Attitude and Purchase Intention to Generic Drugs
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar]
- Smith, R.D.; Correa, C.; Oh, C. Trade, TRIPS, and pharmaceuticals. Lancet 2009, 373, 684–691. [Google Scholar] [CrossRef]
- Brekke, K.R.; Grasdal, A.L.; Holmas, T.H. Regulation and pricing of pharmaceuticals: Reference pricing or price cap regulation? Eur. Econ. Rev. 2009, 53, 170–185. [Google Scholar] [CrossRef]
- Lionberger, R.A. FDA Critical Path Initiatives: Opportunities for generic drug development. AAPS J. 2008, 10, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Kanavos, P. Measuring performance in off-patent drug markets: A methodological framework and empirical evidence from twelve EU member states. Health Policy 2014, 118, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Sherer, F.M. The Pharmaceutical Industry. In Handbook of Health Economics; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Dunne, S.; Shannon, B.; Dunne, C.; Cullen, W. A Review of the differences and similarities between generic drugs and their originator counterparts, including economic benefits associated with usage of generic medicines, using Ireland as a case study. BMC Pharmacol. Toxicol. 2013, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, J.A.; Zeckhauser, R. Generic script share and the price of brand-name drugs: The role of consumer choice. Int. J. Health Care Financ. Econ. 2009, 9, 291–316. [Google Scholar] [CrossRef] [Green Version]
- Desai, R.J.; Sarpatwari, A.; Dejene, S.; Khan, N.F.; Lii, J.; Rogers, J.R.; Dutcher, S.K.; Raofi, S.; Bohn, J.; Connolly, J.; et al. Differences in rates of switchbacks after sitching from branded to authorized generic and branded to generic drug products: Cohort study. BMJ 2018, 361, k1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration. Generic Drugs Facts. 2018. Available online: https://www.fda.gov/drugs/generic-drugs/generic-drug-facts (accessed on 12 January 2021).
- Hornecker, J.R. Generic drugs: History, approval process, and current challenges. U S Pharmacist. 2009, 34, 26–30. [Google Scholar]
- Ghislandi, S.; Krulichova, I.; Garattini, L. Pharmaceutical policy in Italy: Towards a structural change? Health Policy 2005, 72, 53–63. [Google Scholar] [CrossRef]
- Ferreira, V.L.; Da Veiga, C.R.P.; Kudlawicz-Franco, C.; Scalercio, P.; Da Veiga, C.P. Generic drugs in times of economic crisis: Are there changes in consumer purchase intention? J. Retail. Consum. Serv. 2017, 37, 1–7. [Google Scholar] [CrossRef]
- Aufegger, L.; Yanar, C.; Darzi, A.; Bicknell, C. The risk-value trade-off: Price and brand information impact consumers’ intentions to purchase OTC drugs. J. Pharm. Policy Pract. 2021, 14, 11. [Google Scholar] [CrossRef]
- Shekhar, S.K.; Jose, T.P.; Rehin, K.R. Consumer buying behaviour and attitude towards pharmaceuticals. Int. J. Res. Pharm. Sci. 2019, 10, 4. [Google Scholar]
- Greene, J.A.; Kesselheim, A.S. Why do the same drugs look different? Pills, trade dress, and public health. N. Engl. J. Med. 2011, 365, 1. [Google Scholar] [CrossRef]
- Madahi, A.; Sukati, I. The effect of external factors on purchase intention amongst young generation in Malaysia. Int. Bus. Res. 2012, 5, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Keller, K.L. Building customer-based brand equity. Mark. Manag. 2001, 10, 14–19. [Google Scholar]
- John, A.H.; Jagdish, N.S. Theory of Buyer Behavior (Marketing); John Wiley & Sons: New York, NY, USA, 1969. [Google Scholar]
- Domeyer, P.J.; Katsari, V.; Sarafis, P.; Aletras, V.; Niakas, D. Greek students’ attitudes, perception and knowledge regarding generic medicines in times of economic crisis: A cross-sectional study. BMC Med. Educ. 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Ghosh, A. Retail Management Dryden Press; Fort Worth: Chicago, IL, USA, 1990. [Google Scholar]
- Warayuanti, W.; Suyanto, A. The influence of lifestyles and consumers attitudes on product purchasing decision via online shopping in Indonesia. Eur. J. Bus. Manag. 2015, 7, 74–80. [Google Scholar]
