Contribution of Plantar Fascia and Intrinsic Foot Muscles in a Single-Leg Drop Landing and Repetitive Rebound Jumps: An Ultrasound-Based Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. B-Mode Ultrasonography and Real-Time Tissue Elastography
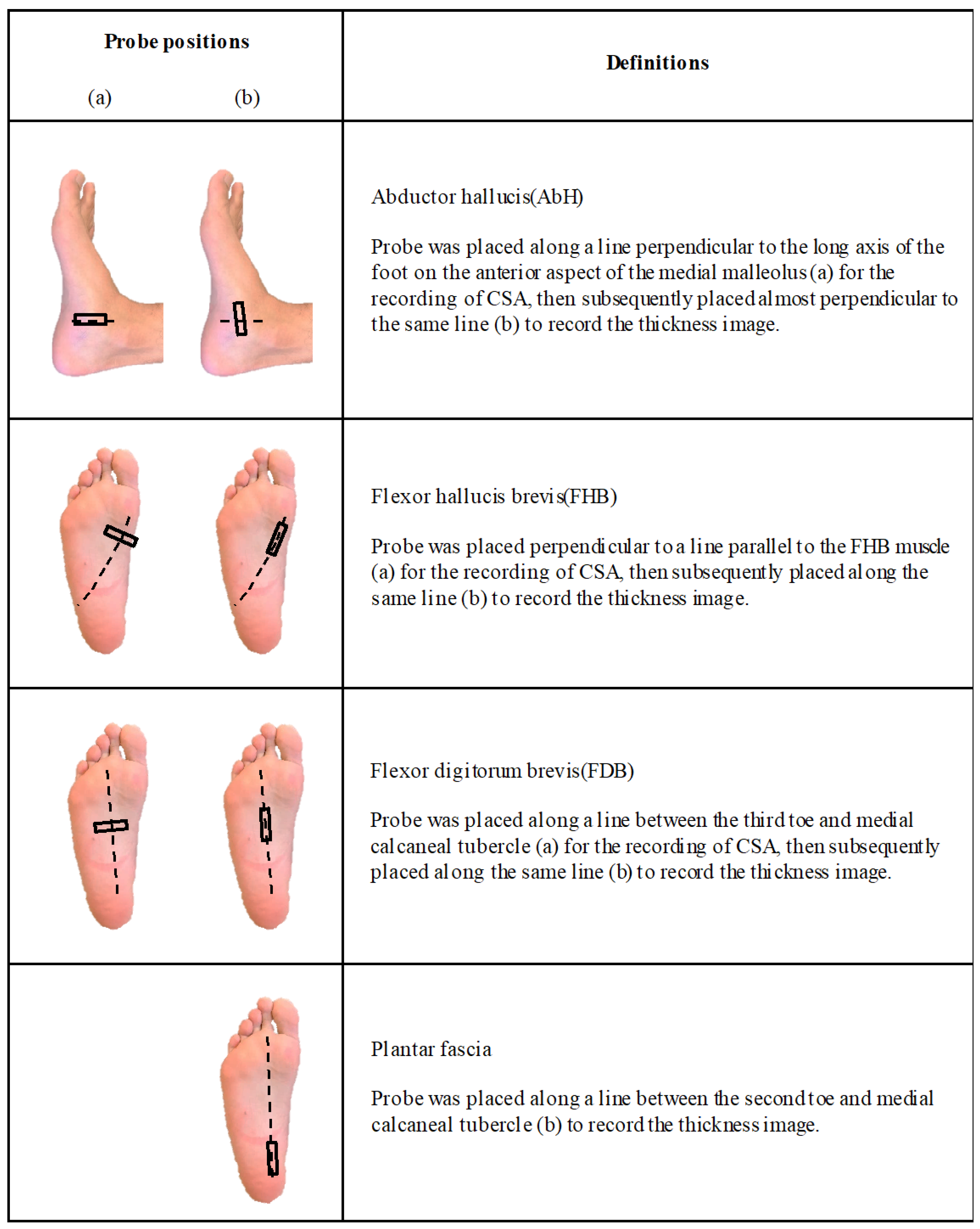
2.2. Evaluation of Thickness and CSA
- Thickness of the abductor hallucis (AbH), flexor hallucis brevis (FHB), and flexor digitorum brevis (FDB) muscles;
- CSA of AbH, FHB, and FDB, and;
- Thickness of the plantar fascia in the calcaneus.
