Abstract
Dermacentor silvarum is an obligate blood sucking arthropod and transmits various pathogens to humans and domestic animals. Recently several new viruses were detected in D. silvarum as an emerging disease threat. In this study, we aimed to analyze its geographical distribution and associated pathogens. Data were collected from multiple sources, including a field survey, reference book, and literature review. We searched various electronic databases with the terms “Dermacentor silvarum” OR “D. silvarum” for studies published since 1963 and the positive rates for Dermacentor silvarum-associated pathogens were estimated by meta-analysis. D. silvarum was found only in four countries in Eurasia, ranging from 22° N to 57° N latitude. At least 20 human pathogens were associated with D. silvarum, including five species of spotted fever group rickettsiae, three species in the family of Anaplasmataceae, three genospecies in the complex Borrelia burgdorferi sensu lato, Francisella tularensis, Babesia venatorum, Coxiella buenetii, Borrelia miyamotoi, and five species of virus. Among them, Rickettsia raoultii was widely detected in D. silvarum, showing the highest pooled positive rate (25.15%; 95% CI 13.31–39.27). Our work presents the most comprehensive data and analysis (to our knowledge) for the geographical distribution of D. silvarum and associated pathogens, revealing an emerging threat to public health and stocking farming. Continued surveillance and further investigations should be enhanced.
1. Introduction
Ticks are obligate blood-sucking ectoparasites distributed widely across the world. They are vectors of many human and animal infectious pathogens, such as tick-borne encephalitis, Crimean-Congo hemorrhagic fever, Lyme disease, Q fever, babesiosis, and severe fever with thrombocytopenia syndrome [1,2,3]. Currently, tick-borne diseases have become an increasing public health threat with global climate changing, accelerated urbanization, and altered distribution of tick and their hosts [4,5,6].
Dermacentor silvarum, a species of hard ticks, is widely distributed in North China, Russia, and Mongolia [7,8,9]. They have a hard scutum, the cornua of basis capituli is longer and pointy, and the length is nearly equal to the base width. The dorsal spur of trochanter I is long and pointed, and the apex of the third segment of the pedipalp is flat [10] (Figure S1). They mainly reside in shrubbery, and sometimes can also be found in forests and farmland [11]. The immature ticks mainly infest a variety of small mammals, and adults feed on medium-sized to large mammals, including humans [8]. It has been reported that D. silvarum can carry a large variety of tick-borne pathogens including tick-borne encephalitis virus, Rickettsia slovaca, R. raoultii, Anaplasma phagocytophilum, Babesia caballi, and Theileria equi, as well as the causative agent of human monocytic ehrlichiosis, Ehrlichia chaffeensis [12,13,14,15]. Moreover, with the development of high throughput sequencing, lots of novel tick-borne viruses have been discovered in D. silvarum in recent years, such as Tacheng tick virus 1 and Jingmen tick virus [16,17].
It is of great importance to know the distribution of D. silvarum and its associated pathogens. Previous studies have depicted the distribution of D. silvarum in China and in Eurasia [18,19]. Here, we comprehensively collected the geographical distribution data of D. silvarum and its associated pathogens, and conducted a meta-analysis to assess its potential threat for public health.
2. Materials and Methods
2.1. Data Collection and Field Survey
Data were collected from multiple sources, including a field survey, reference book, and literature. The field survey was carried out in all provinces, autonomous regions, and municipalities of mainland China from April to November in 2016–2019. Questing ticks were collected by dragging a standard 1-m2 flannel flag over vegetation, and infesting ticks were collected from body surfaces of animals (mainly domestic animals). All ticks were transported alive or preserved in 75% alcohol to our laboratory for taxonomic identification. Tick species were identified based on morphological features [10] by an entomologist, and all identified D. silvarum ticks were included in this study. Locations of survey sites are listed in Table S1. All experimental procedures and protocols were approved by the Ethics Committee of Academy of Military Medical Sciences (Permit Number: 2016YFC1201902).
Using the terms “Dermacentor silvarum” OR “D. silvarum” (and the Chinese name for Dermacentor silvarum), we searched multiple electronic databases, including PubMed, China National Knowledge Infrastructure (CNKI), and the WanFang database. The study period was 1 January 1963 to 15 December 2020. A publication (a journal article, conference proceeding, or degree thesis) was retrieved if the terms appeared in any parts of its content. Published full articles were included if they were in English or Chinese and had reported the collection locations of D. silvarum on a county or prefecture level (some Russian papers might be missed). Articles which report only laboratory findings, describe identification of tick-borne diseases, or do not include any geographical information were excluded. The key information extracted from the literature included: (i) location associated with tick occurrence (and its geographical scale), (ii) time of tick collection, (iii) species of pathogens identified (if any), and (iv) number of tests and number of positives. After the data were collected, a second person checked the dataset thoroughly to avoid errors and duplications. We also included the distribution data of D. silvarum from the reference book, Fauna Sinica-Arachnida Ixodida by YS [20]. All the data from literature search and our field survey are provided in Table S1.
2.2. Distribution of D. Silvarum and Associated Pathogens
To map geographical distribution of D. silvarum and associated pathogens, we obtained the raster-type data with a resolution of one kilometer on vegetation types from the China National Forestry Bureau and a global map of altitude topography. Thematic maps were produced with ArcGIS 10.7 software (ESRI Inc., Redlands, CA, USA). The particular geographical locations of D. silvarum were used for mapping, and the administrative region centroids were used when exact locations were not available.
We did a meta-analysis to estimate the combined positive rate and 95% CI of each D. silvarum-associated pathogen. Studies without exact numbers of ticks were excluded. If there was only one study included for a certain species of pathogen, the positive rate was calculated by dividing the number of positive ticks by total number without a 95% CI. If the number of studies was two or more, the combined positive rate and 95% CI were estimated. Given the data variance in multiple studies, we chose the random effects model for our meta-analysis [21]. The meta-analysis was done with R (version 3.6.0, meta package).