- Abzakh, A.A.; Ling, K.C.; Alkilani, K. The impact of perceived risks on the consumer resistance towards generic drugs in the Malaysia pharmaceutical industry. Int. J. Bus. Manag. 2013, 8, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Straka, R.J.; Keohane, D.J.; Liu, L.Z. Potential clinical and economic impact of switching branded medications to generics. Am. J. Ther. 2017, 24, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Salhia, H.O.; Ali, A.; Rezk, N.L.; El Metwally, A. Perception and attitude of physicians toward local generic medicines in Saudi Arabia: A questionnaire-based study. Saudi Pharm. J. 2015, 23, 397–404. [Google Scholar] [CrossRef] [Green Version]
- Ganther, J.M.; Kreling, D.H. Consumer perceptions of risk and required cost savings for generic prescription drugs. J. Am. Pharm. Assoc. 2000, 40, 378–383. [Google Scholar] [CrossRef]
- Hughes, J.W.; Moore, M.J.; Snyder, E.A. “Napsterizing” Pharmaceuticals: Access, Innovation, and Consumer Welfare. Natl. Bur. Econ. Res. 2002. [Google Scholar] [CrossRef]
- Sewell, K.; Andreae, S.; Luke, E.; Safford, M.M. Perceptions of and Barriers to Use of Generic Medications in a Rural African American Population, Alabama, 2011. Prev. Chronic Dis. 2012, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Muzumdar, J.M.; Schommer, J.C.; Hadsall, R.S.; Huh, J. Effects of anthropomorphic images and narration styles in promotional messages for generic prescription drugs. Res. Soc. Adm. Pharm. 2013, 9, 60–79. [Google Scholar] [CrossRef]
- Figueiras, M.J.; Marcelino, D.; Cortes, M.A. People’s view on the level of agreement of generic medicines for different illnesses Pharmacy. World Sci. 2008, 30, 590–594. [Google Scholar]
- Fraeyman, J.; Peeters, L.; Van Hal, G.; Beutels, P.; De Meyer, G.R.Y.; Loof, H. Consumer Choice Between Common Generic and Brand Medicines in a Country with a Small Generic Market. J. Manag. Care Spec. Pharm. 2015, 21, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.; Karigome, H.; Sakurada, T.; Satoh, N.; Ueda, S. Patients’ attitudes towards generic drug substitution in Japan. Health Policy 2011, 99, 60–65. [Google Scholar] [CrossRef]
- Gillbody, S.; Wilson, P.; Watt, I. Benefits and harms of direct to consumer advertising: A systematic review. BMJ Qual. Saf. 2005, 14, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Rida, N.A.; Zaidan, M.; Ibrahim, M.I.M. An exploratory insight on pharmaceutical sector and pricing policies in Qatar. Glob. J. Pharm. Pharm. Sci. 2017, 1, 4. [Google Scholar]
- Pruszydlo, M.G.; Walk-Fritz, S.U.; Hoppe-Tichy, T.; Kaltschmidht, J.; Haefeli, W.E. Development and evaluation of a computerised clinical decision support system for switching drugs at the interface between primary and tertiary care. BMC Med. Inform. Decis. Mak. 2012, 12, 137. [Google Scholar] [CrossRef] [Green Version]
- Dylst, P.; Vulto, A.; Godman, B.; Simoens, S. Generic medicines: Solutions for a sustainable drug market? Appl. Health Econ. Health Policy 2013, 11, 437–443. [Google Scholar] [CrossRef]
- Fischer, K.E.; Stargardt, T. The diffusion of generics after patent expiry in Germany. Eur. J. Health Econ. 2016, 17, 1027–1040. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.N.; Harris, I.; Frank, G.; Kiptanui, Z.; Qian, J.; Hansen, R. Influencers of generic drug utilization: A systematic review. Res. Soc. Adm. Pharm. 2018, 14, 619–627. [Google Scholar] [CrossRef] [PubMed]
- U.S. Government Accountability Office. Drug Pricing: Research on Savings from Generic Drug Use. 2012. Available online: http://www.gao.gov/assets/590/588064.pdf (accessed on 17 March 2021).
- US Department of Health and Human Services. Guidance for Industry: ANDAsubmissions—Amendments and Easily Correctable Deficiencies under GDUFA. 2014. Available online: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM404440.pdf (accessed on 17 March 2021).
