 0.30 mm, anterior maxilla: 0.82 ± 0.32 mm, and posterior maxilla: 0.71 ± 0.27 mm.
- (2)
- The cortical bone thickness at dental implant sites in the maxilla did not differ across age in male patients.
- (3)
- Among male patients, age was correlated moderately and negatively with the cortical bone thickness in the anterior mandible region (r = −0.552, p = 0.001) and weakly and negatively with the posterior mandible region (r = −0.173, p = 0.048).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lekholm, U.; Zarb, G.A. Patient selection and preparation. In Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry; Brånemark, P.I., Zarb, G.A., Albrektsson, T., Eds.; Quintessence: Chicago, IL, USA, 1985; pp. 199–209. [Google Scholar]
- Gapski, R.; Wang, H.L.; Mascarenhas, P.; Lang, N.P. Critical review of immediate implant loading. Clin. Oral Implant. Res. 2003, 14, 515–527. [Google Scholar] [CrossRef]
- Attard, N.J.; Zarb, G.A. Immediate and early implant loading protocols: A literature review of clinical studies. J. Prosthet. Dent. 2005, 94, 242–258. [Google Scholar] [CrossRef]
- Hsu, J.T.; Fuh, L.J.; Tu, M.G.; Li, Y.F.; Chen, K.T.; Huang, H.L. The effects of cortical bone thickness and trabecular bone strength on noninvasive measures of the implant primary stability using synthetic bone models. Clin. Implant Dent. Relat. Res. 2013, 15, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-C.; Tsai, M.-T.; Fuh, L.-J.; Tsai, M.-J.; Wang, X.-H.; Huang, H.-L.; Hsu, J.-T. Association between Age of Menopause and Thickness of Crestal Cortical Bone at Dental Implant Site: A Cross-Sectional Observational Study. Int. J. Environ. Res. Public Health 2020, 17, 5868. [Google Scholar] [CrossRef]
- Wang, S.-H.; Shen, Y.-W.; Fuh, L.-J.; Peng, S.-L.; Tsai, M.-T.; Huang, H.-L.; Hsu, J.-T. Relationship between Cortical Bone Thickness and Cancellous Bone Density at Dental Implant Sites in the Jawbone. Diagnostics 2020, 10, 710. [Google Scholar] [CrossRef]
- Javed, F.; Romanos, G.E. The role of primary stability for successful immediate loading of dental implants. A literature review. J. Dent. 2010, 38, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Chang, Y.Y.; Lin, D.J.; Li, Y.F.; Chen, K.T.; Hsu, J.T. Initial stability and bone strain evaluation of the immediately loaded dental implant: An in vitro model study. Clin. Oral Implant. Res. 2011, 22, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.-T.; Huang, H.-L.; Tsai, M.-T.; Wu, A.-J.; Tu, M.-G.; Fuh, L.-J. Effects of the 3D bone-to-implant contact and bone stiffness on the initial stability of a dental implant: Micro-CT and resonance frequency analyses. Int. J. Oral Maxillofac. Surg. 2013, 42, 276–280. [Google Scholar] [CrossRef]
- Marquezan, M.; Osório, A.; Sant’Anna, E.; Souza, M.M.; Maia, L. Does bone mineral density influence the primary stability of dental implants? A systematic review. Clin. Oral Implant. Res. 2012, 23, 767–774. [Google Scholar] [CrossRef]
- Kanis, J.A.; Melton III, L.J.; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef]
- Alswat, K.A. Gender disparities in osteoporosis. J. Clin. Med. Res. 2017, 9, 382. [Google Scholar] [CrossRef] [Green Version]
- Borrud, L.G.; Dawson-Hughes, B.; Looker, A.C.; Shepherd, J.A.; Wright, N.C. Osteoporosis or Low Bone Mass at the Femur Neck or Lumbar Spine in Older Adults, United States, 2005–2008; NCHS aata brief no 93; National Center for Health Statistics: Hyattsville, MD, USA, 2012.