2.3. Evaluation of IFM and Plantar Fascia Hardness
2.4. Single-Leg Drop Landing Test
2.5. Repetitive Rebound Jump Test
2.6. Data Collection
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKeon, P.O.; Hertel, J.; Bramble, D.; Davis, I. The foot core system: A new paradigm for understanding intrinsic foot muscle function. Br. J. Sports Med. 2015, 49, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, L.A.; Cresswell, A.G.; Farris, D.J. The energetic behaviour of the human foot across a range of running speeds. Sci. Rep. 2018, 8, 10576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, L.A.; Cresswell, A.G.; Racinais, S.; Whiteley, R.; Lichtwark, G. Intrinsic foot muscles have the capacity to control deformation of the longitudinal arch. J. R. Soc. Interface 2014, 11, 20131188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, L.A.; Lichtwark, G.; Cresswell, A.G. Active regulation of longitudinal arch compression and recoil during walking and running. J. R. Soc. Interface 2015, 12, 20141076. [Google Scholar] [CrossRef] [Green Version]
- Pedley, J.S.; Lloyd, R.S.; Read, P.J.; Moore, I.S.; Croix, M.D.S.; Myer, G.D.; Oliver, J.L. Utility of Kinetic and Kinematic Jumping and Landing Variables as Predictors of Injury Risk: A Systematic Review. J. Sports Sci. Med. 2020, 2, 287–304. [Google Scholar] [CrossRef]
- Kipp, K.; Kiely, M.T.; Giordanelli, M.D.; Malloy, P.J.; Geiser, C.F. Biomechanical Determinants of the Reactive Strength Index During Drop Jumps. Int. J. Sports Physiol. Perform. 2018, 13, 44–49. [Google Scholar] [CrossRef]
- Vázquez-Guerrero, J.; Jones, B.; Fernández-Valdés, B.; Moras, G.; Reche, X.; Sampaio, J. Physical demands of elite basketball during an official U18 international tournament. J. Sports Sci. 2019, 37, 2530–2537. [Google Scholar] [CrossRef]
- Okamura, K.; Fukuda, K.; Oki, S.; Ono, T.; Tanaka, S.; Kanai, S. Effects of plantar intrinsic foot muscle strengthening exercise on static and dynamic foot kinematics: A pilot randomized controlled single-blind trial in individuals with pes planus. Gait Posture 2020, 75, 40–45. [Google Scholar] [CrossRef]
- Welte, L.; Kelly, L.A.; Kessler, S.E.; Lieberman, D.E.; D’Andrea, S.E.; Lichtwark, G.A.; Rainbow, M.J. The extensibility of the plantar fascia influences the windlass mechanism during human running. Proc. Biol. Sci. 2021, 288, 20202095. [Google Scholar] [CrossRef]
- Zhang, X.; Schütte, K.H.; Vanwanseele, B. Foot muscle morphology is related to center of pressure sway and control mechanisms during single-leg standing. Gait Posture 2017, 57, 52–56. [Google Scholar] [CrossRef]
- Maeda, N.; Hirota, A.; Komiya, M.; Morikawa, M.; Mizuta, R.; Fujishita, H.; Nishikawa, Y.; Kobayashi, T.; Urabe, Y. Intrinsic foot muscle hardness is related to dynamic postural stability after landing in healthy men. Gait Posture 2021, 86, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.S.; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Wolters Kluwer/Lippincott Williams & Wilkins Health: Philadelphia, PA, USA, 2014. [Google Scholar]
- Tas, S.; Unluer, N.O.; Cetin, A. Thickness, cross-sectional area, and stiffness of intrinsic foot muscles affect performance in single-leg stance balance tests in healthy sedentary young females. J. Biomech. 2020, 99, 109530. [Google Scholar] [CrossRef] [PubMed]