3. Results
3.1. Dataset
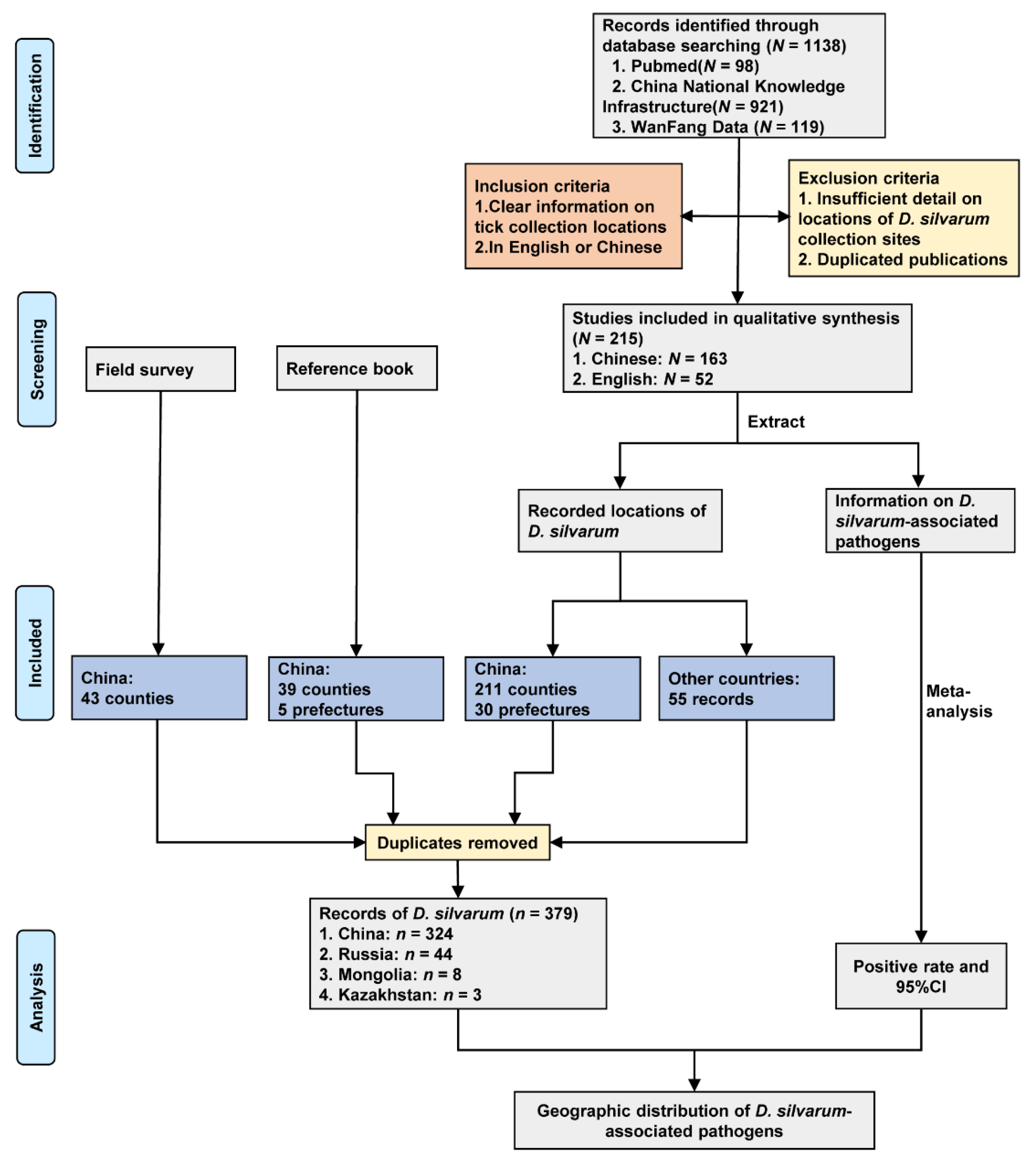
We obtained 43 records from field survey, 40 records from the reference book, and 296 records from publications. After removing duplicate locations, there were 379 records of D. silvarum in four countries (Figure 1), including China (324 records), Russia (44 records), Mongolia (8 records), and Kazakhstan (3 records).
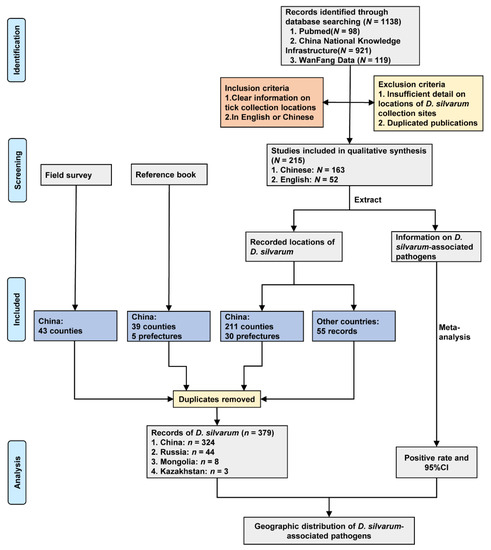
Figure 1.
Study design and data sources. Data were collected from a field survey, reference book and literature review. N indicates the number of studies, and n represents the number of records of D. silvarum.