- Bongers, F.; Carradinha, H. How to increase patient access to generic medicines in European healthcare systems. EGA Health Economics Committee. Eur. Generic Med. Assoc. 2009, 1–52. [Google Scholar]
- Kauppinen-Räisänen, H.; Owusu Richard, A.; Abeeku, B.B. Brand salience of OTC pharmaceuticals through package appearance. Int. J. Pharm. Health Mark. 2012, 6, 230–249. [Google Scholar] [CrossRef]
- Love, J. The production of generic drugs in India. BMJ 2011, 342, d1694. [Google Scholar] [CrossRef] [PubMed]
- Caliari, T.; Ruiz, R.M. Brazilian pharmaceutical industry and generic drugs policy: Impacts on structure and innovation and recent developments. Sci. Public Policy 2014, 41, 245–256. [Google Scholar] [CrossRef]
- Tranfield, D.; Denyer, D.; Smart, P. Towards a methodology for developing evidence-informed management knowledge by means of systematic review. Br. J. Manag. 2003, 14, 207–222. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Petersen, K.; Feldt, R.; Mujtaba, S.; Mattsson, M. Systematic mapping studies in software engineering. In Proceedings of the 12th International Conference on Evaluation and Assessment in Software Engineering, Christchurch, New Zealand, 28–29 June 2018; pp. 71–80. [Google Scholar]
- Zupic, I.; Cater, T. Bibliometric methods in management and organization. Organ. Res. Methods 2015, 18, 429–472. [Google Scholar] [CrossRef]
- Marx, W.; Bornmann, L.; Barth, A.; Leydesdorff, L. Detecting the historical roots of research fields by reference publication year spectroscopy (RPYS). J. Am. Soc. Inf. Sci. Technol. 2014, 65, 751–764. [Google Scholar] [CrossRef] [Green Version]
- Glänzel, W. Bibliometric methods for detecting and analysing emerging research topics. Prof. Inf. 2012, 21, 194–201. [Google Scholar] [CrossRef]
- Cobo, M.J.; López-Herrera, A.G.; Herrera-Viedma, E.; Herrera, F. Science mapping software tools: Review, analysis, and cooperative study among tools. J. Am. Soc. Inf. Sci. Technol. 2011, 62, 7. [Google Scholar] [CrossRef]
- Schmidt, M. The Sankey Diagram in energy and material flow management. Part II: Methodology and current applications. J. Ind. Ecol. 2008, 12, 173–185. [Google Scholar] [CrossRef]
- Prashanth, D.S.; Elias, M.A.; Pati, M.K.; Praveenkumar, A.; Munegovda, C.M.; Bhanuprakash, S.; Sadhana, S.M.; Criel, B.; Migdeli, M.; Devadasan, N. Improving access to medicines for non-communicable diseases in rural India: A mixed methods study protocol using quasi-experimental design. BMC Health Serv. Res. 2016, 16, 421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Zhou, H. From bottom line to consumer’s mind: The framing effects of accounting information. Account. Organ. Soc. 2015, 43, 56–66. [Google Scholar] [CrossRef]
- Newman, C.; Ajay, V.S.; Srinivas, R.; Bhalla, S.; Prabhakaran, D.; Banerjee, A. Drugs for cardiovascular disease in India perspectives of pharmaceutical executives and government officials on access and development a qualitative analysis. J. Pharm. Policy Pract. 2016, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerbini, C.; Luceri, B.; Vergura, D.T. Leveraging consumer’s behaviour to promote generic drugs in Italy. Health Policy 2017, 4, 397–406. [Google Scholar] [CrossRef]
- Dunne, S.S.; Dunne, C.P. What do people really think of generic medicines? A systematic review and critical appraisal of literature on stakeholder perceptions of generic drugs. BMC Med. 2015, 13, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, P.; Barbosa, H. Choice of mandatory prescribed drugs in Portugal: A consumers’ perspective International. J. Pharm. Healthc. Mark. 2017, 11, 439–454. [Google Scholar] [CrossRef]
- Gómez, M.; Rozano, M. Consumer dynamics in a nonmature generic drugs market: A causal model explaining intention to purchase in Spain. Drug Inf. J. 2012, 46, 207–215. [Google Scholar] [CrossRef]
- Dongre, Y.; Mahadevappa, B.; Rohini, R. Building access to healthcare in rural India: Possibility and reasibility of low-cost medicine. Int. J. Pharm. Healthc. Mark. 2010, 4, 396–407. [Google Scholar] [CrossRef]