- Crofts, G.; Angin, S.; Mickle, K.J.; Hill, S.; Nester, C.J. Reliability of ultrasound for measurement of selected foot structures. Gait Posture 2014, 39, 35–39. [Google Scholar] [CrossRef]
- Angin, S.; Mickle, K.J.; Nester, C.J. Contributions of foot muscles and plantar fascia morphology to foot posture. Gait Posture 2018, 61, 238–242. [Google Scholar] [CrossRef]
- Yanagisawa, O.; Sakuma, J.; Kawakami, Y.; Suzuki, K.; Fukubayashi, T. Effect of exercise-induced muscle damage on muscle hardness evaluated by ultrasound real-time tissue elastography. Springerplus 2015, 4, 308. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, O.; Niitsu, M.; Kurihara, T.; Fukubayashi, T. Evaluation of human muscle hardness after dynamic exercise with ultrasound real-time tissue elastography: A feasibility study. Clin. Radiol. 2011, 66, 815–819. [Google Scholar] [CrossRef]
- Comyns, T.M.; Flanagan, E.P.; Fleming, S.; Fitzgerald, E.; Harper, D.J. Interday Reliability and Usefulness of a Reactive Strength Index Derived From 2 Maximal Rebound Jump Tests. Int. J. Sports Physiol. Perform. 2019, 14, 1–17. [Google Scholar] [CrossRef]
- Pfeiffer, S.J.; Blackburn, J.T.; Luc-Harkey, B.; Harkey, M.S.; Stanley, L.E.; Frank, B.; Padua, D.; Marshall, S.W.; Spang, J.T.; Pietrosimone, B. Peak knee biomechanics and limb symmetry following unilateral anterior cruciate ligament reconstruction: Associations of walking gait and jump-landing outcomes. Clin. Biomech. 2018, 53, 79–85. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Olsen, M.T.; Bruening, D.A.; Johnson, A.W.; Ridge, S.T. The Role of the Midfoot in Drop Landings. Med. Sci. Sports Exerc. 2019, 51, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Martin, B.F. Observations on the muscles and tendons of the medial aspect of the sole of the foot. J. Anat. 1964, 98, 437–453. [Google Scholar] [PubMed]
- Cheng, H.Y.; Lin, C.L.; Wang, H.W.; Chou, S.W. Finite element analysis of plantar fascia under stretch-the relative contribution of windlass mechanism and Achilles tendon force. J. Biomech. 2008, 41, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Morita, N.; Yamauchi, J.; Kurihara, T.; Fukuoka, R.; Otsuka, M.; Okuda, T.; Ishizawa, N.; Nakajima, T.; Nakamichi, R.; Matsuno, S.; et al. Toe flexor strength and foot arch height in children. Med. Sci. Sports Exerc. 2015, 47, 350–356. [Google Scholar] [CrossRef]
- Kura, H.; Luo, Z.P.; Kitaoka, H.B.; An, K.N. Quantitative analysis of the intrinsic muscles of the foot. Anat. Rec. 1997, 249, 143–151. [Google Scholar] [CrossRef]
- Rubio-Peirotén, A.; García-Pinillos, F.; Jaén-Carrillo, D.; Cartón-Llorente, A.; Roche-Seruendo, L.E. Is there a relationship between the morphology of connective tissue and reactivity during a drop jump? Influence of sex and athletic performance level. Int. J. Environ. Res. Public Health 2021, 18, 1969. [Google Scholar] [CrossRef]
- Latey, P.J.; Burns, J.; Nightingale, E.J.; Clarke, J.L.; Hiller, C.E. Reliability and correlates of cross-sectional area of abductor hallucis and the medial belly of the flexor hallucis brevis measured by ultrasound. J. Foot Ankle Res. 2018, 11, 28. [Google Scholar] [CrossRef] [Green Version]