3.2. Distribution of Dermacentor Silvarum
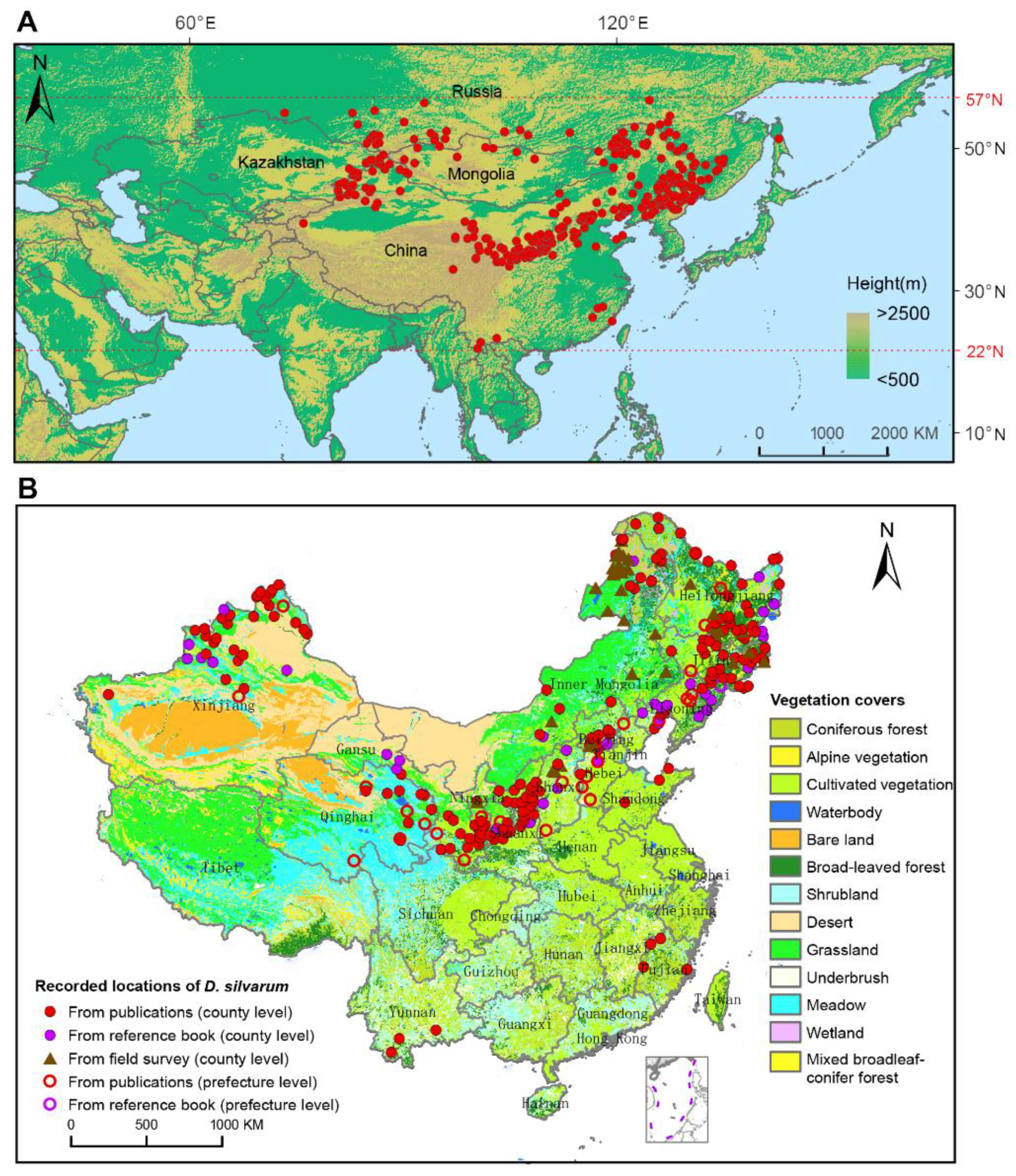
D. silvarum was found only in the northern hemisphere, ranging from 22° N to 57° N latitude (Figure 2A). The easternmost location was the Pacific coast in the southern Sakhalin [9]. The southernmost location was as far as 22° N latitude, which was recently confirmed by Liu et al. [22]. The site of 73.36° E/54.99° N in Omsk, Russia, was documented as the westernmost location [9]. The northernmost distribution could be reasonably defined as the southern districts of the Yakutia Republic, Russia, where D. silvarum was observed up to 56.8° N. D. silvarum was mainly distributed in high altitudinal areas around the mountains. In Russia, D. silvarum was found in the highland of southern Siberia, including the Altai, the mountains around Lake Baikal and the Stanovoy Range. In the Far East, the locations of D. silvarum were connected to the locations in northeastern China. In Mongolia, D. silvarum was recorded in the north region, surrounding the Khentii mountains, and in the southwest area, close to the Altai Mountains. In Kazakhstan, D. silvarum was documented in three locations, which also belong to the Altai Mountains (Figure S2).
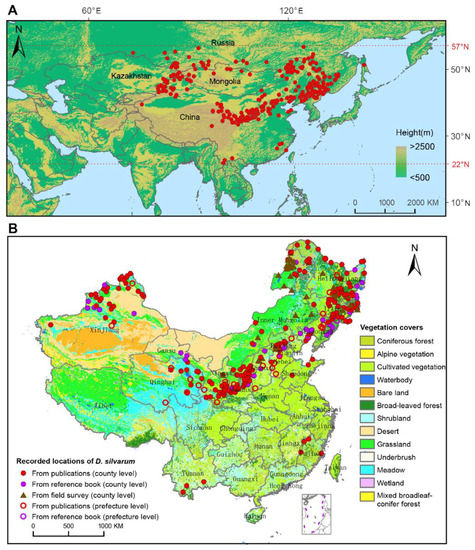
Figure 2.
Geographical distribution of Dermacentor silvarum. (A) Recorded locations of D. silvarum worldwide on an elevation map marked with red dots. It was found to be distributed only in Eurasia, ranging from 22° N to 57° N latitude. (B) Recorded locations of D. silvarum and vegetation coverage in China. Dots represent the county-level regions, and circles represent the prefecture-level regions.
Figure 2B shows the spatial distribution of D. silvarum and vegetation coverage in China. D. silvarum was detected in 11 provinces, three autonomous regions, and one municipality, presenting in 290 counties and 34 prefectures. D. silvarum was mostly reported in the north of China, but it can be found in southeast of China (Fujian province) and southwest of China (Yunnan province). The distribution of D. silvarum was usually close to mountain ranges, including Greater Khingan Mountains, Taihang Mountains, Qinling Mountains, Qilian Mountains, and Tianshan Mountains. In addition, D. silvarum prefers broad-leaved forest, coniferous forest, cultivated vegetation, and shrubland vegetations. The distribution of D. silvarum in different periods of years in China is shown in Figure S3. It reveals a permanent distribution in the north of China, especially in northeast China. Interestingly, before 2000, D. silvarum was sporadically documented in Guangze county, Fuqing County, Ninghua County, and Wuyi Mountain in Fujian Province, but disappeared in the record after 2000. In contrast, D. silvarum had never been reported in Yunnan province until 2019 [22]. However, we only collected D. silvarum in 6 provinces or autonomous regions, despite fieldwork in most parts of mainland China (Figure S4; Table S1). A total of 4268 D. silvarum were collected, most of which were collected in April and May, each year. About 40% of the D. silvarum were free adult ticks, and the hosts were mainly domestic animals, cattle, goat, and sheep.