- Nardi, E.P.; Ferraz, M.B. Perception of the value of generic drugs in São Paulo. Cad. Saúde Pública 2016, 32, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bornmann, L.; Anegón, F.M.; Leydesdorff, L. Do scientific advancements lean on the shoulders of giants? A bibliometric investigation of the Ortega Hypothesis. PLoS ONE 2010, 5, e13327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szasz, T.S.; Hollender, M.H. The basic models of the doctor-patient relationship. AMA Arch. Intern. Med. 1956, 97, 585–592. [Google Scholar] [CrossRef]
- Himmel, W.; Simmenroth-Nayda, A.; Niebling, W.; Ledig, T.; Jansen, R.-D.; Kochem, M.M.; Gleiter, C.H.; Hummers-Pradier, E. What do primary care patients think about generic drugs? Int. J. Clin. Pharmacol. Ther. 2005, 43, 472–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintal, C.; Mendes, P. Underuse of generic medicines in Portugal: An empirical study on the perceptions and attitudes of patients and pharmacists. Health Policy 2012, 104, 61–68. [Google Scholar] [CrossRef]
- Kohli, E.; Buller, A. Factors influencing consumer purchasing patterns of generic versus brand name over-the-counter drugs. South. Med. J. 2013, 106, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Gauld, R.; Norris, P.; Rades, T. “This body does not want free medicines”: South African consumer perceptions of drug quality. Health Policy Plan. 2010, 25, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Shrank, W.H.; Cadarette, S.M.; Cox, E.; Fischer, M.A.; Mehta, J.; Brookhart, A.M.; Avorn, J.; Choudhry, N.K. Is there a relationship between patient beliefs or communication about generic drugs and medication utilization? Med. Care 2009, 47, 319–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrank, W.H.; Liberman, J.N.; Fischer, M.A.; Girdish, C.; Brennan, T.A.; Choudhry, N.K. Physician perceptions about generic drugs. Ann. Pharmacother. 2011, 45, 31–38. [Google Scholar] [CrossRef]
- Shrank, W.H.; Cox, E.R.; Fischer, M.A.; Mehta, J.; Choudhry, N.K. Patient’s perceptions of generic medications. Health Aff. 2009, 28, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babar, Z.U.D.; Stewart, J.; Reddy, S.; Alzaher, W.; Vareed, P.; Yacoub, N.; Dhroptee, B.; Rew, A. An evaluation of consumers’ knowledge, perceptions and attitudes regarding generic medicines in Auckland. Pharm. World Sci. 2010, 32, 440–448. [Google Scholar] [CrossRef]
- Azhar, S.; Hassali, M.A.; Ibrahim, M.I.M.; Ahmad, M.; Masood, I.; Shafie, A. The role of pharmacists in developing countries: The current scenario in Pakistan. Hum. Resour. Health 2009, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Kjoenniksen, I.; Lindbaek, M.; Granas, A.G. Patients’ attitudes towards and experiences of generic drug substitution in Norway. Pharm. World Sci. 2006, 28, 284. [Google Scholar]
- Heikkilä, R.; Mäntyselka, P.; Hartikainen-Herranen, K.; Ahonen, R. Customers’ and physicians’ opinions of and experiences with generic substitution during the first year in Finland. Health Policy 2007, 82, 366–374. [Google Scholar] [CrossRef]
- Bearden, W.O.; Mason, J.B. Consumer-perceived risk and attitudes toward generically prescribed drugs. J. Appl. Psychol. 1978, 63, 741–746. [Google Scholar] [CrossRef]
- Chawla, K.; Tofighi, T.; Agarwal, A.; Thomas, J.; Mondal, T. A global comparison between brand-name and generic drugs. Indian J. Pharm. Pract. 2014, 7, 23–28. [Google Scholar] [CrossRef] [Green Version]
- IQVIA. US Generic Market-Evolution of Indian Players. 2019. Available online: https://www.iqvia.com/-/media/iqvia/pdfs/asia-pacific/india/us-generics-market-evolution-of-indian-players.pdf (accessed on 8 August 2020).
- Food and Drug Administration. FDA Generic Drugs Facts. 2020. Available online: https://www.fda.gov/drugs/buying-using-medicine-safely/generic-drugs (accessed on 12 January 2021).