- Campion, J.M.; Maricic, M.J. Osteoporosis in men. Am. Fam. Physician 2003, 67, 1521–1526. [Google Scholar] [PubMed]
- Luo, Y.; Wu, X. Bone quality is dependent on the quantity and quality of organic–inorganic phases. J. Med. Biol. Eng. 2020, 40, 273–281. [Google Scholar] [CrossRef]
- Iwasaki, M.; Nakamura, K.; Yoshihara, A.; Miyazaki, H. Change in bone mineral density and tooth loss in Japanese community-dwelling postmenopausal women: A 5-year cohort study. J. Bone Miner. Metab. 2012, 30, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Rosano, G.; Taschieri, S. Implant survival rates after maxillary sinus augmentation. Eur. J. Oral Sci. 2008, 116, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, X.; Fujita, H.; Onozuka, M.; Kubo, K.-Y. Age-related changes in trabecular and cortical bone microstructure. Int. J. Endocrinol. 2013, 2013, 213234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaffin, R.A.; Berman, C.L. The excessive loss of Branemark fixtures in type IV bone: A 5-year analysis. J. Periodontol. 1991, 62, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Jemt, T.; Lekholm, U. Implant treatment in edentulous maxillae: A 5-year follow-up report on patients with different degrees of jaw resorption. Int. J. Oral Maxillofac. Implant. 1995, 10, 17–33. [Google Scholar]
- Miyamoto, I.; Tsuboi, Y.; Wada, E.; Suwa, H.; Iizuka, T. Influence of cortical bone thickness and implant length on implant stability at the time of surgery—clinical, prospective, biomechanical, and imaging study. Bone 2005, 37, 776–780. [Google Scholar] [CrossRef]
- Song, Y.-D.; Jun, S.-H.; Kwon, J.-J. Correlation between bone quality evaluated by cone-beam computerized tomography and implant primary stability. Int. J. Oral Maxillofac. Implant. 2009, 24, 59–64. [Google Scholar]
- Gupta, A.; Rathee, S.; Agarwal, J.; Pachar, R.B. Measurement of Crestal Cortical Bone Thickness at Implant Site: A Cone Beam Computed Tomography Study. J. Contemp. Dental Pract. 2017, 18, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.C.; Huang, H.L.; Shen, Y.W.; Cai, J.Y.; Fuh, L.J.; Hsu, J.T. Variations in crestal cortical bone thickness at dental implant sites in different regions of the jawbone. Clin. Implant. Dent. Relat. Res. 2017, 19, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, R.; Nackaerts, O.; Bellaiche, N.; Stamatakis, H.; Tsiklakis, K.; Walker, A.; Bosmans, H.; Bogaerts, R.; Jacobs, R.; Horner, K. Variability of dental cone beam CT grey values for density estimations. Br. J. Radiol. 2013, 86, 20120135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsa, A.; Ibrahim, N.; Hassan, B.; van der Stelt, P.; Wismeijer, D. Bone quality evaluation at dental implant site using multislice CT, micro-CT, and cone beam CT. Clin. Oral Implant. Res. 2015, 26, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Isoda, K.; Ayukawa, Y.; Tsukiyama, Y.; Sogo, M.; Matsushita, Y.; Koyano, K. Relationship between the bone density estimated by cone-beam computed tomography and the primary stability of dental implants. Clin. Oral Implant. Res. 2012, 23, 832–836. [Google Scholar] [CrossRef]
- Silva, I.M.d.C.C.; de Freitas, D.Q.; Ambrosano, G.M.B.; Bóscolo, F.N.; Almeida, S.M. Bone density: Comparative evaluation of Hounsfield units in multislice and cone-beam computed tomography. Braz. Oral Res. 2012, 26, 550–556. [Google Scholar] [CrossRef]
- Varshowsaz, M.; Goorang, S.; Ehsani, S.; Azizi, Z.; Rahimian, S. Comparison of tissue density in Hounsfield units in computed tomography and cone beam computed tomography. J. Dent. (Tehran Iran) 2016, 13, 108. [Google Scholar]
- Naitoh, M.; Hirukawa, A.; Katsumata, A.; Ariji, E. Prospective study to estimate mandibular cancellous bone density using large-volume cone-beam computed tomography. Clin. Oral Implant. Res. 2010, 21, 1309–1313. [Google Scholar] [CrossRef]