3.3. Dermacentor Silvarum-Associated Pathogens
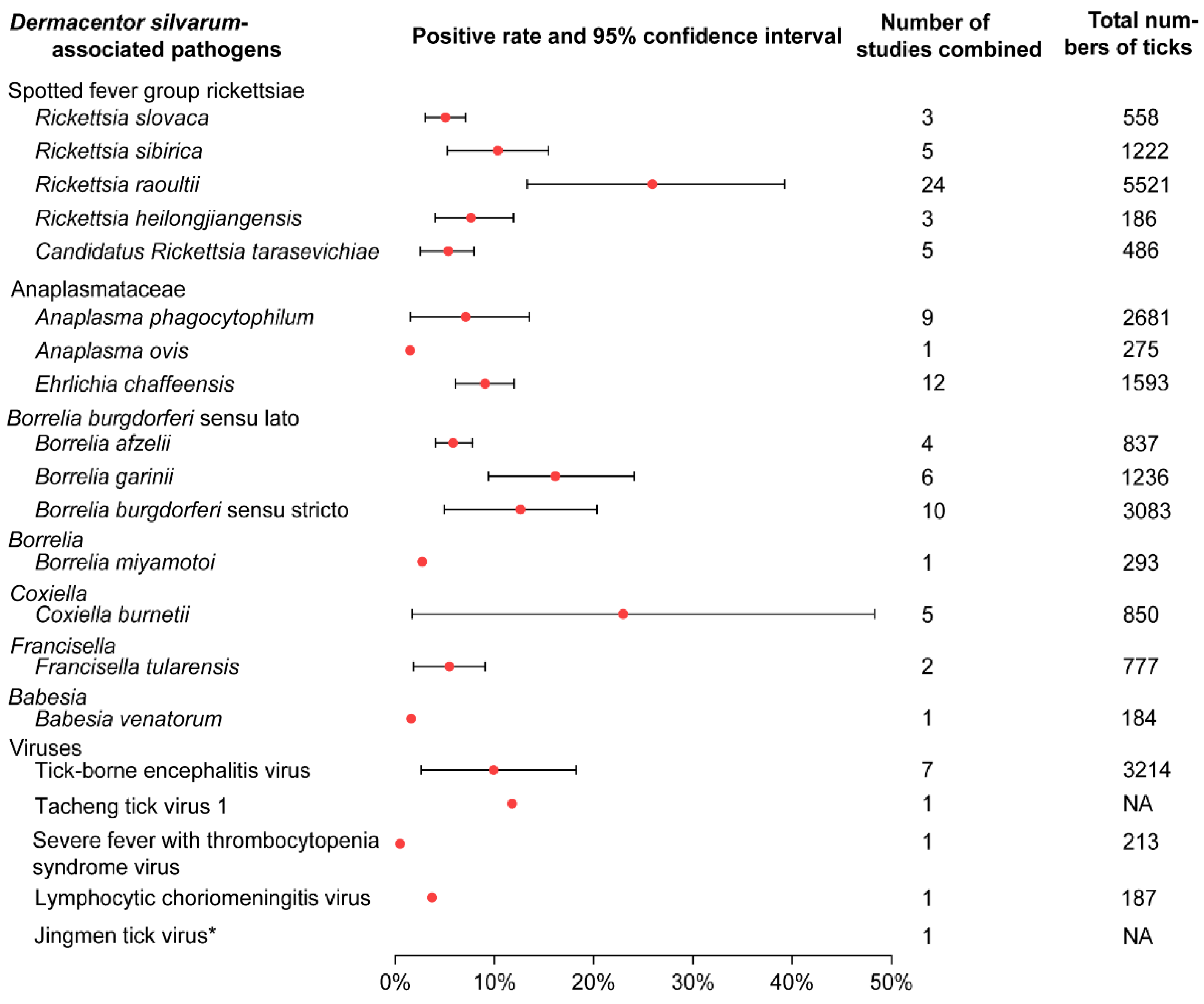
There were 20 human pathogens detected in D. silvarum (Figure 3; Table S2). Among them, five species were spotted fever group rickettsiae (SFGR). Rickettsia raoultii showed the highest pooled positive rate (25.15%; 95% CI 13.31–39.27), while Candidatus Rickettsia tarasevichiae showed the lowest (4.12%; 95% CI 1.08–7.15; Table S3). There were three species in the family of Anaplasmataceae, and the positive rate of E. chaffeensis was 9.03% (95% CI 6.04–12.01), relatively higher than A. phagocytophilum (6.14%; 95% CI 1.53–13.54) and A. ovis (1.5%). Three genospecies were detected in the complex Borrelia burgdorferi sensu lato (s.l.), among which the pooled positive rates of B. garinii and B. burgdorferi sensu stricto (s.s.) were 15.03% (95% CI 9.39–24.08) and 12.64% (95% CI 4.93–20.35), respectively. Five species of the viruses were detected in D. silvarum, while Tacheng tick virus 1 had the highest positive rate of 11.8%. Other pathogens D. silvarum harboured were Francisella tularensis, Babesia venatorum, Coxiella buenetii, and Borrelia miyamotoi, and the positive rates range from 1.6% to 19%. Additionally, animal pathogens, such as Bartonella, Hepatozoon, Theileria, and agents with unknown pathogenicity were also detected (Table S3; Figure S5).
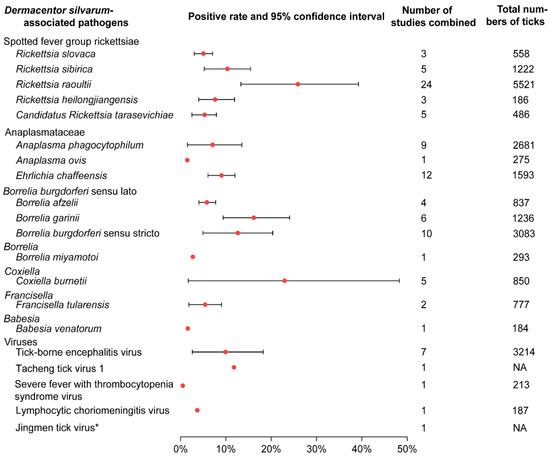
Figure 3.
Prevalence of pathogens associated with Dermacentor silvarum. If there was only one study included for a certain pathogen, CI was not calculated. CI: Confidence Interval. * indicates the virus detected by deep sequencing, and the original article did not give the specific number of tests and positive numbers. NA: no date.