- India Brand Equity Foundation (IBEF). Indian Pharmaceutical Industry. 2020. Available online: https://www.ibef.org/industry/pharmaceutical-india.aspx (accessed on 8 November 2020).
- Barros, P.P. Pharmaceutical policies in European countries. Pharm. Mark. Insur. 2010, 22, 3–27. [Google Scholar]
- Hassali, M.A.; Alrasheedy, A.A.; Aljadhey, H. The experiences of implementing generic medicine policy in eight countries: A review and recommendations for a successful promotion of generic medicine use. Saudi Pharm. J. 2014, 22, 491–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuncay, B.; Pagano, S.; De Santis, M.; Cavallo, P. Prescribing Behavior of General Practitioners for Generic Drugs. Int. J. Environ. Res. Public Health 2020, 17, 5919. [Google Scholar] [CrossRef] [PubMed]
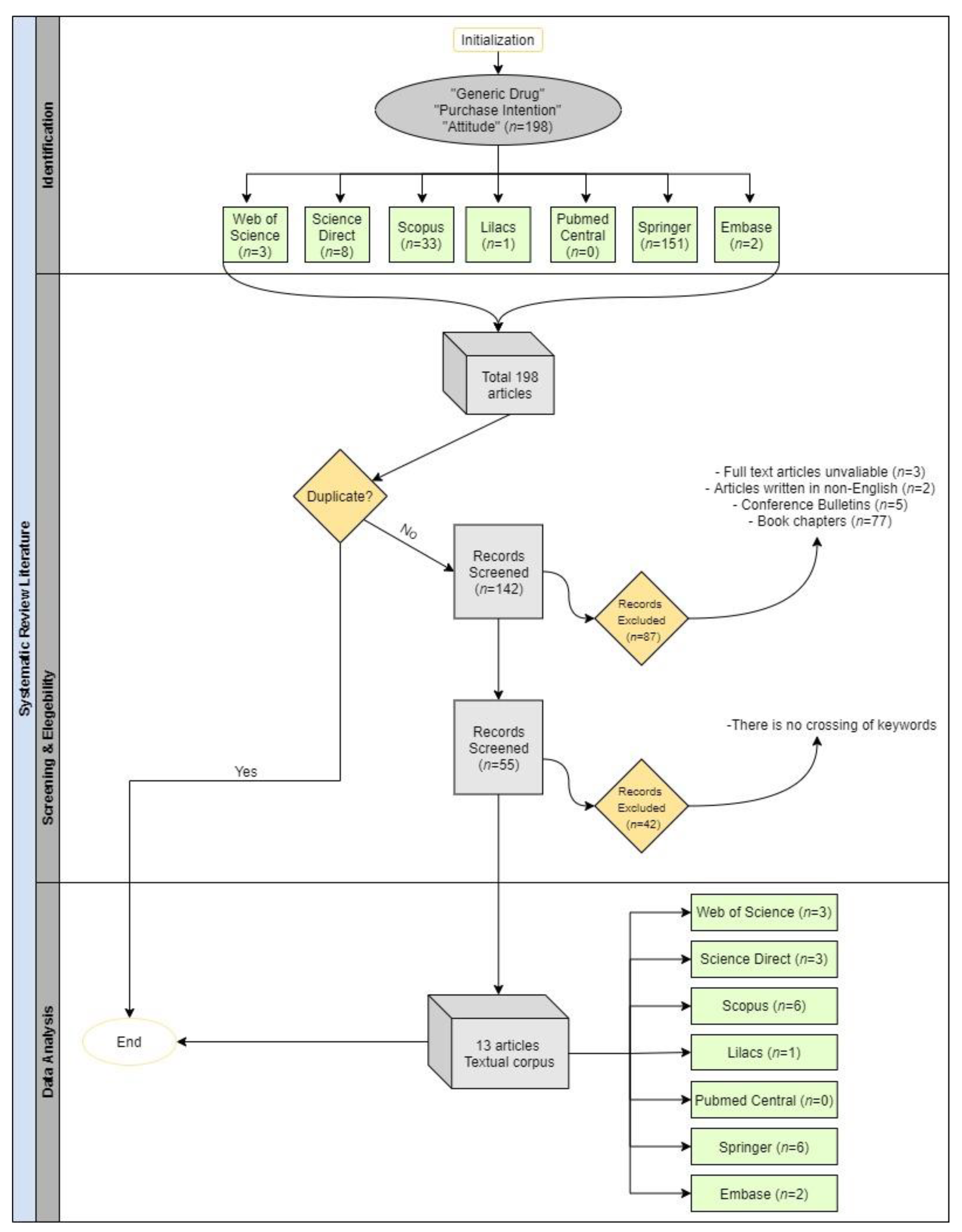





| Search Strings of the Papers in the Corpus | ||
|---|---|---|
| Database | Search String | Results |
| Web of Science | TS = (generic drug and purchase intention and attitude) | 3 |
| Science Direct | “generic drug” and “purchase intention” and “attitude” | 8 |
| Scopus | ALL (“generic drug” AND “purchase intention” AND “attitude”) AND (LIMIT-TO (DOCTYPE, “ar”)) AND (LIMIT-TO (LANGUAGE, “English”)) | 33 |
| Lilacs | “generic drug” and “purchase intention” and “attitude” | 1 |
| Pubmed Central | ((“generic drug”) AND “purchase intention”) AND “attitude” | 0 |
| Springer | purchase intention AND attitude AND “generic drug” | 151 |
| Embase | (‘generic drug’/exp OR ‘generic drug’) AND ‘purchase intention’ AND (‘attitude’/exp OR ‘attitude’) | 2 |
| TOTAL | 198 | |
| Subject | n | Type | Focus | Main Findings | References |
|---|---|---|---|---|---|
| Consumers | Panel data drug utilization 1996–2001 | Secondary Data | How the mix of consumer choices between generic and brand-name drugs might affect the average price of those brand-name drugs that are purchased. | We found that the average price paid by consumers for brand-name drugs falls substantially when generic script share rises. | [8] |
| Consumers | 406 | Questionnaire | Moderating role of an economic crisis in consumer purchase intention of generic drugs | Shopping attitude is positively influenced by both perceived quality and past experience. Role of doctors and pharmacist endorsers have no influence. | [13] |
| Consumers, Doctors, and Pharmacists | 1069 | Experimental study | Understand whether public health interventions in India are affecting the use of and access to generic drugs for non-communicable diseases. | Study generated evidence for health planners on how to optimize health services to improve access to medicines in a particular kind of setting. | [53] |
| Consumers | 563 | Secondary Data | How accounting information and message framing jointly impact consumer choice between brand name and generic drugs | Consumer choice between brand name and generic drugs, information on manufacturers’ profit margins, and costs in different frames evokes associations in memory, predisposing consumers to develop more or less favorable attitudes towards firms and their products. | [54] |