- Arisan, V.; Karabuda, Z.C.; Avsever, H.; Özdemir, T. Conventional multi-slice computed tomography (CT) and cone-beam CT (CBCT) for computer-assisted implant placement. Part I: Relationship of radiographic gray density and implant stability. Clin. Implant Dent. Relat. Res. 2013, 15, 893–906. [Google Scholar] [CrossRef]
- Monje, A.; Monje, F.; González-García, R.; Galindo-Moreno, P.; Rodriguez-Salvanes, F.; Wang, H.L. Comparison between microcomputed tomography and cone-beam computed tomography radiologic bone to assess atrophic posterior maxilla density and microarchitecture. Clin. Oral Implant. Res. 2014, 25, 723–728. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.-Y.; DoDo, H.; Yousef, H.; Firestone, A.R.; Chaudhry, J.; Johnston, W.M.; Lee, D.J.; Emam, H.A.; Kim, D.-G. Efficacy of cone-Beam computed tomography in evaluating bone quality for optimum implant treatment planning. Implant Dent. 2017, 26, 405–411. [Google Scholar] [CrossRef]
- Tsutsumi, K.; Chikui, T.; Okamura, K.; Yoshiura, K. Accuracy of linear measurement and the measurement limits of thin objects with cone beam computed tomography: Effects of measurement directions and of phantom locations in the fields of view. Int. J. Oral Maxillofac. Implant. 2011, 26, 91–100. [Google Scholar]
- Deguchi, T.; Nasu, M.; Murakami, K.; Yabuuchi, T.; Kamioka, H.; Takano-Yamamoto, T. Quantitative evaluation of cortical bone thickness with computed tomographic scanning for orthodontic implants. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 721.e7–721.e12. [Google Scholar] [CrossRef]
- Baumgaertel, S.; Hans, M.G. Buccal cortical bone thickness for mini-implant placement. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 230–235. [Google Scholar] [CrossRef]
- Baumgaertel, S.; Palomo, J.M.; Palomo, L.; Hans, M.G. Reliability and accuracy of cone-beam computed tomography dental measurements. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 19–25. [Google Scholar] [CrossRef]
- Sugiura, T.; Yamamoto, K.; Kawakami, M.; Horita, S.; Murakami, K.; Kirita, T. Influence of bone parameters on peri-implant bone strain distribution in the posterior mandible. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e66. [Google Scholar] [CrossRef]
- Abirami, G. Residual ridge resorption in complete denture wearers. J. Pharm. Sci. Res. 2016, 8, 565. [Google Scholar]
- Fanghänel, J.; Proff, P.; Dietze, S.; Bayerlein, T.; Mack, F.; Gedrange, T. The morphological and clinical relevance of mandibular and maxillary bone structures for implantation. Folia Morphol. 2006, 65, 49–53. [Google Scholar]
- Woelfel, J.B.; Winter, C.M.; Igarashi, T. Five-year cephalometric study of mandibular ridge resorption with different posterior occlusal forms. Part I. Denture construction and initial comparison. J. Prosthet. Dent. 1976, 36, 602–623. [Google Scholar] [CrossRef]
- D’Souza, D. Residual ridge resorption–revisited. Oral Health Care Prosthodont. Periodontol. Biol. Res. Syst. Cond. 2012, 2, 15–24. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-H.; Ko, Y.-C.; Tsai, M.-T.; Fuh, L.-J.; Huang, H.-L.; Shen, Y.-W.; Hsu, J.-T. Can Male Patient’s Age Affect the Cortical Bone Thickness of Jawbone for Dental Implant Placement? A Cohort Study. Int. J. Environ. Res. Public Health 2021, 18, 4284. https://doi.org/10.3390/ijerph18084284
Wang S-H, Ko Y-C, Tsai M-T, Fuh L-J, Huang H-L, Shen Y-W, Hsu J-T. Can Male Patient’s Age Affect the Cortical Bone Thickness of Jawbone for Dental Implant Placement? A Cohort Study. International Journal of Environmental Research and Public Health. 2021; 18(8):4284. https://doi.org/10.3390/ijerph18084284
Chicago/Turabian StyleWang, Shiuan-Hui, Yi-Chun Ko, Ming-Tzu Tsai, Lih-Jyh Fuh, Heng-Li Huang, Yen-Wen Shen, and Jui-Ting Hsu. 2021. "Can Male Patient’s Age Affect the Cortical Bone Thickness of Jawbone for Dental Implant Placement? A Cohort Study" International Journal of Environmental Research and Public Health 18, no. 8: 4284. https://doi.org/10.3390/ijerph18084284