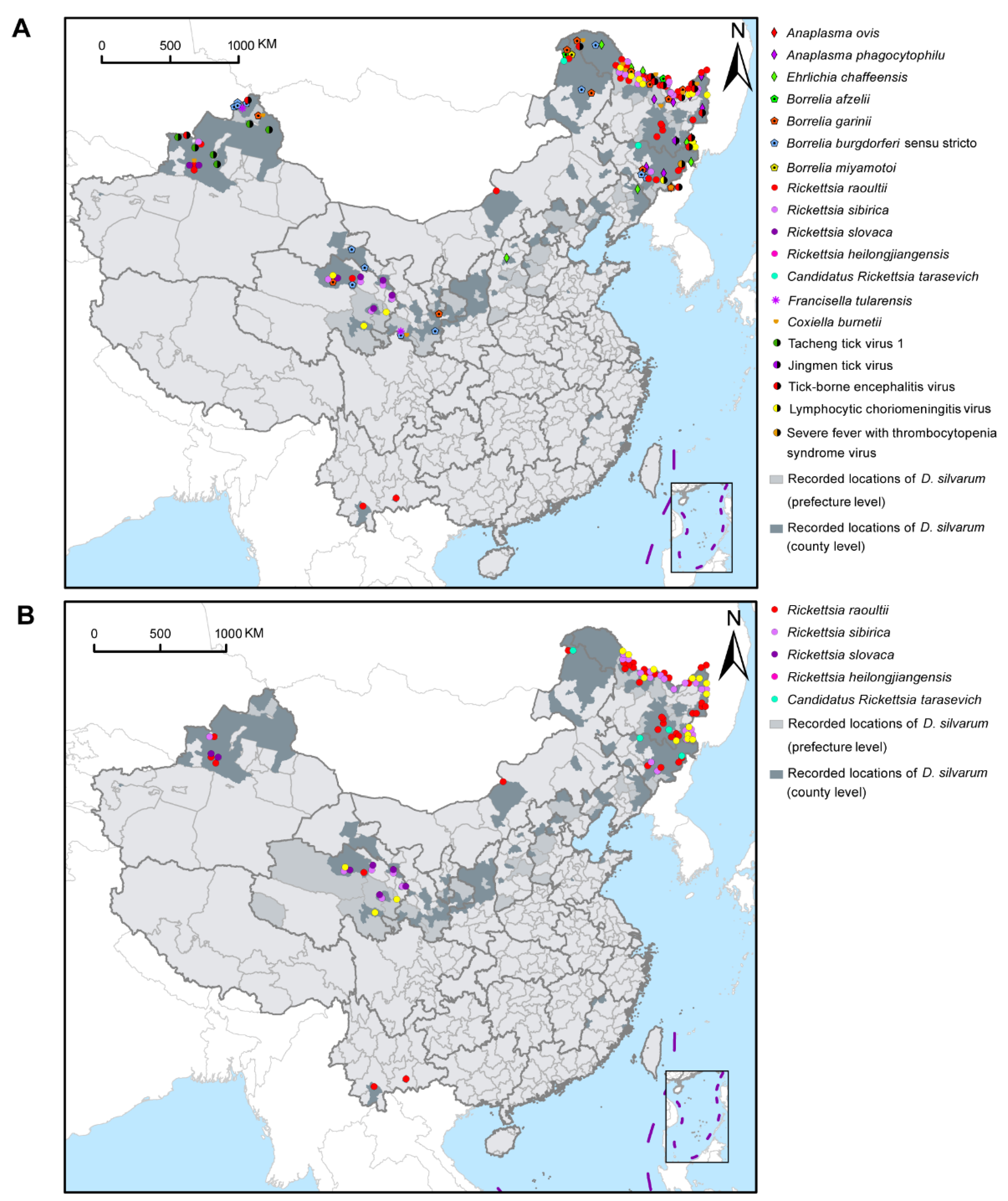
Figure 4A showed the geographical distribution of D. silvarum-associated human pathogens in China. Although D. silvarum was widely distributed in northern China, various pathogens were mainly reported in the northeast regions of China, including four species of SFGR, three species in the family Anaplasmataceae, three genospecies in the complex B. burgdorferi s.l., four species of the viruses, and Coxiella burnetii. Most species of SFGR and Borrelia were widely reported, especially R. raoultii, in northeast, central-north, northwest, and southwest areas of China (Figure 4B). Some other pathogens, however, were only detected in certain regions, for example, R. slovaca only reported in Xinjiang Uygur Autonomous Region and Qinghai province, Tacheng tick virus 1 in Xinjiang Uygur Autonomous Region, and Jingmen tick virus in Heilongjiang province, so far (Figure S6). In Russia, at least three human pathogens, including R. raoultii, Candidatus Rickettsia tarasevichiae, and tick-borne encephalitis virus, were sporadically distributed in the southern border region. Only Ba. caballi was reported in D. silvarum in Mongolia (Figure S7).
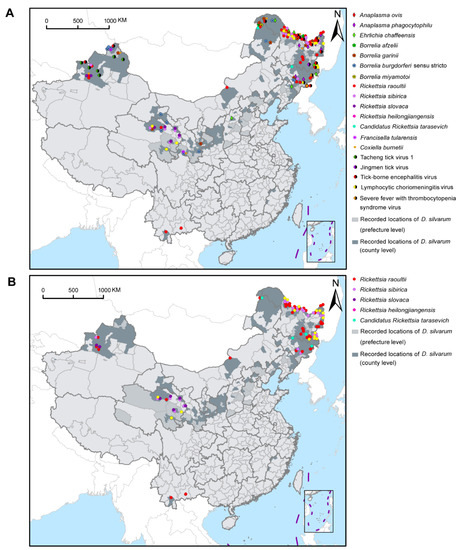
Figure 4.
Geographical distribution of pathogens with Dermacentor silvarum in China. (A) Distribution of 20 human pathogens presented in D. silvarum in China. (B) Geographical distribution of five SFGR species detected in D. silvarum in China. Rickettsia raoultii was the predominant SFGR found in D. silvarum and widely distributed. SFGR: spotted fever group rickettsiae.
4. Discussion
This study confirmed previous distribution data, updating information with more recent reports, as well as a field survey carried out in China during 2016–2019. Our results showed that D. silvarum were present in four countries of the world, and the locations were usually surrounded with mountains within the belt of 22–57° N latitude. At least 20 human pathogens were carried by D. silvarum, including various species of Rickettsia, Anaplasma, Borrelia, and viruses.
Although the presence of a pathogen in a vector does not prove its transmission capability, the vector competence of D. silvarum for R. raoultii and Tick-borne encephalitis virus (TBEV) have been proved [23,24], as well as R. sibirica and Ba. caballi [25,26]. R. raoultii, R. sibirica, and TBEV as the causative agents of tick-borne lymphadenopathy, Siberian tick typhus, and tick-borne encephalitis, respectively, can cause the patient fever, headache, and other symptoms [27]. Ba. caballi as the causative agent of equine piroplasmosis, can cause haemolytic anaemia and abortions [28]. These pathogens can be transmitted by D. silvarum and cause severe harm to human health, as well as economic loss to livestock production.