| Students | 162 | Questionnaire | To evaluate the effects of anthropomorphic images and information narration styles. | The search revealed that anthropomorphism of medications and narration styles could play a significant role in promotional messages for pharmaceuticals. | [55] |
| Government Officials and Pharmaceutical Executives | 10 interviews | Interviews | Evaluate through the stakeholders of the pharmaceutical industry the determinants of cardiovascular generic drugs. | Whilst interviewees suggested that government policy plays an important role in shaping the industry, a significant force for change was ascribed to patient-derived factors. | [56] |
| Consumers | 2222 | Survey | Consumers’ purchase intention of generic drugs based on the theoretical framework of the theory of planned behavior | Positive effects of attitude, subjective norm, and past behavior on generic drugs purchase intention, while there are no positive effects on perceived behavioral control. Risk, trust in the pharmacist, brand sensitivity, and self-identity explained consumers’ intention to buy generic drugs. | [29] |
| Doctors, Pharmacists, and Consumers/Patients | 58 Papers | Review article | Systematic search of published studies that focuses on physician, pharmacist, and patient/consumer perspectives. | A key factor in improving the confidence of these cohorts is the provision of information and education, particularly in the areas of equivalency, regulation, and in dispelling myths about generic medicines. Improving opinions regarding generics within the physician cohort may be of critical importance to improve the usage and acceptance of generic medicines in the future. | [57] |
| Patients | 218 | Questionnaire | Substitution of branded drugs by generics | Participative decision-making has no impact on purchase intention of generics, while perceived risk and price consciousness have a significant impact. | [58] |
| Consumers/Patients, Doctors, and Pharmacists | 542 | Questionnaire | To study the causal relationships influencing consumer purchase intention, including perceived risk, experience, and information provided by a physician and pharmacist as antecedents. | We found that the greater the perceived risk, the lower the motivation to request generic drugs, an effect that is reduced by the positive effect of experience. | [59] |
| Stockists, Doctors, and Consumers | 155 | Questionnaire | To evaluate the possibility of marketing specific low-cost drugs. | Cost of medicine is a significant factor influencing patients’ attitude towards the consumption of medicine. | [60] |
| Consumers | 1278 | Questionnaire | Influence of diseases on the level of agreement of prescription of generic drugs and sociodemographic factors. | Beliefs about the use of generic medicines are associated with the nature of the illness for which they are prescribed. | [30] |