- Venkadesan, M.; Yawar, A.; Eng, C.M.; Dias, M.A.; Singh, D.K.; Tommasini, S.M.; Haims, A.H.; Bandi, M.M.; Mandre, S. Stiffness of the human foot and evolution of the transverse arch. Nature 2020, 579, 97–100. [Google Scholar] [CrossRef] [Green Version]
- Pascual Huerta, J. The effect of the gastrocnemius on the plantar fascia. Foot Ankle Clin. 2014, 19, 701–718. [Google Scholar] [CrossRef]
- Granado, M.J.; Lohman, E.B., 3rd; Daher, N.S.; Gordon, K.E. Effect of gender, toe extension position, and plantar fasciitis on plantar fascia thickness. Foot Ankle Int. 2019, 40, 439–446. [Google Scholar] [CrossRef]

| Parameters | Median (Interquartile Range) | Mean (Standard Deviation) | |
|---|---|---|---|
| Age | (years) | 23.0 (22.0–24.5) | 23.3 ± 1.9 |
| Body height | (cm) | 169.0 · (164.5–173.0) | 169.8 ± 0.8 |
| Body weight | (kg) | 60.0 · (54.0–65.5) | 59.9 ± 9.0 |
| BMI | (kg/m2) | 20.3 · (19.0–23.1) | 20.7 ± 2.2 |
| Foot length | (cm) | 24.7 · (24.1–25.7) | 24.8 ± 1.4 |
| Thickness of selected tissues (mm) | |||
| Abductor hallucis | 11.0 · (9.5–12.4) | 11.1 ± 1.9 | |
| Flexor hallucis brevis | 12.2 · (9.6–13.5) | 11.4 ± 2.4 | |
| Flexor digitorum brevis | 9.6 · (6.3–11.3) | 8.8 ± 2.7 | |
| Plantar fascia | 3.0 · (2.9–3.2) | 3.0 ± 0.4 | |
| Cross-sectional area of selected tissues (mm2) | |||
| Abductor hallucis | 261.3 · (179.0–285.8) | 245.0 ± 66.2 | |
| Flexor hallucis brevis | 236.2 · (220.8–273.7) | 244.8 ± 39.0 | |
| Flexor digitorum brevis | 204.0 · (175.2–259.2) | 214.3 ± 50.4 | |
| Ultrasound elastography of selected tissues (muscle/coupler) | |||
| Abductor hallucis | 1.20 · (0.65–1.95) | 1.28 ± 0.76 | |
| Flexor hallucis brevis | 1.60 · (1.30–2.21) | 1.95 ± 1.03 | |
| Flexor digitorum brevis | 2.60 · (1.70–3.70) | 2.70 ± 1.24 | |
| Plantar fascia | 0.30 · (0.20–0.55) | 0.40 ± 0.29 | |
| Drop landing test | |||
| COP length | (%foot length) | 24.6 · (21.6–27.3) | 25.4 ± 4.8 |
| VGRFmax | (%BW) | 439.2 · (398.0–540.8) | 458.4 ± 90.6 |
| Peak time | (ms) | 54.0 · (48.8–62.8) | 57.2 ± 10.4 |
| Loading rate | (%BW/s) | 7.7 · (6.5–10.1) | 8.3 ± 2.5 |
| Repetitive rebound jump test | |||
| Contact time | (s) | 0.30 · (0.27–0.33) | 0.30 ± 0.05 |
| Jump height | (cm) | 12.9 · (10.8–16.3) | 13.5 ± 3.5 |
| Reactive jump index | (cm/s) | 45.6 · (34.9–53.4) | 46.0 ± 15.1 |
| Variables | Drop Landing Test | Repetitive Rebound Jump Test | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COP Length | VGRF Max | Time to Peak | Loading Rate | Contact Time | Jump Height | Reactive Jump Index | |||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | ||
| Thickness of selected tissues | (mm) | ||||||||||||||
| Abductor hallucis a | 0.030 | 0.897 | −0.043 | 0.854 | 0.093 | 0.687 | −0.059 | 0.800 | −0.230 | 0.316 | 0.215 | 0.349 | 0.212 | 0.357 | |
| Flexor hallucis brevis a | −0.229 | 0.319 | −0.055 | 0.811 | −0.166 | 0.472 | 0.137 | 0.555 | −0.050 | 0.830 | 0.253 | 0.269 | 0.194 | 0.399 | |
| Flexor digitorum brevis b | −0.311 | 0.169 | −0.211 | 0.360 | 0.205 | 0.373 | −0.216 | 0.346 | 0.104 | 0.654 | 0.360 | 0.109 | 0.311 | 0.169 | |
| Plantar fascia of calcaneal portion a | −0.513 * | 0.018 | 0.051 | 0.825 | 0.028 | 0.905 | 0.083 | 0.721 | −0.425 | 0.055 | 0.615 ** | 0.003 | 0.645 ** | 0.002 | |