It is well known that the spatial distributions of ticks are influenced by many factors, including host availability, vegetation, ambient temperatures, and other abiotic and biotic environmental conditions [29].The life cycle of D. silvarum takes approximately one year. The egg hatching starts when the mean ambient temperature is higher than 16 °C, and is active from late February to early September under field conditions. Then, adults enter a behavioral diapause, which lasts until the following spring [30]. In our study, D. silvarum was mainly clustered in northern China and southern Russia, where most climate types are boreal (continental) climates, especially boreal climates with precipitation all year round and boreal climates with dry winters [19]. The oviposition of females and egg hatching was synchronized, irrespective of the month in which the females engorged to ensure that the emergence of eggs and larvae coincide with optimal conditions for development. In fact, D. silvarum is particularly well adapted to cold winter temperatures; the lowest temperature points (at which body fluids spontaneously freeze) of larvae and adults averaged −20.0 °C and −23.9 °C, respectively [31]. Moreover, unfed adults can enter a behavioral diapause, remaining quiescent under the leaf litter without actively questing for host, which can last about 9–10 months to over winter [30]. All of these may help it survive the cold boreal climates.
Figure 2B shows that the D. silvarum mainly reside in broad-leaved forest, coniferous forest, cultivated vegetation, and shrubland, most of which are in the high altitudinal regions. D. silvarum is a three-host tick species with a broad host range, involving domestic and wild animals. In our field survey and literature search, we found that the adults mainly feed on domestic goats, cattle, horses, and wild-boar, while the immature ticks prefer to infest a variety of small mammals, such as rodents, hares, and hedgehogs [11]. Obviously, there could be a correlation between these hosts and the habitats. In the high altitudes surrounding the mountains, it can provide a suitable environment and easy access to a blood meal.
Although, several species of ticks have been expanding their geographical ranges in recent decades, largely due to climate change [29,32], the distribution of D. silvarum does not seem to be significantly changed in China (Figure 2B; Figure S3). It is noteworthy that D. silvarum occurred sporadically in Fujian and Yunnan provinces, southeast and southwest China, respectively. In Fujian province, D. silvarum was documented in four locations next to the Wuyi mountains [33,34,35]. Moderate temperature, rich vegetation species, and suitable hosts could explain its existence. However, all records of D. silvarum in Fujian province were around 20 years ago, and further investigation should be performed. The appearance of D. silvarum in Yunnan province was possibly linked to birds’ migration, highlighting the possibility of D. silvarum transported on host and subsequently establishing in new areas. Liu et al. [22] collected 56 free adult D. silvarum in three locations of Yunnan province in 2019, where covering with forest and meadow steppe favor a range of wild and domestic animals and provide suitable habitats [36]. The report of D. silvarum in new areas may pose a significant public health problem. Further work on its genome sequence may be able to shed some light on where it came from and how it transported here. As our field survey in Fujian and Yunnan were conducted in September, the peak period might be missed [8].
We identified 38 agents carried by D. silvarum globally, among which at least 20 are pathogenic to humans. Most species of Rickettsia carried by D. silvarum are human pathogens. R. raoultii was the predominant Rickettsia found in D. silvarum of China and Russia, with wide distribution and high infection rate (25.15%; 95% CI 13.31–39.27). R. raoultii, as the causative agent of tick-borne lymphadenopathy or Dermacentor-borne necrosis erythema lymphadenopathy [37,38], could be maintained in Dermacentor ticks for 4–7 generations with a high level of transovarial and transstadial transmission [23]. So, D. silvarum may play a major role in transmission of R. raoultii to human, and a patient bitten by D. silvarum and with an inoculation eschar on the scalp or cervical lymphadenopathies should be suspected with R. raoultii infection. TBEV, with the positive rate of 8.85% (95% CI 2.62–18.26) in D. silvarum in China and Russia, can attack the human central nervous system, leading to tick-borne encephalitis [39]. Although Ixodes persulcatus and I. ricinus are the main TBEV vectors, D. silvarum has also been proven to be the vector [24]. Therefore, in northeast China and the far east of Russia more attention should be paid to tick-borne encephalitis surveillance and prevention.
Lots of viruses were detected by next-generation sequencing (NGS) in D. silvarum in recent years, such as Tacheng tick virus 1, Jingmen tick virus, Blacklegged tick phlebovirus, and Deer tick Mononegavirales-like virus [16,17,40]. Tacheng tick virus 1 as a causative pathogen, has been isolated from a patient with fever and identified in several species of ticks, near the residence. JMTV as a segmented RNA virus have been identified in arthropods and mammals [41,42,43], and isolated from the tick, Amblyomma javanense [16], showing a remarkable diversity and a global geographical distribution. All these data reveal their potential pathogenicity to humans. As a new detection method, NGS is more efficient than traditional methods, and is helpful to promote the discovery of new pathogens in D. silvarum and other tick species. Further laboratory studies are required to test the potential pathogenicity and vector capacities of some other unexplored pathogens detected in D. silvarum.
The study has following limitations: first, articles published in languages other than Chinese and English were not included, which may lead to some data omissions about D. silvarum in some regions, especially in Mongolia and Russia. Second, it must also be considered that the absence of reports in some areas may be caused by lack of collection effort rather than true absence. Third, D. silvarum and associated pathogens have been reported to be identified at the China-North Korea border. According to the prevailing climate, it can be assumed that D. silvarum is also endemic in North Korea, although there is no evidence for this in the literature so far. Finally, the accuracy of risk assessment based on published data might be limited by the varying sensitivities of detection methods in each study.
5. Conclusions
In summary, we showed that D. silvarum was found only in four countries in Eurasia, ranging from 22° N to 57° N latitude, and at least 20 human pathogens were associated with D. silvarum. Our work presents the most comprehensive data and analysis (to our knowledge) for the geographical distribution of D. silvarum and its associated pathogens, some of which can cause human and animal diseases, and some are new pathogens, revealing an emerging threat to public health and stocking farming. It should be noted that some Russian papers might be missed in this study using our approach. Further surveillance and investigation should be strengthened in areas where D. silvarum and associated pathogens are presented.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph18094430/s1, Figure S1: Dermacentor silvarum, Figure S2: Geographical distribution of Dermacentor silvarum in Russia, Mongolia, and Kazakhstan, Figure S3: Recorded locations of Dermacentor silvarum in different periods of years in China, Figure S4: Map of field survey sites in China from 2016 to 2019, Figure S5: Meta-analysis of positive rate of each Dermacentor silvarum-associated agent, Figure S6: Geographical distribution of each Dermacentor silvarum-associated agent in China, Figure S7: Geographical distribution of Dermacentor silvarum-associated agents in Russia and Mongolia, Table S1: The dataset, Table S2: References of each agent for meta-analysis, Table S3: Prevalence and pathogenicity of Dermacentor silvarum-associated agents.