| Opinion-leaders, Consumers/Patients | 150 | Questionnaire | Consumers, doctors, and pharmacists’ perception of generic drugs | Multiple factors may contribute to the decision to buy a generic drug. Price seems to be an important factor as well as effectiveness, safety, and trust. | [61] |
| Sources | Articles | SJR Best Quartil |
|---|---|---|
| International Journal of Pharmaceutical and Healthcare Marketing | 2 | Q3 |
| Accounting Organizations and Society | 1 | Q1 |
| BMC Health Services Research | 1 | Q1 |
| BMC Medicine | 1 | Q1 |
| Cadernos de Saúde Publica | 1 | Q2 |
| Drug Information Journal | 1 | Q1 |
| Health Policy | 1 | Q1 |
| International Journal of Health Care Finance & Economics | 1 | Q1 |
| Journal of Pharmaceutical Policy and Practice | 1 | Q1 |
| Journal of Retailing and Consumer Services | 1 | Q1 |
| Pharmacy World & Science | 1 | - |
| Research in Social & Administrative Pharmacy | 1 | Q1 |
| Clusters | Authors | Year | Journal | Total Link Strength | Citations |
|---|---|---|---|---|---|
| Cluster 1–Consumer attitude and behavior | Himmel W. | 2005 | Int. J. Clin. Pharm. Th. | 41 | 6 |
| Quintal C. | 2012 | Health Policy | 39 | 5 | |
| Kobayashi E. | 2011 | Health Policy | 33 | 4 | |
| Kohli E. | 2013 | South Med. J. | 27 | 3 | |
| Patel A. | 2010 | Health Policy | 27 | 3 | |
| Shrank W.H. | 2009 | Med. Care | 23 | 5 | |
| Shrank W.H. | 2011 | Ann. Pharmacother. | 22 | 5 | |
| Cluster 2–Views of patients and health professionals | Shrank W.H. | 2009 | Health Affair | 28 | 4 |
| Babar Z. | 2010 | Pharm. World Sci. | 27 | 3 | |
| Hassali M. | 2009 | Int. J. Clin. Pharm. Pract. | 27 | 3 | |
| Kjoenniksen I. | 2006 | Pharm. World Sci. | 23 | 3 | |
| Cluster 3–Risks associated with generic drugs | Heikkila R. | 2007 | Health Policy | 23 | 3 |
| Ganther J.M. | 2000 | J. Am. Pharm. Assoc. | 19 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arcaro, R.; da Veiga, C.R.P.; da Silva, W.V.; Pereira da Veiga, C. Attitude and Purchase Intention to Generic Drugs. Int. J. Environ. Res. Public Health 2021, 18, 4579. https://doi.org/10.3390/ijerph18094579
Arcaro R, da Veiga CRP, da Silva WV, Pereira da Veiga C. Attitude and Purchase Intention to Generic Drugs. International Journal of Environmental Research and Public Health. 2021; 18(9):4579. https://doi.org/10.3390/ijerph18094579
Chicago/Turabian StyleArcaro, Ricardo, Cássia Rita Pereira da Veiga, Wesley Vieira da Silva, and Claudimar Pereira da Veiga. 2021. "Attitude and Purchase Intention to Generic Drugs" International Journal of Environmental Research and Public Health 18, no. 9: 4579. https://doi.org/10.3390/ijerph18094579
APA StyleArcaro, R., da Veiga, C. R. P., da Silva, W. V., & Pereira da Veiga, C. (2021). Attitude and Purchase Intention to Generic Drugs. International Journal of Environmental Research and Public Health, 18(9), 4579. https://doi.org/10.3390/ijerph18094579