| Cross-sectional area of selected tissues | (mm2) | ||||||||||||||
| Abductor hallucis a | −0.055 | 0.814 | −0.051 | 0.825 | 0.358 | 0.111 | −0.176 | 0.447 | −0.478 * | 0.028 | 0.258 | 0.260 | 0.376 | 0.093 | |
| Flexor hallucis brevis a | −0.163 | 0.481 | −0.472 * | 0.031 | 0.200 | 0.384 | −0.339 | 0.132 | 0.117 | 0.613 | −0.066 | 0.776 | −0.059 | 0.801 | |
| Flexor digitorum brevis a | −0.291 | 0.201 | −0.279 | 0.221 | 0.278 | 0.222 | −0.351 | 0.119 | 0.088 | 0.706 | 0.333 | 0.141 | 0.240 | 0.294 | |
| Ultrasound elastography of selected tissues | (tissue/ coupler) | ||||||||||||||
| Abductor hallucis a | 0.012 | 0.957 | −0.054 | 0.815 | −0.159 | 0.491 | −0.011 | 0.962 | 0.159 | 0.491 | 0.063 | 0.786 | 0.008 | 0.971 | |
| Flexor hallucis brevis b | −0.023 | 0.920 | −0.017 | 0.942 | −0.147 | 0.526 | 0.032 | 0.891 | −0.259 | 0.257 | 0.190 | 0.410 | 0.227 | 0.322 | |
| Flexor digitorum brevis a | 0.004 | 0.987 | 0.292 | 0.199 | −0.220 | 0.338 | 0.267 | 0.242 | −0.194 | 0.400 | 0.073 | 0.753 | 0.153 | 0.507 | |
| Plantar fascia b | 0.219 | 0.341 | −0.071 | 0.760 | −0.150 | 0.515 | −0.028 | 0.903 | −0.092 | 0.692 | −0.299 | 0.187 | −0.211 | 0.358 | |
| Variables | Contact Time | Reactive Jump Index | ||||||
|---|---|---|---|---|---|---|---|---|
| β | 95% CI interval | p | β | 95% CI interval | p | |||
| Lower | Upper | Lower | Upper | |||||
| CSA of abductor hallucis | −0.369 | −0.001 | 0.000 | 0.101 | 0.152 | −0.057 | 0.126 | 0.437 |
| Thickness of plantar facia | −0.284 | −0.086 | 0.019 | 0.200 | 0.587 | 7.219 | 38.743 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morikawa, M.; Maeda, N.; Komiya, M.; Hirota, A.; Mizuta, R.; Kobayashi, T.; Kaneda, K.; Nishikawa, Y.; Urabe, Y. Contribution of Plantar Fascia and Intrinsic Foot Muscles in a Single-Leg Drop Landing and Repetitive Rebound Jumps: An Ultrasound-Based Study. Int. J. Environ. Res. Public Health 2021, 18, 4511. https://doi.org/10.3390/ijerph18094511
Morikawa M, Maeda N, Komiya M, Hirota A, Mizuta R, Kobayashi T, Kaneda K, Nishikawa Y, Urabe Y. Contribution of Plantar Fascia and Intrinsic Foot Muscles in a Single-Leg Drop Landing and Repetitive Rebound Jumps: An Ultrasound-Based Study. International Journal of Environmental Research and Public Health. 2021; 18(9):4511. https://doi.org/10.3390/ijerph18094511
Chicago/Turabian StyleMorikawa, Masanori, Noriaki Maeda, Makoto Komiya, Arisu Hirota, Rami Mizuta, Toshiki Kobayashi, Kazuki Kaneda, Yuichi Nishikawa, and Yukio Urabe. 2021. "Contribution of Plantar Fascia and Intrinsic Foot Muscles in a Single-Leg Drop Landing and Repetitive Rebound Jumps: An Ultrasound-Based Study" International Journal of Environmental Research and Public Health 18, no. 9: 4511. https://doi.org/10.3390/ijerph18094511
APA StyleMorikawa, M., Maeda, N., Komiya, M., Hirota, A., Mizuta, R., Kobayashi, T., Kaneda, K., Nishikawa, Y., & Urabe, Y. (2021). Contribution of Plantar Fascia and Intrinsic Foot Muscles in a Single-Leg Drop Landing and Repetitive Rebound Jumps: An Ultrasound-Based Study. International Journal of Environmental Research and Public Health, 18(9), 4511. https://doi.org/10.3390/ijerph18094511