Author Contributions
Data curation, W.-B.G., Q.W., X.-M.C., Y.-H.Z., Y.-S.P., B.-G.J., Q.-C.C., Y.S., J.-F.J., and J.-X.C.; software, J.-T.W.; writing—original draft preparation, W.-B.G.; writing—review and editing, N.J. and W.-Q.S.; supervision, N.J. and W.-C.C.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (grant number 81621005).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Academy of Military Medical Sciences (Permit Number: 2016YFC1201902).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We acknowledge Hong-Ze Shao (Animal Husbandry and Veterinary Science Research Institute of Jilin Province, Changchun, China) and Jin-Guo Zhu (ManZhouLi Customs District, Inner Mongolia, China) for their support on filed survey.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fang, L.Q.; Liu, K.; Li, X.L.; Liang, S.; Yang, Y.; Yao, H.W.; Sun, R.X.; Sun, Y.; Chen, W.J.; Zuo, S.Q.; et al. Emerging tick-borne infections in mainland China: An increasing public health threat. Lancet Infect. Dis. 2015, 15, 1467–1479. [Google Scholar] [CrossRef]
- Grzeszczuk, A.; Stańczak, J.; Kubica-Biernat, B. Serological and molecular evidence of human granulocytic ehrlichiosis focus in the Białowieza Primeval Forest (Puszcza Białowieska), northeastern Poland. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2002, 21, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Selinger, M.; Wilkie, G.; Tong, L.; Gu, Q.; Schnettler, E.; Grubhoffer, L.; Kohl, A. Analysis of tick-borne encephalitis virus-induced host responses in human cells of neuronal origin and interferon-mediated protection. J. Gen. Virol. 2017, 98, 2043–2060. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.; Leach, S. Effect of climate change on vector-borne disease risk in the UK. Lancet Infect. Dis. 2015, 15, 721–730. [Google Scholar] [CrossRef]
- Parola, P.; Paddock, C.; Raoult, D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin. Microbiol. Rev. 2005, 18, 719–756. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; He, B.; Huang, S.; Wei, F.; Zhu, X. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect. Dis. 2014, 14, 763–772. [Google Scholar] [CrossRef]
- Kulik, I.; Vinokurova, N. Range of the tick Dermacentor silvarum in the USSR. Meditsinskaia Parazitol. Parazit. Bolezn. 1983, 52, 23–28. [Google Scholar]
- Yu, Z.; Zheng, H.; Yang, X.; Chen, Z.; Wang, D.; Hao, M.; Yang, Y.; Liu, J. Seasonal abundance and activity of the tick Dermacentor silvarum in northern China. Med. Vet. Entomol. 2011, 25, 25–31. [Google Scholar] [CrossRef]
- Kiefer, D.; Pfister, K.; Tserennorov, D.; Bolormaa, G.; Otgonbaatar, D.; Samjaa, R.; Burmeister, E.G.; Kiefer, M.S. Current state of Ixodidae research in Mongolia. Explor. Biol. Resour. Mong. 2010, 11, 405–418. [Google Scholar]
- Deng, G.F.; Jiang, Z.J. Economic Insect Fauna of China, Acari: Ixidae; Science Press: Beijing, China, 1992; Volume 39, p. 52. [Google Scholar]
- Liu, J.; Liu, Z.; Zhang, Y.; Yang, X.; Gao, Z. Biology of Dermacentor silvarum (Acari: Ixodidae) under laboratory conditions. Exp. Appl. Acarol. 2005, 36, 131–138. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, B.; Yu, J.; Zhang, W.; Gao, H.; Zhan, L.; Sun, Y.; Zhang, X.; Zhang, P.; Liu, W.; et al. Anaplasma phagocytophilum infection in ticks, China-Russia border. Emerg. Infect. Dis. 2011, 17, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, L.; Vapalahti, O. Tick-borne encephalitis. Lancet Lond. Engl. 2008, 371, 1861–1871. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, G.; Shen, H.; Xie, J.; Luo, J.; Tian, M. First report on the occurrence of Rickettsia slovaca and Rickettsia raoultii in Dermacentor silvarum in China. Parasites Vectors 2012, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Cao, W.; Pan, H. Ehrlichiae and ehrlichial diseases in china. Ann. N.Y. Acad. Sci. 2003, 990, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Liu, H.; Ni, X.; Bell-Sakyi, L.; Zheng, Y.; Song, J.; Li, J.; Jiang, B.; Wang, Q.; Sun, Y.; et al. Emergence of human infection with Jingmen tick virus in China: A retrospective study. EBioMedicine 2019, 43, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, X.; Wang, Z.; Dong, Z.; Xie, S.; Jiang, M.; Song, R.; Ma, J.; Chen, S.; Chen, K.; et al. A Tentative Tamdy Orthonairovirus related to Febrile Illness in Northwestern China. Clin. Infect. Dis. 2020, 70, 2155–2160. [Google Scholar] [CrossRef]
- Zhang, G.; Zheng, D.; Tian, Y.; Li, S. A dataset of distribution and diversity of ticks in China. Sci. Data 2019, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Rubel, F.; Brugger, K.; Belova, O.A.; Kholodilov, I.S.; Didyk, Y.M.; Kurzrock, L.; Garcia-Perez, A.L.; Kahl, O. Vectors of disease at the northern distribution limit of the genus Dermacentor in Eurasia: D. reticulatus and D. silvarum. Exp. Appl. Acarol. 2020, 82, 95–123. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, R.M. Fauna Sinica-Arachnida Ixodida; Science Press: Beijing, China, in press.
- Borenstein, M.; Hedges, L.; Higgins, J.; Rothstein, H. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- Liu, H.; Liang, X.; Wang, H.; Sun, X.; Bai, X.; Hu, B.; Shi, N.; Wang, N.; Zhang, X.; Huang, L.; et al. Molecular evidence of the spotted fever group Rickettsiae in ticks from Yunnan Province, Southwest China. Exp. Appl. Acarol. 2020, 80, 339–348. [Google Scholar] [CrossRef]
- Samoylenko, I.; Shpynov, S.; Raoult, D.; Rudakov, N.; Fournier, P. Evaluation of Dermacentor species naturally infected with Rickettsia raoultii. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2009, 305–306. [Google Scholar] [CrossRef]
- Lu, Z.; Bröker, M.; Liang, G. Tick-borne encephalitis in mainland China. Vector Borne Zoonotic Dis. Larchmt. N.Y. 2008, 8, 713–720. [Google Scholar] [CrossRef]
- Podboronov, V.M.; Pchelkina, A.A. Characteristics of the transphase and transovarial transmission of Rickettsia sibirica by ixodid and argasid ticks (in Russian). Med. Parazitol. Mosk 1989, 4, 8–14. [Google Scholar]
- Ristic, M. Transmission of Babesia. In Babesiosis of Domestic Animals and Man; CRC Press: Boca Raton, MA, USA, 1988; pp. 23–52. [Google Scholar]
- Socolovschi, C.; Parola, P.; Raoult, D. Tick-Borne Spotted Fever Rickettsioses. In Hunter’s Tropical Medicine and Emerging Infectious Disease, 9th ed.; Magill, A.J., Hill, D.R., Solomon, T., Ryan, E.T., Eds.; W.B. Saunders: London, UK, 2013; Volume 64, pp. 546–552. [Google Scholar]
- Love, S.; Mair, T.S. Infectious Diseases and Parasitology. In Equine Medicine, Surgery and Reproduction, 9th ed.; Mair, T.S., Love, S., Schumacher, J., Smith, R.K.W., Frazer, G., Eds.; W.B. Saunders: Oxford, UK, 2012; Volume 19, pp. 399–422. [Google Scholar]
- Sonenshine, D. Range Expansion of Tick Disease Vectors in North America: Implications for Spread of Tick-Borne Disease. Int. J. Env. Res. Public Health 2018, 15, 478. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zheng, H.; Chen, Z.; Zheng, B.; Ma, H.; Liu, J. The life cycle and biological characteristics of Dermacentor silvarum Olenev (Acari: Ixodidae) under field conditions. Vet. Parasitol. 2010, 168, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, X.; Jia, Q.; Dong, N.; Wang, H.; Hu, Y.; Yu, Z.; Liu, J. Cold tolerance and biochemical response of unfed Dermacentor silvarum ticks to low temperature. Ticks Tick Borne Dis. 2017, 8, 757–763. [Google Scholar] [CrossRef]
- Gray, J.S.; Dautel, H.; Estrada-Peña, A.; Kahl, O.; Lindgren, E. Effects of climate change on ticks and tick-borne diseases in europe. Interdiscip. Perspect. Infect. Dis. 2009, 2009, 593232. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Huang, Y.P.; Zheng, H.N.; Liu, J.Y.; Wu, H.S.; Liu, C.C.; Zheng, X.Y. Investigation of ticks and Tabanidae distribution and their carrier status in Lyme disease epidemic area of Fujian Province (in Chinese). Chin. J. Vector Biol. Control 1993, 2, 58. [Google Scholar]
- Liao, H.R.; Yu, X. Investigation of ticks in Fujian (in Chinese). Endem. Dis. Bull. 1995, 10, 50–52. [Google Scholar]
- Chen, Z.G.; Xu, R.M.; Pan, L.; Lin, H.; Chen, M.; Bi, D.Z.; Zhang, M.Y.; Zhong, J.P. Study on the Host Animals and Transmission Vectors of SFGR in Ninghua County, Fujian (in Chinese). Strait J. Prev. Med. 1999, 2, 3–5. [Google Scholar]
- Du, C.; Liu, H.; Wei, R.; Jongejan, F.; Gao, Z.; Shao, Z.; Duan, X.; Jiang, B.; Liu, Y.; Jiang, J.; et al. Investigation on Ehrlichia Infection in Small Mammals and Ticks from Tengchong, Yunnan Province, Southern China. Vector Borne Zoonotic Dis. 2018, 18, 563–566. [Google Scholar] [CrossRef]
- Mediannikov, O.; Matsumoto, K.; Samoylenko, I.; Drancourt, M.; Roux, V.; Rydkina, E.; Davoust, B.; Tarasevich, I.; Brouqui, P.; Fournier, P. Rickettsia raoultii sp. nov., a spotted fever group rickettsia associated with Dermacentor ticks in Europe and Russia. Int. J. Syst. Evol. Microbiol. 2008, 58, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Rovery, C.; Rolain, J.; Brouqui, P.; Davoust, B.; Raoult, D. Rickettsia slovaca and R. raoultii in tick-borne Rickettsioses. Emerging Infect. Dis. 2009, 15, 1105–1108. [Google Scholar] [CrossRef] [PubMed]
- Kollaritsch, H.; Paulke-Korinek, M.; Holzmann, H.; Hombach, J.; Bjorvatn, B.; Barrett, A. Vaccines and vaccination against tick-borne encephalitis. Expert Rev. Vaccines 2012, 11, 1103–1119. [Google Scholar] [CrossRef]
- Meng, F.; Ding, M.; Tan, Z.; Zhao, Z.; Xu, L.; Wu, J.; He, B.; Tu, C. Virome analysis of tick-borne viruses in Heilongjiang Province, China. Ticks Tick Borne Dis. 2019, 10, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Ladner, J.; Wiley, M.; Beitzel, B.; Auguste, A.; Dupuis, A.; Lindquist, M.; Sibley, S.; Kota, K.; Fetterer, D.; Eastwood, G.; et al. A Multicomponent Animal Virus Isolated from Mosquitoes. Cell Host Microbe 2016, 20, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Sameroff, S.; Tokarz, R.; Charles, R.; Jain, K.; Oleynik, A.; Che, X.; Georges, K.; Carrington, C.; Lipkin, W.; Oura, C. Viral Diversity of Tick Species Parasitizing Cattle and Dogs in Trinidad and Tobago. Sci. Rep. 2019, 9, 10421. [Google Scholar] [CrossRef] [PubMed]
- Temmam, S.; Bigot, T.; Chrétien, D.; Gondard, M.; Pérot, P.; Pommelet, V.; Dufour, E.; Petres, S.; Devillers, E.; Hoem, T.; et al. Insights into the Host Range, Genetic Diversity, and Geographical Distribution of Jingmenviruses. mSphere 2019, 4. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).